Lung Nodules in Melanoma Patients: Morphologic Criteria to Differentiate Non-Metastatic and Metastatic Lesions
Abstract
:1. Introduction
2. Methods
2.1. Overview and Study Design
2.2. Patients—Baseline Characteristics and Follow-Up
2.3. Baseline CT Scan Protocol
2.4. Lung Nodules
2.5. Lung Nodules on Follow-Up Chest CT Scan
2.6. Statistics
3. Results
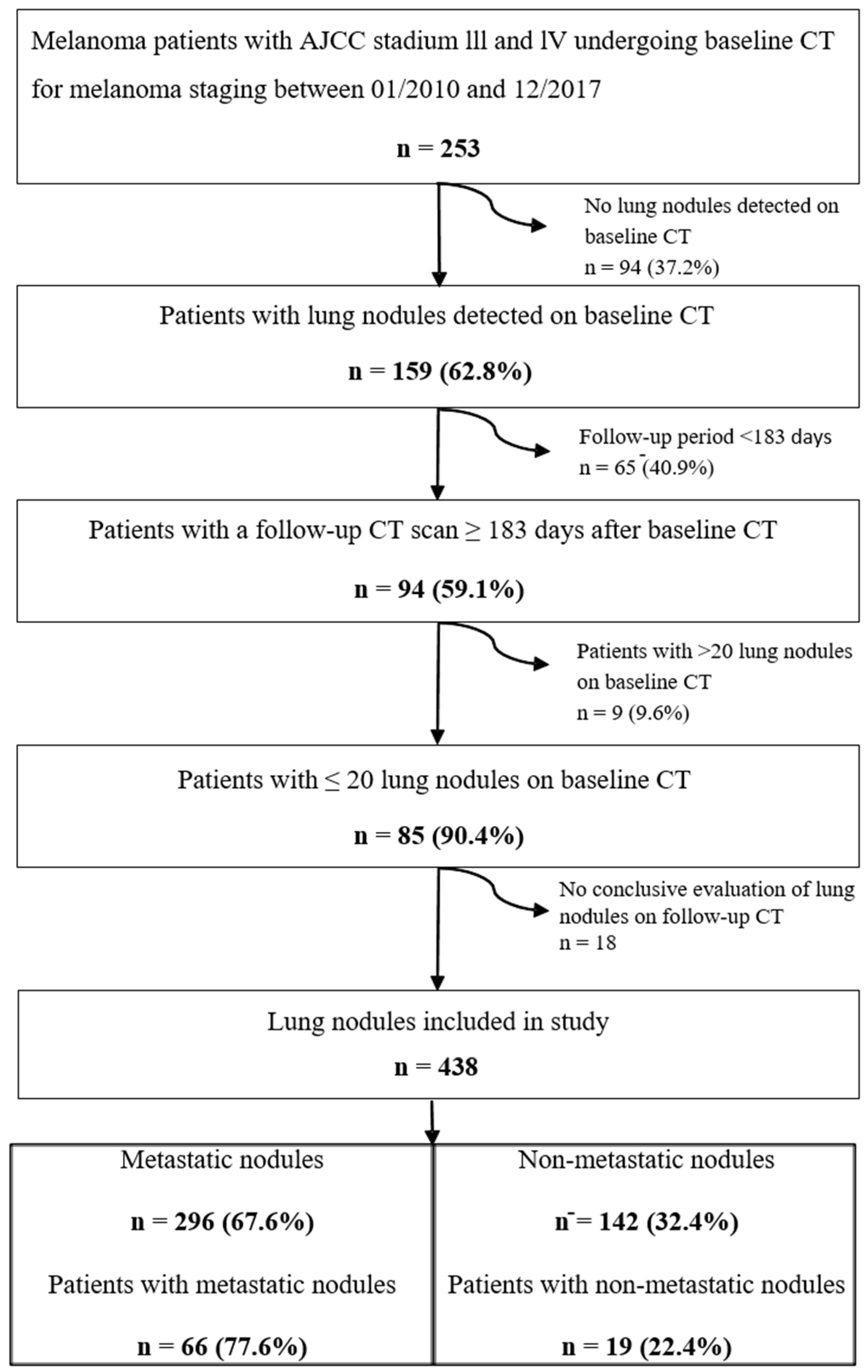
3.1. Patients
3.2. Evaluation of Lung Nodules
3.3. Metastatic Lung Nodules
3.4. Indicators for Metastatic or Non-Metastatic Nodules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guy, G.P., Jr.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C.; Centers for Disease Control and Prevention (CDC). Vital signs: Melanoma incidence and mortality trends and projections—United States, 1982–2030. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 591–596. [Google Scholar] [PubMed]
- Knackstedt, T.; Knackstedt, R.W.; Couto, R.; Gastman, B. Malignant Melanoma: Diagnostic and Management Update. Plast. Reconstr. Surg. 2018, 142, 202e–216e. [Google Scholar] [CrossRef] [PubMed]
- Belhocine, T.Z.; Scott, A.M.; Even-Sapir, E.; Urbain, J.L.; Essner, R. Role of nuclear medicine in the management of cutaneous malignant melanoma. J. Nucl. Med. 2006, 47, 957–967. [Google Scholar] [PubMed]
- Neuman, H.B.; Patel, A.; Hanlon, C.; Wolchok, J.D.; Houghton, A.N.; Coit, D.G. Stage-IV melanoma and pulmonary metastases: Factors predictive of survival. Ann. Surg. Oncol. 2007, 14, 2847–2853. [Google Scholar] [CrossRef]
- Sandru, A.; Voinea, S.; Panaitescu, E.; Blidaru, A. Survival rates of patients with metastatic malignant melanoma. J. Med. Life 2014, 7, 572–576. [Google Scholar] [PubMed]
- Manola, J.; Atkins, M.; Ibrahim, J.; Kirkwood, J. Prognostic factors in metastatic melanoma: A pooled analysis of Eastern Cooperative Oncology Group trials. J. Clin. Oncol. 2000, 18, 3782–3793. [Google Scholar] [CrossRef]
- Swetter, S.M.; Tsao, H.; Bichakjian, C.K.; Curiel-Lewandrowski, C.; Elder, D.E.; Gershenwald, J.E.; Guild, V.; Grant-Kels, J.M.; Halpern, A.C.; Johnson, T.M.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2019, 80, 208–250. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.S.; Dercle, L.; Goldmacher, G.V.; Yang, H.; Connors, D.; Tang, Y.; Karovic, S.; Zhao, B.; Carvajal, R.D.; Robert, C.; et al. Comparing RECIST 1.1 and iRECIST in advanced melanoma patients treated with pembrolizumab in a phase II clinical trial. Eur. Radiol. 2021, 31, 1853–1862. [Google Scholar] [CrossRef]
- Callister, M.E.; Baldwin, D.R. How should pulmonary nodules be optimally investigated and managed? Lung Cancer 2016, 91, 48–55. [Google Scholar] [CrossRef]
- Martini, K.; Bluthgen, C.; Eberhard, M.; Schonenberger, A.L.N.; De Martini, I.; Huber, F.A.; Barth, B.K.; Euler, A.; Frauenfelder, T. Impact of Vessel Suppressed-CT on Diagnostic Accuracy in Detection of Pulmonary Metastasis and Reading Time. Acad. Radiol. 2020. [Google Scholar] [CrossRef]
- Milanese, G.; Eberhard, M.; Martini, K.; Vittoria De Martini, I.; Frauenfelder, T. Vessel suppressed chest Computed Tomography for semi-automated volumetric measurements of solid pulmonary nodules. Eur. J. Radiol. 2018, 101, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Horeweg, N.; van Rosmalen, J.; Heuvelmans, M.A.; van der Aalst, C.M.; Vliegenthart, R.; Scholten, E.T.; ten Haaf, K.; Nackaerts, K.; Lammers, J.W.; Weenink, C.; et al. Lung cancer probability in patients with CT-detected pulmonary nodules: A prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol. 2014, 15, 1332–1341. [Google Scholar] [CrossRef]
- Borghesi, A.; Michelini, S.; Scrimieri, A.; Golemi, S.; Maroldi, R. Solid Indeterminate Pulmonary Nodules of Less Than 300 mm(3): Application of Different Volume Doubling Time Cut-offs in Clinical Practice. Diagnostics 2019, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Muller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Park, J.H.; Kim, Y.H.; Lee, K.W.; Kim, J.W.; Oh, H.K.; Jeon, J.J.; Yoon, H.; Kim, J.; Lee, K.H. Diagnostic Yield and False-Referral Rate of Staging Chest CT in Patients with Colon Cancer. Radiology 2018, 289, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Bankier, A.A.; MacMahon, H.; Goo, J.M.; Rubin, G.D.; Schaefer-Prokop, C.M.; Naidich, D.P. Recommendations for Measuring Pulmonary Nodules at CT: A Statement from the Fleischner Society. Radiology 2017, 285, 584–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohr, P.; Eggermont, A.M.; Hauschild, A.; Buzaid, A. Staging of cutaneous melanoma. Ann. Oncol. 2009, 20, vi14–vi21. [Google Scholar] [CrossRef]
- Gould, M.K.; Tang, T.; Liu, I.L.; Lee, J.; Zheng, C.; Danforth, K.N.; Kosco, A.E.; Di Fiore, J.L.; Suh, D.E. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am. J. Respir. Crit. Care Med. 2015, 192, 1208–1214. [Google Scholar] [CrossRef]
- Petersen, R.P.; Hanish, S.I.; Haney, J.C.; Miller, C.C., III; Burfeind, W.R., Jr.; Tyler, D.S.; Seigler, H.F.; Wolfe, W.; D’Amico, T.A.; Harpole, D.H., Jr. Improved survival with pulmonary metastasectomy: An analysis of 1720 patients with pulmonary metastatic melanoma. J. Thorac. Cardiovasc. Surg. 2007, 133, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanamiya, M.; Aoki, T.; Yamashita, Y.; Kawanami, S.; Korogi, Y. Frequency and significance of pulmonary nodules on thin-section CT in patients with extrapulmonary malignant neoplasms. Eur. J. Radiol. 2012, 81, 152–157. [Google Scholar] [CrossRef]
- Caparica, R.; Mak, M.P.; Rocha, C.H.; Velho, P.H.I.; Viana, P.; Moura, M.R.L.; Menezes, M.R.; Amato, M.B.P.; Feher, O. Pulmonary Nodules in Patients with Nonpulmonary Cancer: Not Always Metastases. J. Glob. Oncol. 2016, 2, 138–144. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef] [Green Version]
- Borghesi, A.; Tironi, A.; Michelini, S.; Scrimieri, A.; Benetti, D.; Maroldi, R. Two synchronous lung metastases from malignant melanoma: The same patient but different morphological patterns. Eur. J. Radiol. Open 2019, 6, 287–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snoeckx, A.; Reyntiens, P.; Desbuquoit, D.; Spinhoven, M.J.; Van Schil, P.E.; van Meerbeeck, J.P.; Parizel, P.M. Evaluation of the solitary pulmonary nodule: Size matters, but do not ignore the power of morphology. Insights Imaging 2018, 9, 73–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prigent, K.; Lasnon, C.; Ezine, E.; Janson, M.; Coudrais, N.; Joly, E.; Cesaire, L.; Stefan, A.; Depontville, M.; Aide, N. Assessing immune organs on (18)F-FDG PET/CT imaging for therapy monitoring of immune checkpoint inhibitors: Inter-observer variability, prognostic value and evolution during the treatment course of melanoma patients. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Hindie, E.; Sarandi, F.; Banayan, S.; Groheux, D.; Rubello, D.; Vercellino, L.; Toubert, M.E.; Moretti, J.L.; Lebbe, C. Nuclear Medicine in Early-Stage Melanoma: Sentinel Node Biopsy-FDG-PET/CT. PET Clin. 2011, 6, 9–25. [Google Scholar] [CrossRef]
- Banerjee, S.; Singh, S.K.; Chakraborty, A.; Das, A.; Bag, R. Melanoma Diagnosis Using Deep Learning and Fuzzy Logic. Diagnostics 2020, 10, 577. [Google Scholar] [CrossRef]
- Li, D.; Mikela Vilmun, B.; Frederik Carlsen, J.; Albrecht-Beste, E.; Ammitzbol Lauridsen, C.; Bachmann Nielsen, M.; Lindskov Hansen, K. The Performance of Deep Learning Algorithms on Automatic Pulmonary Nodule Detection and Classification Tested on Different Datasets That Are Not Derived from LIDC-IDRI: A Systematic Review. Diagnostics 2019, 9, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannil, M.; Eberhard, M.; von Spiczak, J.; Heindel, W.; Alkadhi, H.; Baessler, B. Artificial Intelligence and Texture Analysis in Cardiac Imaging. Curr. Cardiol. Rep. 2020, 22, 131. [Google Scholar] [CrossRef]


| Baseline Characteristics of Patients (n = 85) | ||
|---|---|---|
| Sex | ||
| Male | 53 | 62% |
| Female | 32 | 38% |
| Age (years) | 58 | 32–80 |
| Smoking status | ||
| Never-smokers | 60 | 71% |
| Current smoker | 11 | 13% |
| Former smokers | 14 | 17% |
| Melanoma staging | ||
| T1 | 8 | 9% |
| T2 | 13 | 15% |
| T3 | 27 | 32% |
| T4 | 19 | 22% |
| n/a | 18 | 21% |
| N0 | 9 | 11% |
| N1 | 20 | 24% |
| N2 | 22 | 26% |
| N3 | 21 | 25% |
| n/a | 13 | 15% |
| M0 | 22 | 26% |
| M1 | 63 | 74% |
| Primary tumor site | ||
| Cutaneous | 71 | 84% |
| Head and Neck | 15 | 18% |
| Body trunk | 26 | 31% |
| Upper extremities | 12 | 14% |
| Lower extremities | 18 | 21% |
| Connective tissue (choroidal) | 2 | 2% |
| Non-keratinized stratified squamous epithelium (vaginal) | 2 | 2% |
| Unknown | 10 | 12% |
| All Lung Nodules | Non-Metastatic Lesions | Metastases | p-Value | ||||
|---|---|---|---|---|---|---|---|
| N = 438 | N = 142 | 32% | N = 296 | 68% | |||
| Side | |||||||
| Right | 253 | 58% | 82 | 58% | 171 | 58% | 1.00 |
| Left | 185 | 42% | 60 | 42% | 125 | 42% | |
| Lobe | |||||||
| Lower lobe | 218 | 50% | 65 | 46% | 153 | 52% | 0.410 |
| Upper lobe | 177 | 40% | 64 | 45% | 113 | 38% | |
| Middle lobe | 43 | 10% | 13 | 9% | 30 | 10% | |
| Location | |||||||
| Central | 122 | 28% | 28 | 20% | 94 | 32% | 0.009 |
| Periphery | 316 | 72% | 114 | 80% | 202 | 68% | |
| Morphology | |||||||
| Subsolid | 53 | 12% | 29 | 20% | 24 | 8% | <0.001 |
| Non-solid | 8 | 2% | 7 | 5% | 1 | 0.3% | |
| Part-solid | 45 | 10% | 22 | 15% | 23 | 8% | |
| Solid | 385 | 88% | 113 | 80% | 272 | 92% | |
| Margin | |||||||
| Ill-defined | 24 | 6% | 7 | 6% | 17 | 6% | 0.821 |
| Well-defined | 387 | 88% | 129 | 91% | 258 | 87% | |
| Lobulated | 22 | 5% | 5 | 4% | 17 | 26% | |
| Spiculated | 5 | 1% | 1 | 1% | 4 | 1% | |
| Grouping | |||||||
| Solitary | 425 | 97% | 138 | 97% | 287 | 97% | 1.000 |
| Grouped | 13 | 3% | 4 | 3% | 9 | 3% | |
| Pleura retraction | |||||||
| No | 431 | 98% | 142 | 100% | 289 | 98% | 0.102 |
| Yes | 7 | 2% | 0 | 0% | 7 | 2% | |
| Air bronchogram | |||||||
| No | 436 | 99.5% | 142 | 100% | 294 | 99% | 1.000 |
| Yes | 2 | 0.5% | 0 | 0% | 2 | 1% | |
| Feeding Vessel | |||||||
| No | 214 | 49% | 76 | 54% | 138 | 47% | 0.186 |
| Yes | 224 | 51% | 66 | 46% | 158 | 53% | |
| Calcification | |||||||
| No | 425 | 97.0% | 129 | 91% | 296 | 100% | <0.001 |
| Yes | 13 | 3.0% | 13 | 9% | 0 | 0% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stadelmann, S.A.; Blüthgen, C.; Milanese, G.; Nguyen-Kim, T.D.L.; Maul, J.-T.; Dummer, R.; Frauenfelder, T.; Eberhard, M. Lung Nodules in Melanoma Patients: Morphologic Criteria to Differentiate Non-Metastatic and Metastatic Lesions. Diagnostics 2021, 11, 837. https://doi.org/10.3390/diagnostics11050837
Stadelmann SA, Blüthgen C, Milanese G, Nguyen-Kim TDL, Maul J-T, Dummer R, Frauenfelder T, Eberhard M. Lung Nodules in Melanoma Patients: Morphologic Criteria to Differentiate Non-Metastatic and Metastatic Lesions. Diagnostics. 2021; 11(5):837. https://doi.org/10.3390/diagnostics11050837
Chicago/Turabian StyleStadelmann, Simone Alexandra, Christian Blüthgen, Gianluca Milanese, Thi Dan Linh Nguyen-Kim, Julia-Tatjana Maul, Reinhard Dummer, Thomas Frauenfelder, and Matthias Eberhard. 2021. "Lung Nodules in Melanoma Patients: Morphologic Criteria to Differentiate Non-Metastatic and Metastatic Lesions" Diagnostics 11, no. 5: 837. https://doi.org/10.3390/diagnostics11050837
APA StyleStadelmann, S. A., Blüthgen, C., Milanese, G., Nguyen-Kim, T. D. L., Maul, J.-T., Dummer, R., Frauenfelder, T., & Eberhard, M. (2021). Lung Nodules in Melanoma Patients: Morphologic Criteria to Differentiate Non-Metastatic and Metastatic Lesions. Diagnostics, 11(5), 837. https://doi.org/10.3390/diagnostics11050837








