Towards a Treatment for Neuroinflammation in Epilepsy: Interleukin-1 Receptor Antagonist, Anakinra, as a Potential Treatment in Intractable Epilepsy
Abstract
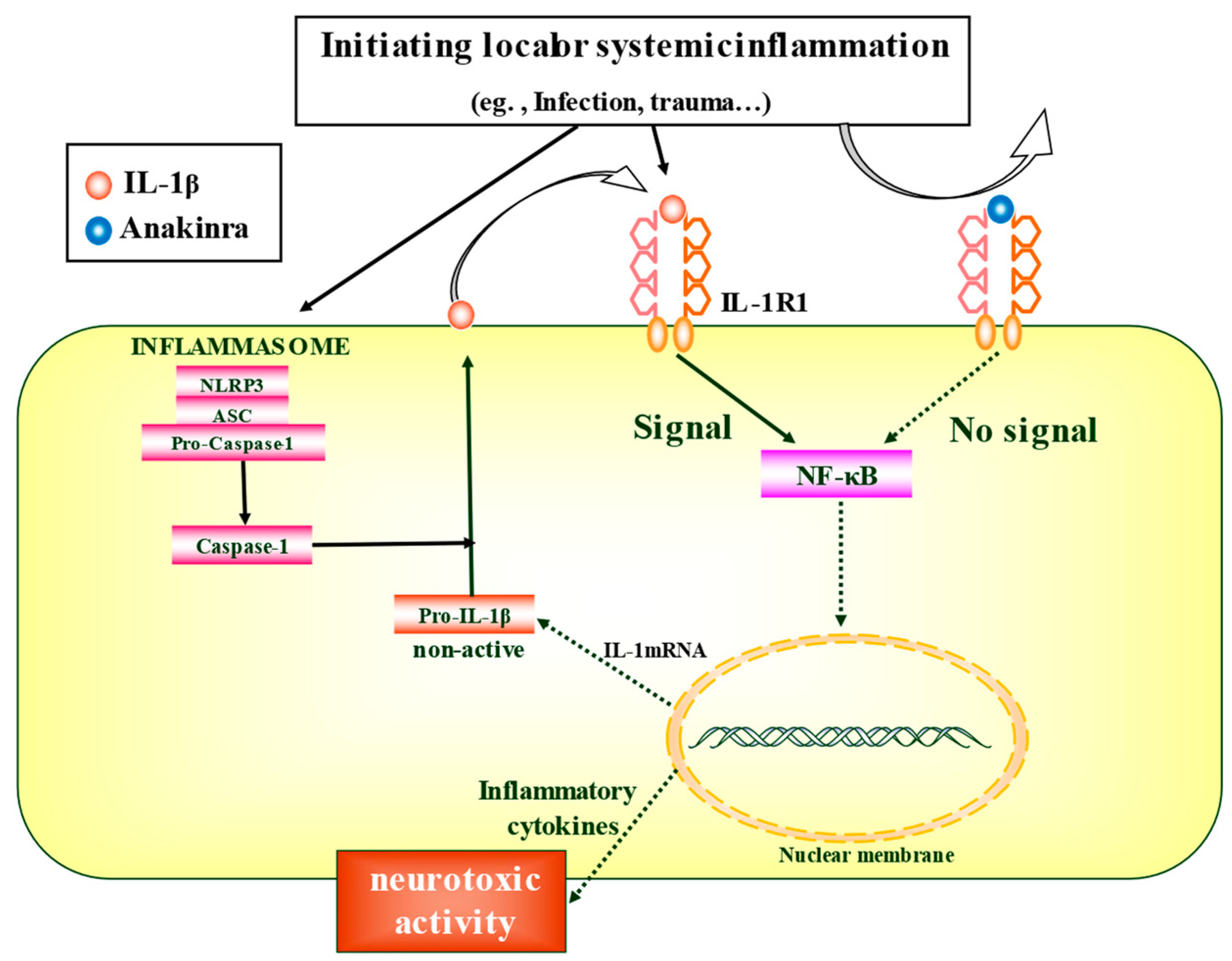
:1. Introduction
2. Materials and Methods
3. Clinical Findings
3.1. Anakinra for FIRES
3.1.1. Case Study
3.1.2. Cohort Study
3.1.3. FIRES Cases Refractory to Anakinra
3.1.4. Adverse Events of Anakinra
3.2. Anakinra for DRE
4. Scientific Findings
4.1. Cytokine Analysis of Clinical Cases
4.2. IL-1RA-Mediated Regulation of Epileptogenesis
4.3. Potential Indicator of Anakinra Administration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Considerations
References
- Hirsch, L.J.; Gaspard, N.; van Baalen, A.; Nabbout, R.; Demeret, S.; Loddenkemper, T.; Navarro, V.; Specchio, N.; Lagae, L.; Rossetti, A.O.; et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia 2018, 59, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, N.; Hirsch, L.J.; Sculier, C.; Loddenkemper, T.; van Baalen, A.; Lancrenon, J.; Emmery, M.; Specchio, N.; Farias-Moeller, R.; Wong, N.; et al. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): State of the art and perspectives. Epilepsia 2018, 59, 745–752. [Google Scholar] [CrossRef]
- van Baalen, A.; Vezzani, A.; Hausler, M.; Kluger, G. Febrile Infection-Related Epilepsy Syndrome: Clinical Review and Hypotheses of Epileptogenesis. Neuropediatrics 2017, 48, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Kramer, U.; Chi, C.S.; Lin, K.L.; Specchio, N.; Sahin, M.; Olson, H.; Nabbout, R.; Kluger, G.; Lin, J.J.; van Baalen, A. Febrile infection-related epilepsy syndrome (FIRES): Pathogenesis, treatment, and outcome: A multicenter study on 77 children. Epilepsia 2011, 52, 1956–1965. [Google Scholar] [CrossRef]
- Caraballo, R.H.; Reyes, G.; Avaria, M.F.; Buompadre, M.C.; Gonzalez, M.; Fortini, S.; Cersosimo, R. Febrile infection-related epilepsy syndrome: A study of 12 patients. Seizure 2013, 22, 553–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.B.; Roy, A.G.; Vinayan, K.P. Clinical profile and treatment outcome of febrile infection-related epilepsy syndrome in South Indian children. Ann. Indian Acad. Neurol. 2016, 19, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Ryvlin, P.; Nashef, L.; Tomson, T. Prevention of sudden unexpected death in epilepsy: A realistic goal? Epilepsia 2013, 54 (Suppl. 2), 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devinsky, O.; Hesdorffer, D.C.; Thurman, D.J.; Lhatoo, S.; Richerson, G. Sudden unexpected death in epilepsy: Epidemiology, mechanisms, and prevention. Lancet Neurol. 2016, 15, 1075–1088. [Google Scholar] [CrossRef]
- Partemi, S.; Vidal, M.C.; Striano, P.; Campuzano, O.; Allegue, C.; Pezzella, M.; Elia, M.; Parisi, P.; Belcastro, V.; Casellato, S.; et al. Genetic and forensic implications in epilepsy and cardiac arrhythmias: A case series. Int. J. Leg. Med. 2015, 129, 495–504. [Google Scholar] [CrossRef]
- Coll, M.; Allegue, C.; Partemi, S.; Mates, J.; Del Olmo, B.; Campuzano, O.; Pascali, V.; Iglesias, A.; Striano, P.; Oliva, A.; et al. Genetic investigation of sudden unexpected death in epilepsy cohort by panel target resequencing. Int. J. Leg. Med. 2016, 130, 331–339. [Google Scholar] [CrossRef]
- Sakuma, H.; Tanuma, N.; Kuki, I.; Takahashi, Y.; Shiomi, M.; Hayashi, M. Intrathecal overproduction of proinflammatory cytokines and chemokines in febrile infection-related refractory status epilepticus. J. Neurol. Neurosurg. Psychiatry 2015, 86, 820–822. [Google Scholar] [CrossRef]
- Kenney-Jung, D.L.; Vezzani, A.; Kahoud, R.J.; LaFrance-Corey, R.G.; Ho, M.L.; Muskardin, T.W.; Wirrell, E.C.; Howe, C.L.; Payne, E.T. Febrile infection-related epilepsy syndrome treated with anakinra. Ann. Neurol. 2016, 80, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, B.D.S.; LaFrance-Corey, R.G.; Kahoud, R.J.; Farias-Moeller, R.; Payne, E.T.; Howe, C.L. Functional deficiency in endogenous interleukin-1 receptor antagonist in patients with febrile infection-related epilepsy syndrome. Ann. Neurol. 2019, 85, 526–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothur, K.; Bandodkar, S.; Wienholt, L.; Chu, S.; Pope, A.; Gill, D.; Dale, R.C. Etiology is the key determinant of neuroinflammation in epilepsy: Elevation of cerebrospinal fluid cytokines and chemokines in febrile infection-related epilepsy syndrome and febrile status epilepticus. Epilepsia 2019, 60, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, A.M.; DeWolfe, J.L.; Miller, D.W.; Szaflarski, J.P. New-onset refractory status epilepticus (NORSE)--The potential role for immunotherapy. Epilepsy Behav. 2015, 47, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Balosso, S.; Ravizza, T. The role of cytokines in the pathophysiology of epilepsy. Brain. Behav. Immun. 2008, 22, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef] [Green Version]
- van Vliet, E.A.; Aronica, E.; Vezzani, A.; Ravizza, T. Review: Neuroinflammatory pathways as treatment targets and biomarker candidates in epilepsy: Emerging evidence from preclinical and clinical studies. Neuropathol. Appl. Neurobiol. 2018, 44, 91–111. [Google Scholar] [CrossRef]
- de Vries, E.E.; van den Munckhof, B.; Braun, K.P.; van Royen-Kerkhof, A.; de Jager, W.; Jansen, F.E. Inflammatory mediators in human epilepsy: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 63, 177–190. [Google Scholar] [CrossRef]
- Vezzani, A.; Conti, M.; De Luigi, A.; Ravizza, T.; Moneta, D.; Marchesi, F.; De Simoni, M.G. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: Functional evidence for enhancement of electrographic seizures. J. Neurosci. 1999, 19, 5054–5065. [Google Scholar] [CrossRef] [Green Version]
- Vezzani, A.; Moneta, D.; Conti, M.; Richichi, C.; Ravizza, T.; De Luigi, A.; De Simoni, M.G.; Sperk, G.; Andell-Jonsson, S.; Lundkvist, J.; et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11534–11539. [Google Scholar] [CrossRef] [Green Version]
- Bernardino, L.; Balosso, S.; Ravizza, T.; Marchi, N.; Ku, G.; Randle, J.C.; Malva, J.O.; Vezzani, A. Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: A crucial role of P2X7 receptor-mediated IL-1beta release. J. Neurochem. 2008, 106, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Iori, V.; Iyer, A.M.; Ravizza, T.; Beltrame, L.; Paracchini, L.; Marchini, S.; Cerovic, M.; Hill, C.; Ferrari, M.; Zucchetti, M.; et al. Blockade of the IL-1R1/TLR4 pathway mediates disease-modification therapeutic effects in a model of acquired epilepsy. Neurobiol. Dis. 2017, 99, 12–23. [Google Scholar] [CrossRef]
- Feng, B.; Chen, Z. Generation of Febrile Seizures and Subsequent Epileptogenesis. Neurosci. Bull. 2016, 32, 481–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef]
- Aronica, E.; Crino, P.B. Inflammation in epilepsy: Clinical observations. Epilepsia 2011, 52 (Suppl. 3), 26–32. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Aronica, E.; Mazarati, A.; Pittman, Q.J. Epilepsy and brain inflammation. Exp. Neurol. 2013, 244, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ravizza, T.; Balosso, S.; Vezzani, A. Inflammation and prevention of epileptogenesis. Neurosci. Lett. 2011, 497, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kamaşak, T.; Dilber, B.; özer yaman, S.; Durgut, B.; Kurt, T.; Çoban, E.; Arslan, E.; Şahin, S.; Karahan, S.; Cansu, A. HMGB-1, TLR4, IL-1R1, TNF-α, and IL-1β: Novel epilepsy markers? Epileptic Disord. 2020, 22, 183–193. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.Y.; Kim, H.; Lim, B.C.; Hwang, H.; Chae, J.H.; Kim, K.J.; Oh, S.; Kim, E.Y.; Shin, J.S. Serum α-synuclein and IL-1β are increased and correlated with measures of disease severity in children with epilepsy: Potential prognostic biomarkers? BMC Neurol. 2020, 20, 85. [Google Scholar] [CrossRef]
- Yamanaka, G.; Takamatsu, T.; Morichi, S.; Yamazaki, T.; Mizoguchi, I.; Ohno, K.; Watanabe, Y.; Ishida, Y.; Oana, S.; Suzuki, S.; et al. Interleukin-1β in peripheral monocytes is associated with seizure frequency in pediatric drug-resistant epilepsy. J. Neuroimmunol. 2021, 352, 577475. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Simon, A.; van der Meer, J.W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov 2012, 11, 633–652. [Google Scholar] [CrossRef] [Green Version]
- Jyonouchi, H.; Geng, L. Intractable Epilepsy (IE) and Responses to Anakinra, a Human Recombinant IL-1 Receptor Agonist (IL-1ra): Case Reports. J. Clin. Cell. Immunol. 2016, 7, 1–5. [Google Scholar] [CrossRef] [Green Version]
- De Sena, A.D.; Do, T.; Schulert, G.S. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J. Neuroinflamm. 2018, 15, 38. [Google Scholar] [CrossRef] [Green Version]
- Jyonouchi, H.; Geng, L. Resolution of EEG findings and clinical improvement in a patient with encephalopathy and ESES with a combination of immunomodulating agents other than corticosteroids: A case report. Epilepsy Behav. Rep. 2020, 14, 100379. [Google Scholar] [CrossRef] [PubMed]
- Dilena, R.; Mauri, E.; Aronica, E.; Bernasconi, P.; Bana, C.; Cappelletti, C.; Carrabba, G.; Ferrero, S.; Giorda, R.; Guez, S.; et al. Therapeutic effect of Anakinra in the relapsing chronic phase of febrile infection-related epilepsy syndrome. Epilepsia Open 2019, 4, 344–350. [Google Scholar] [CrossRef]
- Saffari, A.; Brösse, I.; Wiemer-Kruel, A.; Wilken, B.; Kreuzaler, P.; Hahn, A.; Bernhard, M.K.; van Tilburg, C.M.; Hoffmann, G.F.; Gorenflo, M.; et al. Safety and efficacy of mTOR inhibitor treatment in patients with tuberous sclerosis complex under 2 years of age—A multicenter retrospective study. Orphanet J. Rare Dis. 2019, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, C.; Subramaniam, T.; Seagren, R.M.; Tarula, E.; Co, D.; Furstenberg-Knauff, M.; Wallace, A.; Hsu, D.; Payne, E. Febrile Infection-Related Epilepsy Syndrome Treated Successfully with Anakinra in a 21-Year-Old Woman. WMJ 2019, 118, 135–139. [Google Scholar]
- Stredny, C.M.; Case, S.; Sansevere, A.J.; Son, M.; Henderson, L.; Gorman, M.P. Interleukin-6 Blockade with Tocilizumab in Anakinra-Refractory Febrile Infection-Related Epilepsy Syndrome (FIRES). Child. Neurol. Open 2020, 7, 2329048x20979253. [Google Scholar] [CrossRef]
- Yang, J.H.; Nataraj, S.; Sattar, S. Successful Treatment of Pediatric FIRES with Anakinra. Pediatr. Neurol. 2021, 114, 60–61. [Google Scholar] [CrossRef]
- Koh, S.; Wirrell, E.; Vezzani, A.; Nabbout, R.; Muscal, E.; Kaliakatsos, M.; Wickström, R.; Riviello, J.J.; Brunklaus, A.; Payne, E.; et al. Proposal to optimize evaluation and treatment of Febrile infection-related epilepsy syndrome (FIRES): A Report from FIRES workshop. Epilepsia Open 2021, 6, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Muscal, E.; Wells, E.; Shukla, N.; Eschbach, K.; Hyeong Lee, K.; Kaliakatsos, M.; Desai, N.; Wickström, R.; Viri, M.; et al. Anakinra usage in febrile infection related epilepsy syndrome: An international cohort. Ann. Clin. Transl. Neurol. 2020, 7, 2467–2474. [Google Scholar] [CrossRef]
- Sa, M.; Singh, R.; Pujar, S.; D’Arco, F.; Desai, N.; Eltze, C.; Hughes, E.; Al Obaidi, M.; Eleftheriou, D.; Tisdall, M.; et al. Centromedian thalamic nuclei deep brain stimulation and Anakinra treatment for FIRES—Two different outcomes. Eur. J. Paediatr. Neurol. 2019, 23, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.S.; Lee, S.T.; Kim, R.; Chu, K.; Lee, S.K. Tocilizumab treatment for new onset refractory status epilepticus. Ann. Neurol. 2018, 84, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Kasatwar, N.; Hafeez, S.; Seifi, A.; Gilbert, A.; Barthol, C.; Small, C.; Ákos Szabó, C. Resolution of cryptogenic new onset refractory status epilepticus with tocilizumab. Epilepsy Behav. Rep. 2021, 15, 100431. [Google Scholar] [CrossRef]
- Cantarín-Extremera, V.; Jiménez-Legido, M.; Duat-Rodríguez, A.; García-Fernández, M.; Ortiz-Cabrera, N.V.; Ruiz-Falcó-Rojas, M.L.; González-Gutiérrez-Solana, L. Tocilizumab in pediatric refractory status epilepticus and acute epilepsy: Experience in two patients. J. Neuroimmunol. 2020, 340, 577142. [Google Scholar] [CrossRef]
- Vastert, S.J.; Jamilloux, Y.; Quartier, P.; Ohlman, S.; Osterling Koskinen, L.; Kullenberg, T.; Franck-Larsson, K.; Fautrel, B.; de Benedetti, F. Anakinra in children and adults with Still’s disease. Rheumatology 2019, 58, vi9–vi22. [Google Scholar] [CrossRef]
- Gofshteyn, J.S.; Wilfong, A.; Devinsky, O.; Bluvstein, J.; Charuta, J.; Ciliberto, M.A.; Laux, L.; Marsh, E.D. Cannabidiol as a Potential Treatment for Febrile Infection-Related Epilepsy Syndrome (FIRES) in the Acute and Chronic Phases. J. Child. Neurol. 2017, 32, 35–40. [Google Scholar] [CrossRef]
- Samueli, S.; Abraham, K.; Dressler, A.; Gröppel, G.; Mühlebner-Fahrngruber, A.; Scholl, T.; Kasprian, G.; Laccone, F.; Feucht, M. Efficacy and safety of Everolimus in children with TSC- associated epilepsy -Pilot data from an open single-center prospective study. Orphanet J. Rare Dis. 2016, 11, 145. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Lo, C.M.; Yeung, O.W.H.; Li, C.X.; Liu, X.B.; Qi, X.; Ng, K.T.P.; Liu, J.; Ma, Y.Y.; Lam, Y.F.; et al. NLRP3 inflammasome induced liver graft injury through activation of telomere-independent RAP1/KC axis. J. Pathol. 2017, 242, 284–296. [Google Scholar] [CrossRef]
- de Jager, W.; Hoppenreijs, E.P.; Wulffraat, N.M.; Wedderburn, L.R.; Kuis, W.; Prakken, B.J. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: A cross-sectional study. Ann. Rheum. Dis. 2007, 66, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, G.; Kawashima, H.; Oana, S.; Ishida, Y.; Miyajima, T.; Kashiwagi, Y.; Hoshika, A. Increased level of serum interleukin-1 receptor antagonist subsequent to resolution of clinical symptoms in patients with West syndrome. J. Neurol. Sci. 2010, 298, 106–109. [Google Scholar] [CrossRef]
- Lehtimaki, K.A.; Keranen, T.; Palmio, J.; Makinen, R.; Hurme, M.; Honkaniemi, J.; Peltola, J. Increased plasma levels of cytokines after seizures in localization-related epilepsy. Acta Neurol. Scand. 2007, 116, 226–230. [Google Scholar] [CrossRef]
- Alapirtti, T.; Rinta, S.; Hulkkonen, J.; Makinen, R.; Keranen, T.; Peltola, J. Interleukin-6, interleukin-1 receptor antagonist and interleukin-1beta production in patients with focal epilepsy: A video-EEG study. J. Neurol. Sci. 2009, 280, 94–97. [Google Scholar] [CrossRef]
- Lehtimaki, K.A.; Keranen, T.; Palmio, J.; Peltola, J. Levels of IL-1beta and IL-1ra in cerebrospinal fluid of human patients after single and prolonged seizures. Neuroimmunomodulation 2010, 17, 19–22. [Google Scholar] [PubMed]
- Jackman, K.; Kahles, T.; Lane, D.; Garcia-Bonilla, L.; Abe, T.; Capone, C.; Hochrainer, K.; Voss, H.; Zhou, P.; Ding, A.; et al. Progranulin deficiency promotes post-ischemic blood-brain barrier disruption. J. Neurosci. 2013, 33, 19579–19589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka, G.; Morishita, N.; Morichi, S.; Takeshita, M.; Tomomi, U.; Ishida, Y.; Tomoko, T.; Oana, S.; Watanabe, Y.; Go, S.; et al. Serial Analysis of Multiple Serum Cytokine Responses to Adrenocorticotropic Hormone Therapy in Patients With West Syndrome. J. Child. Neurol. 2018, 33, 528–533. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Auvin, S.; Jeljeli, M.; Desnous, B.; Soussi-Yanicostas, N.; Dournaud, P.; Sterkers, G. Altered vaccine-induced immunity in children with Dravet syndrome. Epilepsia 2018, 59, e45–e50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, B.; Tang, Y.; Chen, B.; Xu, C.; Wang, Y.; Dai, Y.; Wu, D.; Zhu, J.; Wang, S.; Zhou, Y.; et al. Transient increase of interleukin-1β after prolonged febrile seizures promotes adult epileptogenesis through long-lasting upregulating endocannabinoid signaling. Sci. Rep. 2016, 6, 21931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitoh, M.; Kobayashi, K.; Ohmori, I.; Tanaka, Y.; Tanaka, K.; Inoue, T.; Horino, A.; Ohmura, K.; Kumakura, A.; Takei, Y.; et al. Cytokine-related and sodium channel polymorphism as candidate predisposing factors for childhood encephalopathy FIRES/AERRPS. J. Neurol. Sci. 2016, 368, 272–276. [Google Scholar] [CrossRef]
- Gallentine, W.B.; Shinnar, S.; Hesdorffer, D.C.; Epstein, L.; Nordli, D.R., Jr.; Lewis, D.V.; Frank, L.M.; Seinfeld, S.; Shinnar, R.C.; Cornett, K.; et al. Plasma cytokines associated with febrile status epilepticus in children: A potential biomarker for acute hippocampal injury. Epilepsia 2017, 58, 1102–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virta, M.; Hurme, M.; Helminen, M. Increased plasma levels of pro- and anti-inflammatory cytokines in patients with febrile seizures. Epilepsia 2002, 43, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kwak, B.O.; Kwon, A.; Ha, J.; Kim, S.J.; Bae, S.W.; Son, J.S.; Kim, S.N.; Lee, R. Analysis of plasma multiplex cytokines and increased level of IL-10 and IL-1Ra cytokines in febrile seizures. J. Neuroinflamm. 2017, 14, 200. [Google Scholar] [CrossRef] [PubMed]
- Dubé, C.M.; Ravizza, T.; Hamamura, M.; Zha, Q.; Keebaugh, A.; Fok, K.; Andres, A.L.; Nalcioglu, O.; Obenaus, A.; Vezzani, A.; et al. Epileptogenesis provoked by prolonged experimental febrile seizures: Mechanisms and biomarkers. J. Neurosci. 2010, 30, 7484–7494. [Google Scholar] [CrossRef] [Green Version]
- Wulffraat, N.M.; Ruperto, N.; Brunner, H.I.; Oliveira, S.; Uziel, Y.; Nistala, K.; Cimaz, R.; Ferrandiz, M.A.; Flato, B.; Gamir, M.L.; et al. OP0180 Maintenance of Efficacy by Canakinumab Treatment in Systemic Juvenile Idiopathic Arthritis Patients. Ann. Rheum. Dis. 2014, 73, 130. [Google Scholar] [CrossRef]
- Nabbout, R.; Vezzani, A.; Dulac, O.; Chiron, C. Acute encephalopathy with inflammation-mediated status epilepticus. Lancet Neurol. 2011, 10, 99–108. [Google Scholar] [CrossRef]
- Vitaliti, G.; Pavone, P.; Mahmood, F.; Nunnari, G.; Falsaperla, R. Targeting inflammation as a therapeutic strategy for drug-resistant epilepsies: An update of new immune-modulating approaches. Hum. Vaccin Immunother. 2014, 10, 868–875. [Google Scholar] [CrossRef] [Green Version]
| Case * /Ref. | Onset Age (Years)/Sex | ASM | Special Additions | Sz Onset to Anakinra (Days) | Anakinra Dose (mg/kg/Day) | Anakinra Duration (Days) |
|---|---|---|---|---|---|---|
| 4/[44] | 5/M | PB, MDZ, TH, KETA, CBD | KD CMN-DBS | 22 | titrated up to 10 mg/kg/day | 90 |
| 6/[40] | 6/M | LEV, PHT, PB, MDZ, KE, PENT, VPA, TPM, CBD, LZP | IVIg, steroid, Tocilizumab, KD | 6 | titrated to 20 mg/kg/day | 15 |
| 9/[43] | 9/M | MDZ, PENT, LID, CBD, DBS | IVIg, steroid, PE, KD | 42 | 4 mg/kg/day | >114 |
| 10/[43] | 5/M | MDZ, PENT, LID, KE, CBD, DBS | IVIg, steroid, PE, KD | 21 | 10 mg/kg/day | >124 |
| 24/[43] | 8/M | MDZ, PENT, CBD | IVIg, steroid, HYPO | 6 | 3.8 mg/kg/day | 9 |
| 32/[43] | 4/M | MDZ, PENT, KE, CBD | IVIg, steroid, PE, rituximab | 33 | 5 mg/kg/day | 5 |
| Case/Ref. | Clinical Diagnosis of Epilepsy | Onset Age (Years)/Sex | ASM */Special Additions | Sz Onset to Anakinra (Years) | Anakinra Dose (mg/kg/Day) | Clinical Findings | Developmental Prognosis |
|---|---|---|---|---|---|---|---|
| 1/[34] | (1) focal onset impaired awareness (2) unknown onset tonic -clonic | 13/M | IVIG, VNS | 4 | 2 mg/kg/day → 4 mg/kg/day | decreased grand mal seizures from 1–2x/week to 1x/3–4 weeks | More alert, expressive, and attentive, with improved social interactions and three-dimensional vision. |
| 2/[34] | (1) focal onset impaired awareness (2) unknown onset tonic -clonic | 8/F | IVIG, Steroid | 2 | 3 mg/kg/day | seizure-free for 1 year while receiving 100 mg of anakinra. When reduced to 75 mg, cluster seizures occurred. | ND |
| 3/[34] | Landau-Kleffner syndrome | 6/M | IVIG, Steroid, VNS | 4 | 3 mg/kg/day | Thalidomide and VNS reduced seizure frequency until discontinuation due to adverse reactions. Seizures resolved with addition of anakinra for over 4 years | Improvement in motor skills and cognitive skills. Photophobia and persistently dilated pupils also resolved. |
| 4/[34] | ND | 9/M | IVIG, Steroid | 1 | 1.5 mg/kg/day | decreased grand mal seizure once a year | Improved cognitive skills (more attentive and focused), reduced irritability, and hyperactivity. |
| 5/[35] | Generalized onset absence | 14/F | dexamethasone | ND | 100 mg daily → 100 mg/day twice daily | 100 mg of anakinra once daily, 80% reduction in seizure frequency 100 mg twice daily, no clinically evident seizures for two months. | Profound improvements in her fatigue, general malaise, quality of life, and academic performance |
| 6/[36] | ESES (nocturnal seizure) | 6/M | IVIG, Steroid, mTOR | 2 | 100 mg/day | reduced behavioural symptoms, but not the ESES pattern (N60% SWI in all areas). Adding Sirolimus for 7 weeks improved the ESES pattern on EEG. | Hyperlexia and echolalia remained |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanaka, G.; Ishida, Y.; Kanou, K.; Suzuki, S.; Watanabe, Y.; Takamatsu, T.; Morichi, S.; Go, S.; Oana, S.; Yamazaki, T.; et al. Towards a Treatment for Neuroinflammation in Epilepsy: Interleukin-1 Receptor Antagonist, Anakinra, as a Potential Treatment in Intractable Epilepsy. Int. J. Mol. Sci. 2021, 22, 6282. https://doi.org/10.3390/ijms22126282
Yamanaka G, Ishida Y, Kanou K, Suzuki S, Watanabe Y, Takamatsu T, Morichi S, Go S, Oana S, Yamazaki T, et al. Towards a Treatment for Neuroinflammation in Epilepsy: Interleukin-1 Receptor Antagonist, Anakinra, as a Potential Treatment in Intractable Epilepsy. International Journal of Molecular Sciences. 2021; 22(12):6282. https://doi.org/10.3390/ijms22126282
Chicago/Turabian StyleYamanaka, Gaku, Yu Ishida, Kanako Kanou, Shinji Suzuki, Yusuke Watanabe, Tomoko Takamatsu, Shinichiro Morichi, Soken Go, Shingo Oana, Takashi Yamazaki, and et al. 2021. "Towards a Treatment for Neuroinflammation in Epilepsy: Interleukin-1 Receptor Antagonist, Anakinra, as a Potential Treatment in Intractable Epilepsy" International Journal of Molecular Sciences 22, no. 12: 6282. https://doi.org/10.3390/ijms22126282
APA StyleYamanaka, G., Ishida, Y., Kanou, K., Suzuki, S., Watanabe, Y., Takamatsu, T., Morichi, S., Go, S., Oana, S., Yamazaki, T., & Kawashima, H. (2021). Towards a Treatment for Neuroinflammation in Epilepsy: Interleukin-1 Receptor Antagonist, Anakinra, as a Potential Treatment in Intractable Epilepsy. International Journal of Molecular Sciences, 22(12), 6282. https://doi.org/10.3390/ijms22126282






