Ruminant Milk-Derived Extracellular Vesicles: A Nutritional and Therapeutic Opportunity?
Abstract
:1. Introduction
2. Nutritional Composition of Milk
2.1. Carbohydrate
2.2. Fat
2.3. Protein
2.4. Micronutrients
2.5. Other Milk Components
3. Milk-Derived EVs
3.1. Isolation of Milk-Derived EVs
3.2. Protein Composition of Milk-Derived EVs
3.3. Lipid Composition of Milk-Derived EVs
3.4. Nucleic Acid Composition of Milk-Derived EVs
| Species | Technique | Findings | Ref. |
| Extracellular Vesicle | |||
| Human | NGS | Total of 1523 miRNAs identified with more than one read in 70% of samples from the Faroe Islands cohort (364 mothers). | [195] |
| Human | qPCR | Total of 55 lncRNAs identified with 11 lncRNA detected in >50% of the breast milk samples and 5 in >90%. The authors suggested the packing of highly correlated lncRNAs is regulated by the same pathway. | [196] |
| Human | NGS | Total of 5 miRNA stably expressed in all groups. Total of 4 (probiotic+) and 5 (atopic dermatitis+) miRNAs differentially expressed. No evidence of maternal probiotic ingestion altering miRNA abundance, unlikely for probiotic protective effect to be transferred to the infants. | [172] |
| Human + Pig | qPCR, NGS | Identified 309 (human) and 218 (pig) mature miRNAs. In silico analyses demonstrated evolutionary conservation of the top 20 most abundant miRNAs between human, cow, pig, and panda. | [170] |
| Cow | qPCR, NGS | Identified more than 200 cow milk-derived EV miRNAs. | [109] |
| Cow | qPCR, NGS | Enrichment of small RNA profiles in 4 fractions (12 k, 35 k, 70 k, and 100k× g). Distinct differences in small RNA biotypes between fractions. | [174] |
| Cow | qPCR, NGS | Total circRNAs: 39,276 identified, with 17,169 unique to Staphylococcus aureus-infected cows. Demonstrated the selective circRNA packaging mechanism regulated by the infection. | [197] |
| Cow | Microarray | mRNA profiles are altered by viral load and lactate dehydrogenase concentration. | [187] |
| Cow | qPCR, NGS | Total of 276 miRNAs identified with 9 differentially expressed between forage-fed and non-forage fibre source-fed cows. | [175] |
| Cow | qPCR | Demonstrated an enriched subset of miRNAs in EVs prepared at 12,000 and 35,000× g, which were traditionally discarded during preparation. | [133] |
| Cow + Sheep | qPCR, NGS | Identified 685 miRNAs (601 novel) in sheep samples. In silico comparison of the top 20 expressed miRNA in both milks that have immune-related functions. | [182] |
| Exosome | |||
| Human | qPCR, NGS | Identified 221 and 48 mature miRNAs (fresh and 4-week-old milk stored at 4 °C, respectively) detected in 1 mL samples. No reliable detection of miRNAs in infant formula. | [167] |
| Human | qPCR, NGS | Total miRNAs: 631 detected, with 208 novel miRNAs. Total of 9 miRNAs differentially abundant in type 1 diabetes samples. | [168] |
| Human | qPCR, NGS | Identified 602 miRNAs with 59 miRNAs that are immune-related. Demonstrated resistance and stability of exosomal miRNAs against harsh conditions. | [173] |
| Human and Pig | In silico | Reported the presence of plant miRNA in both human and pig milk exosomes based on publicly available sequencing data. | [198] |
| Cow | Qpcr | Demonstrated the bioavailability of cow milk exosomal miRNAs in human plasma without eliciting a cytokine response ex vivo (human PBMCs). | [199] |
| Cow | PCR, NGS | Total miRNAs: 290 detected, with 69 novel miRNAs. Total of 37 miRNAs differentially expressed due to infection. The predicted target genes for 2 miRNAs highly expressed in infected samples, bta-miR-378 and bta-miR-185, were functionally validated with target genes. | [200] |
| Cow | qPCR, NGS | Total miRNAs: 1472 detected, with 480 novel miRNAs. Total of 18 miRNAs differentially expressed due to mastitis. Presented miRNA expression profiles of both healthy and infected cows. bta-miR-223 and bta-miR-142-5 were considered potential early mastitis detection targets. | [158] |
| Cow | qPCR | Reported the effects of fermentation on the expression of miR-29b (unaffected) and miR-21 (significantly reduced by fermentation). | [201] |
| Cow | qPCR, Microarray | Microarray profiling of miRNA (79) and mRNA (19,320) on exosome obtained via ultracentrifugation and its supernatant. | [178] |
| Cow | NGS | Total miRNAs: 417 detected, with 303 novel miRNAs. Two differential expression analyses revealed 6 miRNAs with significant differential presence. Total of 2 miRNAs were proposed as potential biomarkers for early infection. | [179] |
| Cow and Buffalo | NGS, in silico | Total miRNAs: 558 detected in all species (buffalo, cow, pig, human, and panda), with the top 10 highly expressed miRNAs conserved across species. Total of 48 miRNAs were differentially expressed in buffalo, compared to other species. | [183] |
| Pig | qPCR, NGS | Total mRNAs: 16,304 detected, with 2409 novel mRNAs. A random selection of 14 mRNAs among the top 50 was further confirmed using qPCR. | [129] |
| Pig | qPCR, NGS | Total miRNAs: 491 detected, with 315 novel miRNAs. | [180] |
| Pig | qPCR, NGS | Total pre-miRNAs: 180 detected, with 40 novel pre-miRNAs, corresponding to 237 mature and 234 unique miRNAs. Immune-related miRNAs are most abundant in colostrum. | [181] |
| Camel | qPCR | Stable expression of the casein family genes between mid and late lactation periods. | [151] |
| Microvesicle/Nanovesicle | |||
| Human | qPCR Microarray | Total of 281 miRNAs detected. Expression of miR-181a and miR-17 was detected in CD63-positive human milk exosomes. | [202] |
| Cow | qPCR | Six different cow colostrum exosome isolation methods were compared. Method 2 (conventional: differential centrifugation) had the highest purity and greatest amount of microvesicular miRNAs quantified. | [203] |
| Cow | qPCR | Identification of selected mRNA and miRNA in microvesicles, unaffected by acidification, and in vitro transfer of RNA from samples. | [204] |
| Buffalo | qPCR | The expression of 6 nanovesicular miRNAs from three biofluids was evaluated, and 2 of them (miR-21 and miR-500) were reported to be stably expressed during different household storage conditions. | [205] |
4. Stability and Uptake of Milk-Derived EVs
5. Biological Effects of Milk-Derived EVs
| Species | Findings | Ref. |
|---|---|---|
| Extracellular vesicle | ||
| Human | Protective effect in vitro (MA-104 and Hep-2 cell lines) against human rotavirus and respiratory syncytial virus. | [254] |
| Human | In vitro (HFF-1 cell line) antiviral activity against human cytomegalovirus via inhibition of viral replication. | [138] |
| Human | Antiviral activity against Zika and Usutu in vitro (Vero cell line). | [255] |
| Human | Coagulant potential of human milk, owing to the presence of tissue factor (TF)-exposing EVs, but not found in cow milk. | [256] |
| Human | Protective effect against experimental-induced NEC in vitro (IEC-6 and FHs 74 Int cell lines) and in vivo (Sprague Dawley pups). | [257] |
| Human + Cow | Attenuation of inflammatory cytokine expression and nuclear factor (NF)-κB activation in vitro (LPS-stimulated RAW 264.7). | [258] |
| Cow | Promotion of osteogenesis via proliferation and differentiation of osteoblasts in vitro (Saos-2 cell line) and in vivo (Sprague Dawley rats). | [259] |
| Cow | Improved small intestinal dysfunction in malnutrition C57BL/6J mouse model. | [260] |
| Cow | Enhancement of curcumin cell uptake and permeability in an intestinal model in vitro (Caco-2 cell line). | [261] |
| Cow | Osteoprotective effects in vivo (BALB/c and C57BL/6 mice), and decreased the RANKL/OPG ratio in vitro (MLO-Y4 cell line). | [249] |
| Cow | Induction of phenotypical changes in hPAEC and NRCM cell lines. | [109] |
| Cow | Modulation of gut microbiota composition, SCFA profiles, and enhancement of intestinal immune regulation by EVs in vitro (RAW 264.7 cell line) and in vivo (C57BL/6J mice). | [225] |
| Cow | Differential improvements in DSS-induced colitis of two EV subsets via different mechanisms in vivo (C57BL/6J mice). | [188] |
| Cow | Modulation of agricultural dust-induced lung inflammation by EVs in vitro (MH-S cell line) and in vivo (C57BL/6J mice). | [232] |
| Cow | Demonstrated sonication effects on EV skeletal muscle biomarkers in vivo (Fischer 344 rats). | [262] |
| Cow | Biocompatibility and potential use as a non-immunogenic delivery vehicle of EVs in vitro (RAW 264.7) and in vivo (ICR mice). | [111] |
| Cow | Demonstrated EVs do not cause genotoxicity and contain bioactive TGF-β in vitro (NIH/3T3 cell line), and EVs facilitate differentiation of naive T cells into pathogenic Th17 cells (ex vivo DBA/1J mice). The panel of toxicology studies found differences in toxicological profiles in vitro (HL-60, RAW 264.7, and CHO-K1 cell lines) and ex vivo (human blood). | [234] |
| Cow | Increased osteocytes number and osteoblast differentiation in vivo (DBA/1J mice), and increased osteoblast differentiation transitioning into osteocytes in vitro (human MSCs). | [263] |
| Cow | EVs significantly delayed arthritis development in vivo (IL-1Ra-/- and DBA/1J mice). EV uptake demonstrated via ex vivo (mouse ileal cells and splenocytes) and in vitro (RAW 264.7 cells). | [238] |
| Cow | EVs contain bioactive TGF-β in vitro (NIH/3T3 cell line), and EVs facilitate differentiation of naive T cells into pathogenic Th17 cells (ex vivo DBA/1J mice). | [233] |
| Exosome | ||
| Human | Protective effect of both raw and pasteurised exosomes against NEC in vivo (C57BL/6 mice) and ex vivo (neonatal mice intestinal organoids). | [239] |
| Human | Demonstrated that miR-148a influenced the proliferation, morphology, and protein expression of transformed cells more so than normal cells in vitro (LS123 and CCD841 cell lines). The role of miR-148a was validated using a knockdown model in vitro (293T cell line). | [189] |
| Human | Protection against H2O2-induced oxidative stress in NEC in vitro (IEC-6 cell line). | [240] |
| Human | Showed uptake of exosomes, increased expression of miR-148a, and decreased expression of DNA-methyltransferase 1 in vitro (CRL-1831, K-562, and LIM1215 cell lines). | [250] |
| Human | TGF-β2 influences epithelial–mesenchymal transition in vitro (MCF-7 and MCF 10A cell lines). | [244] |
| Human | Inhibition of HIV-1 viral transfer to CD4+ T cells ex vivo (human MDC organoids). | [235] |
| Human | The abundance and composition of exosomes vary due to lactation stage, maternal sensitisation, and lifestyle, which influence the regulation of the allergic outcome in the child. | [247] |
| Human | The presence of MHC classes I and II, CD63, CD81, and CD86 on exosomes, inhibition of anti-CD3-induced cytokine production, and an increase in Foxp3+ CD4+ CD25+ T regulatory cells ex vivo (human PBMCs). | [60] |
| Cow | The loading of miRNA (hsa-miR-148a-3p) as a nanocarrier in vitro (HepG2 and Caco-2 cell lines). | [264] |
| Cow | Activation of immune cells ex vivo (human PBMCs) under inflammatory conditions. | [265] |
| Cow | Restoration of small intestinal epithelial architecture and barrier function in malnourished C57BL/6J mice. | [266] |
| Cow | Exosomes influence macrophage proliferation and protect against cisplatin-induced cytotoxicity in vitro (RAW 264.7 cell line). | [236] |
| Cow | Exosomes have cytoprotective and anti-inflammatory activity in ulcerative colitis in vivo (Kindlin 2−/− mice). | [237] |
| Cow | Protective effects in vitro (IEC-6 cell line) against oxidative stress. | [267] |
| Cow | Osteoporosis prevention in in vitro (MC3T3-E1 and RAW 264.7 cell lines) and in vivo (C57BL/6J mice) models. Additionally, the restoration of gut microbiota affected by osteoarthritis. | [268] |
| Cow | Exosomes can be used as an siRNA delivery vehicle in vitro (A549 cell line) and have anti-tumour activity against lung tumour xenografts in vivo (athymic nude mice) and in vitro (MDA-MB-231, MCF7, A549, H1299, PANC-1, Mia PaCa-2, and A2780 cell lines). | [227] |
| Cow | The use of exosomes as an oral delivery vehicle in xenografts, which enhanced gut absorption and retention involving neonatal Fc receptor in vivo (Balb/c mice, CT26 cells). | [229] |
| Cow | Enhanced goblet cell activity, improved response against NEC in vivo (C57BL/6 mice), and increased mucin production in vitro (LS174T cell line). | [242] |
| Cow | Bilberry anthocyanins encapsulated in exosomes were preferentially taken up by colonic cancer cells in vitro (HCT 116, HT-29, CCD-18Co cell lines), and therapeutic enhancement with encapsulated anthocyanins showed no significant differences in vivo (C57BL/6J mice). | [228] |
| Cow | Depletion in dietary milk exosomes and their miRNA aggravates irritable bowel disease in vivo (Mdr1a−/− mice). | [269] |
| Cow | Exosomes have a minimal effect on skeletal muscle biology in vivo (C57BL/5 mice), suggesting that other tissues may be the targets of exosomes. | [245] |
| Cow | The use of paclitaxel encapsulated in exosomes as a drug delivery vehicle in vivo (athymic nude and C57BL/6 mice). | [213] |
| Cow | Enhancement of skeletal muscle protein synthesis and anabolism in skeletal muscle cells independent of amino acids in vitro (C2C12 myoblast). | [246] |
| Cow | Resistance of exosomes to in vitro digestion and subsequent internalisation and trans-epithelial transport in vitro (Caco-2 cell line). | [159] |
| Cow | The effects on exosomes of in vitro fermentation using three combinations of probiotic bacteria, uptake of these exosomes, and increased proliferation due to the upregulation of ERK1/2 and p38 in vitro (IEC-6 cell line). | [201] |
| Cow | The use of encapsulated celastrol as a drug delivery vehicle, and anti-tumour activity against lung tumour xenografts in vivo (athymic nude mice, A549 and H1299 cell lines). | [231] |
| Cow | The use of both encapsulated hydrophilic and lipophilic small molecules as a delivery vehicle, with tumour targetability, cross-species tolerance, and enhanced drug efficacy compared to free drugs in vivo (athymic nude mice) and in vitro (A549, H1299, MDA-MB-231, T47D, and Beas-2B cell lines). | [212] |
| Cow | The uptake, transport kinetics, and presence of exosomal surface glycoproteins and inhibitors of endocytosis in vitro (Caco-2 and IEC-6 cell lines). | [209] |
| Cow + ASC + Coconut | Promotion of bacterial growth and alteration of gene expression in vitro (Escherichia coli K-12 MG1655 and Lactobacillus plantarum WCFS1 cultures). | [251] |
| Cow + Mice+ Pig | Inter-species and intra-species bioavailability and distribution of exosomes in vivo (Balb/c mice). | [223] |
| Cow + Yak | Higher growth efficiency in vitro (IEC-6 cell line) under hypoxic conditions when supplemented with yak exosomes rather than cow milk-derived exosomes. | [270] |
| Buffalo | Increased stability, solubility, and bioavailability of digested and undigested EV-encapsulated curcumin in vitro (Caco-2 cell line). | [226] |
| Camel | Anticancer effects, via induction of apoptosis, inhibition of oxidative stress, reduced angiogenesis, and metastasis, in vivo (albino rats) and in vitro (MCF7 cell line). | [252] |
| Rat | Rat milk-derived exosomes promote intestinal epithelial cell viability, enhance proliferation, and stimulate intestinal stem cell activity in vitro (IEC-18 cell line). | [243] |
| Pig | Protective effect against deoxynivalenol (DON)-induced intestinal damage in vivo (Kunming mice) and in vitro (IPEC-J2 cell line). | [271] |
| Pig | Protective effects of exosomes against LPS-induced effects in vivo (Kunming mice) and in vitro (IPEC-J2 cell line). | [185] |
| Pig | Promotion of digestive tract development, alteration in the expression of proliferation-related genes in vivo (Kunming mice), and altered cell proliferation, proliferation-related gene expression, and miRNA concentration in vitro (IPEC-J2 cell line). | [272] |
| Pig | Expression of miRNA during different lactation stages, and a higher uptake of colostrum-derived immune-related miRNA in vivo (piglets). | [181] |
| Pig + Cow | Both cow and pig milk exosomes alter serum miRNAs in vivo (piglets), and exosomal miRNA is taken up in vitro (IPEC-J2 cell line). | [162] |
| Microvesicle/Nanovesicle | ||
| Cow | Suitability of nanovesicles and encapsulated siRNA as a therapeutic delivery vehicle in vivo (zebrafish) and ex vivo (C57BL/6 splenocytes). | [184] |
| Cow | Demonstrated successful uptake of PKH67-labelled microvesicles in vitro (RAW 264.7 cell line). | [203] |
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, A.R.; Silva, M.M.; Ribeiro, B.D. Health issues and technological aspects of plant-based alternative milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef] [PubMed]
- Moatsou, G.; Sakkas, L. Sheep milk components: Focus on nutritional advantages and biofunctional potential. Small Rumin. Res. 2019, 180, 86–99. [Google Scholar] [CrossRef]
- Oftedal, O.T. The evolution of lactation in mammalian species. Nestle Nutr. Inst. Workshop Ser. 2020, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Barłowska, J.; Szwajkowska, M.; Litwińczuk, Z.; Król, J. Nutritional Value and Technological Suitability of Milk from Various Animal Species Used for Dairy Production. Compr. Rev. Food Sci. Food Saf. 2011, 10, 291–302. [Google Scholar] [CrossRef]
- Evershed, R.P.; Payne, S.; Sherratt, A.G.; Copley, M.S.; Coolidge, J.; Urem-Kotsu, D.; Kotsakis, K.; Özdoğan, M.; Özdoğan, A.E.; Nieuwenhuyse, O.; et al. Earliest date for milk use in the Near East and southeastern Europe linked to cattle herding. Nature 2008, 455, 528–531. [Google Scholar] [CrossRef]
- FAOSTAT. Livestock Primary (Total World Milk Production Quantity, 2009 and 2019). Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 7 April 2021).
- Scholz-Ahrens, K.E.; Ahrens, F.; Barth, C.A. Nutritional and health attributes of milk and milk imitations. Eur. J. Nutr. 2019, 59, 19–34. [Google Scholar] [CrossRef]
- Medhammar, E.; Wijesinha-Bettoni, R.; Stadlmayr, B.; Nilsson, E.; Charrondiere, U.R.; Burlingame, B. Composition of milk from minor dairy animals and buffalo breeds: A biodiversity perspective. J. Sci. Food Agric. 2011, 92, 445–474. [Google Scholar] [CrossRef]
- Muehlhoff, E.; Bennett, A.; McMahon, D. Milk and Dairy Products in Human Nutrition; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Galley, J.D.; Besner, G.E. The Therapeutic Potential of Breast Milk-Derived Extracellular Vesicles. Nutrients 2020, 12, 745. [Google Scholar] [CrossRef] [Green Version]
- Sanwlani, R.; Fonseka, P.; Chitti, S.V.; Mathivanan, S. Milk-Derived Extracellular Vesicles in Inter-Organism, Cross-Species Communication and Drug Delivery. Proteomes 2020, 8, 11. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Exosomes of pasteurized milk: Potential pathogens of Western diseases. J. Transl. Med. 2019, 17, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Munir, J.; Lee, M.; Ryu, S. Exosomes in Food: Health Benefits and Clinical Relevance in Diseases. Adv. Nutr. 2019, 11, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J. Milk exosomes: Beyond dietary microRNAs. Genes Nutr. 2017, 12, 1–4. [Google Scholar] [CrossRef]
- Gomez, C.D.L.T.; Goreham, R.V.; Bech-Serra, J.J.; Nann, T.; Kussmann, M. “Exosomics”—A Review of Biophysics, Biology and Biochemistry of Exosomes with a Focus on Human Breast Milk. Front. Genet. 2018, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, B.P.; Balassa, T.; Benen, T.D.; Dominovic, M.; Elmadjian, G.K.; Florova, V.; Fransolet, M.D.; Kestlerova, A.; Kmiecik, G.; Kostadinova, I.A.; et al. Extracellular vesicles in blood, milk and body fluids of the female and male urogenital tract and with special regard to reproduction. Crit. Rev. Clin. Lab. Sci. 2016, 53, 379–395. [Google Scholar] [CrossRef]
- Souci, S.W.; Fachmann, W.; Kraut, H. Food Composition and Nutrition Tables, 8th ed.; MedPharm Scientific Publishers: Stuttgart, Germany, 2015; pp. 1–24. ISBN 978-380-475-072-2. [Google Scholar]
- Puwastien, P.; Craven, G. ASEAN Food Composition Database, 1st ed.; Institute of Nutrition, Mahidol University: Nakhon Pathom, Thailand, 2014; pp. 24–25. ISBN 974-664-480-7. [Google Scholar]
- Musaiger, A.R.O. Food Composition Tables for Kingdom of Bahrain; Arab Center for Nutrition: Manama, Bahrain, 2011; pp. 46–49. ISBN 978-999-011-571-0. [Google Scholar]
- Vincent, A.; Grande, F.; Compaoré, E.; Amponsah Annor, G.; Addy, P.S.; Aburime, L.C.; Ahmed, D.; Bih Loh, A.M.; Dahdouh Cabia, S.; Deflache, N.; et al. FAO/INFOODS Food Composition Table for Western Africa (2019) User Guide & Condensed Food Composition Table/Table de Composition des Aliments FAO/INFOODS Pour L’Afrique de L’Ouest (2019); FAO: Rome, Italy, 2020; pp. 352–367. ISBN 978-925-132-223-9. [Google Scholar]
- Pinchen, H.; Powell, N.; Weiner, D.; Finglas, P. Composition of Foods Integrated Dataset (CoFID). Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid (accessed on 27 December 2020).
- TFDA. Food Composition Database 2019 (New Edition). Available online: https://consumer.fda.gov.tw/Food/TFND.aspx?nodeID=178 (accessed on 7 April 2021).
- Rhodes, D.G.; Morton, S.; Martin, C.L.; Adler, M.E.; Hymes, M.A.; Garceau, A.O.; Kovalchik, A.; Sattgast, L.H.; Steinfeldt, L.C.; Clemens, J.C.; et al. 2015–2016 Food and Nutrient Database for Dietary Studies; U.S. Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 2018.
- Sivakumaran, S. The Concise New Zealand Food Composition Tables, 13th ed.; The New Zealand Institute for Plant and Food Research Limited and Ministry of Health: Auckland, New Zealand, 2018; ISBN 978-047-347-690-8.
- FSANZ. Australian Food Composition Database. 2017. Available online: https://www.foodstandards.gov.au/science/monitoringnutrients/afcd/Pages/default.aspx (accessed on 7 April 2021).
- Leung, W.T.W.; Butrum, R.R.; Chang, F.F.H.; Rao, M.N.; Polacchi, W. Food Composition Table for Use in East Asia. 1972. Available online: http://www.fao.org/3/X6878E/X6878E00.htm#TOC (accessed on 7 April 2021).
- Frida. Food ID: 1125 Human Milk, Colostrum. Available online: https://frida.fooddata.dk/food/1125?lang=en (accessed on 27 January 2020).
- Verduci, E.; D’Elios, S.; Cerrato, L.; Comberiati, P.; Calvani, M.; Palazzo, S.; Martelli, A.; Landi, M.; Trikamjee, T.; Peroni, D.G. Cow’s Milk Substitutes for Children: Nutritional Aspects of Milk from Different Mammalian Species, Special Formula and Plant-Based Beverages. Nutrients 2019, 11, 1739. [Google Scholar] [CrossRef] [Green Version]
- Andreas, N.J.; Kampmann, B.; Le-Doare, K.M. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; O’Mahony, J.A. Milk: An Overview. In Milk Proteins, 2nd ed.; Singh, H., Boland, M., Thompson, A., Eds.; Academic Press: San Diego, CA, USA, 2014; p. 622. [Google Scholar]
- Campbell, J.R.; Marshall, R.T. The Science of Providing Milk for Man; McGraw Hill Book Company: New York, NY, USA, 1975; p. 801. [Google Scholar]
- Deng, Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients 2015, 7, 8020–8035. [Google Scholar] [CrossRef] [Green Version]
- Vanga, S.K.; Raghavan, V. How well do plant based alternatives fare nutritionally compared to cow’s milk? J. Food Sci. Technol. 2017, 55, 10–20. [Google Scholar] [CrossRef]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef]
- Boehm, G.; Stahl, B. Oligosaccharides from Milk. J. Nutr. 2007, 137, 847S–849S. [Google Scholar] [CrossRef] [Green Version]
- Bernard, L.; Bonnet, M.; Delavaud, C.; Delosière, M.; Ferlay, A.; Fougère, H.; Graulet, B. Milk Fat Globule in Ruminant: Major and Minor Compounds, Nutritional Regulation and Differences Among Species. Eur. J. Lipid Sci. Technol. 2018, 120, 1700039. [Google Scholar] [CrossRef]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, Production, and Clinical Treatments of Milk Fat Globule Membrane for Infant Nutrition and Well-Being. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef]
- Brink, L.R.; Lönnerdal, B. Milk fat globule membrane: The role of its various components in infant health and development. J. Nutr. Biochem. 2020, 85, 108465. [Google Scholar] [CrossRef]
- German, J.B.; Dillard, C.J. Composition, Structure and Absorption of Milk Lipids: A Source of Energy, Fat-Soluble Nutrients and Bioactive Molecules. Crit. Rev. Food Sci. Nutr. 2006, 46, 57–92. [Google Scholar] [CrossRef]
- Zou, X.; Huang, J.; Jin, Q.; Guo, Z.; Liu, Y.; Cheong, L.-Z.; Xu, X.; Wang, X. Lipid Composition Analysis of Milk Fats from Different Mammalian Species: Potential for Use as Human Milk Fat Substitutes. J. Agric. Food Chem. 2013, 61, 7070–7080. [Google Scholar] [CrossRef] [PubMed]
- Devle, H.; Vetti, I.; Vegarud, G.; Ekeberg, D.; Naess-Andresen, C.F.; Rukke, E.-O. A comparative study of fatty acid profiles in ruminant and non-ruminant milk. Eur. J. Lipid Sci. Technol. 2012, 114, 1036–1043. [Google Scholar] [CrossRef]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Hinz, K.; O’Connor, P.M.; Huppertz, T.; Ross, R.; Kelly, A.L. Comparison of the principal proteins in bovine, caprine, buffalo, equine and camel milk. J. Dairy Res. 2012, 79, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Le, T.T.; Deeth, H.C.; Larsen, L.B. Proteomics of major bovine milk proteins: Novel insights. Int. Dairy J. 2017, 67, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Truchet, S.; Honvo-Houéto, E. Physiology of milk secretion. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Boeren, S.; Smits, M.; van Hooijdonk, T.; Vervoort, J.; Hettinga, K. Proteomic study on the stability of proteins in bovine, camel, and caprine milk sera after processing. Food Res. Int. 2016, 82, 104–111. [Google Scholar] [CrossRef]
- Yang, Y.; Bu, D.; Zhao, X.; Sun, P.; Wang, J.; Zhou, L. Proteomic Analysis of Cow, Yak, Buffalo, Goat and Camel Milk Whey Proteins: Quantitative Differential Expression Patterns. J. Proteome Res. 2013, 12, 1660–1667. [Google Scholar] [CrossRef]
- Wang, X. Isolation of Extracellular Vesicles from Breast Milk. In Extracellular Vesicles: Methods and Protocols; Kuo, W., Jia, S., Walker, J., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1660, pp. 351–353. [Google Scholar]
- Lu, J.; Wang, X.; Zhang, W.; Liu, L.; Pang, X.; Zhang, S.; Lv, J. Comparative proteomics of milk fat globule membrane in different species reveals variations in lactation and nutrition. Food Chem. 2016, 196, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, N.; Wang, W.; Zhao, X.; Zhang, Y.; Han, R.; Ma, L.; Zhao, S.; Li, S.; Guo, T.; et al. N-glycosylation proteomic characterization and cross-species comparison of milk fat globule membrane proteins from mammals. Proteomics 2016, 16, 2792–2800. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, N.; Zhao, X.; Zhang, Y.; Han, R.; Ma, L.; Zhao, S.; Li, S.; Guo, T.; Wang, J. Proteomic characterization and comparison of mammalian milk fat globule proteomes by iTRAQ analysis. J. Proteom. 2015, 116, 34–43. [Google Scholar] [CrossRef]
- Spertino, S.; Cipriani, V.; De Angelis, C.; Giuffrida, M.G.; Marsano, F.; Cavaletto, M. Proteome profile and biological activity of caprine, bovine and human milk fat globules. Mol. BioSyst. 2012, 8, 967–974. [Google Scholar] [CrossRef]
- Roncada, P.; Piras, C.; Soggiu, A.; Turk, R.; Urbani, A.; Bonizzi, L. Farm animal milk proteomics. J. Proteom. 2012, 75, 4259–4274. [Google Scholar] [CrossRef]
- Malacarne, M.; Martuzzi, F.; Summer, A.; Mariani, P. Protein and fat composition of mare’s milk: Some nutritional remarks with reference to human and cow’s milk. Int. Dairy J. 2002, 12, 869–877. [Google Scholar] [CrossRef]
- Graulet, B. Ruminant milk: A source of vitamins in human nutrition. Anim. Front. 2014, 4, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Guéguen, L.; Pointillart, A. The Bioavailability of Dietary Calcium. J. Am. Coll. Nutr. 2000, 19, 119S–136S. [Google Scholar] [CrossRef] [PubMed]
- Graulet, B.; Martin, B.; Agabriel, C.; Girard, C.L. Vitamins in Milks. In Milk and Dairy Products in Human Nutrition; Park, Y.W., Haenlein, G.F.W., Eds.; John Wiley & Sons, Ltd., Publication: West Sussex, UK, 2013; pp. 200–219. ISBN 978-111-853-416-8. [Google Scholar]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with Immune Modulatory Features Are Present in Human Breast Milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Aguilar-Lozano, A.; Sadri, M.; Sukreet, S.; Manca, S.; Wu, D.; Zhou, F.; Mutai, E. Biological Activities of Extracellular Vesicles and Their Cargos from Bovine and Human Milk in Humans and Implications for Infants. J. Nutr. 2016, 147, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Zempleni, J.; Baier, S.R.; Howard, K.M.; Cui, J. Gene regulation by dietary microRNAs. Can. J. Physiol. Pharmacol. 2015, 93, 1097–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zempleni, J.; Sukreet, S.; Zhou, F.; Wu, D.; Mutai, E. Milk-Derived Exosomes and Metabolic Regulation. Annu. Rev. Anim. Biosci. 2019, 7, 245–262. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witwer, K.W.; Soekmadji, C.; Hill, A.F.; Wauben, M.H.; Buzás, E.I.; Di Vizio, D.; Falcon-Perez, J.M.; Gardiner, C.; Hochberg, F.; Kurochkin, I.V.; et al. Updating the MISEV minimal requirements for extracellular vesicle studies: Building bridges to reproducibility. J. Extracell. Vesicles 2017, 6, 1396823. [Google Scholar] [CrossRef] [Green Version]
- Lötvall, J.; Hill, A.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Roux, Q.; van Deun, J.; Dedeyne, S.; Hendrix, A. The EV-TRACK summary add-on: Integration of experimental information in databases to ensure comprehensive interpretation of biological knowledge on extracellular vesicles. J. Extracell. Vesicles 2020, 9, 1699367. [Google Scholar] [CrossRef]
- Van Deun, J.; EV-TRACK Consortium; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]
- Kim, D.-K.; Lee, J.; Kim, S.-H.; Choi, D.-S.; Yoon, Y.J.; Kim, J.H.; Go, G.; Nhung, D.; Hong, K.; Jang, S.C.; et al. EVpedia: A community web portal for extracellular vesicles research. Bioinformatics 2014, 31, 933–939. [Google Scholar] [CrossRef]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2018, 47, D516–D519. [Google Scholar] [CrossRef] [Green Version]
- Kalra, H.; Simpson, R.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borras, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef]
- Murillo, O.; Thistlethwaite, W.; Rozowsky, J.; Subramanian, S.L.; Lucero, R.; Shah, N.; Jackson, A.R.; Srinivasan, S.; Chung, A.; Laurent, C.D.; et al. exRNA Atlas Analysis Reveals Distinct Extracellular RNA Cargo Types and Their Carriers Present across Human Biofluids. Cell 2019, 177, 463–477.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, Q.; Zhang, J.; Li, C.; Miao, Y.-R.; Lei, Q.; Li, Q.; Guo, A.-Y. EVmiRNA: A database of miRNA profiling in extracellular vesicles. Nucleic Acids Res. 2018, 47, D89–D93. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, Y.; Chen, B.; Zhao, J.; Yu, S.; Tang, Y.; Zheng, Q.; Li, Y.; Wang, P.; He, X.; et al. exoRBase: A database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2017, 46, D106–D112. [Google Scholar] [CrossRef] [Green Version]
- Welsh, J.A.; van der Pol, E.; Arkesteijn, G.J.; Bremer, M.; Brisson, A.; Coumans, F.; Dignat-George, F.; Duggan, E.; Ghiran, I.; Giebel, B.; et al. MIFlowCyt-EV: A framework for standardized reporting of extracellular vesicle flow cytometry experiments. J. Extracell. Vesicles 2020, 9, 1713526. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Spidlen, J.; Boyce, K.; Cai, J.; Crosbie, N.; Dalphin, M.; Furlong, J.; Gasparetto, M.; Goldberg, M.; Goralczyk, E.M.; et al. MIFlowCyt: The minimum information about a flow cytometry experiment. Cytom. A 2008, 73, 926–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greening, D.W.; Simpson, R. Understanding extracellular vesicle diversity—current status. Expert Rev. Proteom. 2018, 15, 887–910. [Google Scholar] [CrossRef]
- Greening, D.; Xu, R.; Gopal, S.K.; Rai, A.; Simpson, R. Proteomic insights into extracellular vesicle biology—Defining exosomes and shed microvesicles. Expert Rev. Proteom. 2016, 14, 69–95. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Conigliaro, A.; Corrado, C.; Fontana, S.; Alessandro, R. Exosome Basic Mechanisms. In Exosomes: A Clinical Compendium; Edelstein, L.R., Smythies, J.R., Quesenberry, P.J., Noble, D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–21. ISBN 978-012-816-053-4. [Google Scholar]
- Palmulli, R.; Van Niel, G. To be or not to be… secreted as exosomes, a balance finely tuned by the mechanisms of biogenesis. Essays Biochem. 2018, 62, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Mulcahy, L.; Pink, R.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Kosaka, N.; Ochiya, T. Latest advances in extracellular vesicles: From bench to bedside. Sci. Technol. Adv. Mater. 2019, 20, 746–757. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Weber, S.R.; Zhao, Y.; Chen, H.; Sundstrom, J.M. Methods for Exosome Isolation and Characterization. In Exosomes: A Clinical Compendium; Edelstein, L.R., Smythies, J.R., Quesenberry, P.J., Noble, D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 23–38. ISBN 978-012-816-053-4. [Google Scholar]
- Popovic, M. Routine and novel methods for isolation of extracellular vesicles. Biol. Serb. 2019, 41, 36–43. [Google Scholar] [CrossRef]
- Lane, R.E.; Korbie, D.; Trau, M.; Hill, M.M. Purification Protocols for Extracellular Vesicles. In Extracellular Vesicles: Methods and Protocols, 1st ed.; Kuo, W.P., Jia, S., Walker, J., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1660, pp. 111–130. [Google Scholar]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Zeringer, E.; Barta, T.; Li, M.; Vlassov, A.V. Strategies for Isolation of Exosomes. Cold Spring Harb. Protoc. 2015, 2015, 319–323. [Google Scholar] [CrossRef] [Green Version]
- Lässer, C.; Eldh, M.; Lötvall, J. Isolation and Characterization of RNA-Containing Exosomes. J. Vis. Exp. 2012, e3037. [Google Scholar] [CrossRef] [PubMed]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef] [PubMed]
- Boriachek, K.; Islam, N.; Möller, A.; Salomon, C.; Nguyen, N.-T.; Hossain, S.; Yamauchi, Y.; Shiddiky, M.J.A. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small 2017, 14. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed. Res. Int. 2018, 2018, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Shah, S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef]
- Li, J.; He, X.; Deng, Y.; Yang, C. An Update on Isolation Methods for Proteomic Studies of Extracellular Vesicles in Biofluids. Molecules 2019, 24, 3516. [Google Scholar] [CrossRef] [Green Version]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, M.L.; Vykoukal, J. Sequential Filtration: A Gentle Method for the Isolation of Functional Extracellular Vesicles. In Extracellular Vesicles: Methods and Protocols, 1st ed.; Kuo, W.P., Jia, S., Walker, J., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1660, pp. 33–41. ISBN 978-149-397-253-1. [Google Scholar]
- Meng, Y.; Asghari, M.; Aslan, M.K.; Yilmaz, A.; Mateescu, B.; Stavrakis, S.; Demello, A.J. Microfluidics for extracellular vesicle separation and mimetic synthesis: Recent advances and future perspectives. Chem. Eng. J. 2021, 404, 126110. [Google Scholar] [CrossRef]
- Vogel, R.; Savage, J.; Muzard, J.; Della Camera, G.; Vella, G.; Law, A.; Marchioni, M.; Mehn, D.; Geiss, O.; Peacock, B.; et al. Measuring particle concentration of multimodal synthetic reference materials and extracellular vesicles with orthogonal techniques: Who is up to the challenge? J. Extracell. Vesicles 2021, 10, e12052. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Shpacovitch, V.; Hergenröder, R. Optical and surface plasmonic approaches to characterize extracellular vesicles: A review. Anal. Chim. Acta 2018, 1005, 1–15. [Google Scholar] [CrossRef]
- Chia, B.S.; Low, Y.P.; Wang, Q.; Li, P.; Gao, Z. Advances in exosome quantification techniques. TrAC Trends Anal. Chem. 2017, 86, 93–106. [Google Scholar] [CrossRef]
- Bickmore, D.C.; Miklavcic, J.J. Characterization of Extracellular Vesicles Isolated from Human Milk Using a Precipitation-Based Method. Front. Nutr. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickmore, D.C. Elucidating the Relation between Human Milk Fatty Acids, Extracellular Vesicles, and Infant Developmental Outcomes in the First Year of Life; Chapman University: Orange, CA, USA, 2020. [Google Scholar]
- Zonneveld, M.; Brisson, A.R.; van Herwijnen, M.; Tan, S.; Van De Lest, C.H.A.; Redegeld, F.A.; Garssen, J.; Wauben, M.; Hoen, E.N.M.N. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morozumi, M.; Izumi, H.; Shimizu, T.; Takeda, Y. Comparison of isolation methods using commercially available kits for obtaining extracellular vesicles from cow milk. J. Dairy Sci. 2021, 104, 6463–6471. [Google Scholar] [CrossRef] [PubMed]
- Bedoya, N. Impact of Bovine Milk Extracellular Vesicles and Their MicroRNA Cargoes on the Cardiovascular System; Icahn School of Medicine at Mount Sinai: New York, NY, USA, 2020. [Google Scholar]
- Rahman, M.; Shimizu, K.; Yamauchi, M.; Takase, H.; Ugawa, S.; Okada, A.; Inoshima, Y. Acidification effects on isolation of extracellular vesicles from bovine milk. PLoS ONE 2019, 14, e0222613. [Google Scholar] [CrossRef] [Green Version]
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1440132. [Google Scholar] [CrossRef] [Green Version]
- Pollott, G.; Brito, A.; Gardiner, C.; Lawson, C. A Comparison of Different Methodologies for the Measurement of Extracellular Vesicles and Milk-derived Particles in Raw Milk from Cows. Biomark. Insights 2016, 11, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Ridolfi, A.; Brucale, M.; Montis, C.; Caselli, L.; Paolini, L.; Borup, A.; Boysen, A.T.; Loria, F.; Van Herwijnen, M.J.C.; Kleinjan, M.; et al. AFM-Based High-Throughput Nanomechanical Screening of Single Extracellular Vesicles. Anal. Chem. 2020, 92, 10274–10282. [Google Scholar] [CrossRef]
- Jackson, K.; Powell, R.R.; Bruce, T.F.; Marcus, R.K. Solid-phase extraction of exosomes from diverse matrices via a polyester capillary-channeled polymer (C-CP) fiber stationary phase in a spin-down tip format. Anal. Bioanal. Chem. 2020, 412, 4713–4724. [Google Scholar] [CrossRef]
- Yamauchi, M.; Shimizu, K.; Rahman, M.; Ishikawa, H.; Takase, H.; Ugawa, S.; Okada, A.; Inoshima, Y. Efficient method for isolation of exosomes from raw bovine milk. Drug Dev. Ind. Pharm. 2018, 45, 359–364. [Google Scholar] [CrossRef]
- Vaswani, K.; Koh, Y.Q.; Almughlliq, F.; Peiris, H.; Mitchell, M.D. A method for the isolation and enrichment of purified bovine milk exosomes. Reprod. Biol. 2017, 17, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Inoshima, Y.; Matsuda, T.; Ishiguro, N. Comparison of Methods for Isolating Exosomes from Bovine Milk. J. Vet. Med. Sci. 2012, 74, 1523–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaswani, K.; Mitchell, M.; Holland, O.; Koh, Y.Q.; Hill, R.J.; Harb, T.; Davies, P.S.W.; Peiris, H. A Method for the Isolation of Exosomes from Human and Bovine Milk. J. Nutr. Metab. 2019, 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Blans, K.; Hansen, M.S.; Sørensen, L.V.; Hvam, M.L.; Howard, K.A.; Moeller, A.; Wiking, L.; Larsen, L.B.; Rasmussen, J.T. Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2017, 6, 1294340. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-S.; Kim, D.-K.; Kim, Y.-K.; Gho, Y.S. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom. Rev. 2014, 34, 474–490. [Google Scholar] [CrossRef]
- Kreimer, S.; Belov, A.M.; Ghiran, I.; Murthy, S.K.; Frank, D.A.; Ivanov, A.R. Mass-Spectrometry-Based Molecular Characterization of Extracellular Vesicles: Lipidomics and Proteomics. J. Proteome Res. 2015, 14, 2367–2384. [Google Scholar] [CrossRef] [PubMed]
- Pocsfalvi, G.; Stanly, C.; Fiume, I.; Vékey, K. Chromatography and its hyphenation to mass spectrometry for extracellular vesicle analysis. J. Chromatogr. A 2016, 1439, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Pocsfalvi, G.; Stanly, C.; Vilasi, A.; Fiume, I.; Capasso, G.; Turiák, L.; Buzas, E.I.; Vékey, K. Mass spectrometry of extracellular vesicles. Mass Spectrom. Rev. 2015, 35, 3–21. [Google Scholar] [CrossRef]
- Schmidt, A.; Forne, I.; Imhof, A. Bioinformatic analysis of proteomics data. BMC Syst. Biol. 2014, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Regnault, A.; Garin, J.; Wolfers, J.; Zitvogel, L.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Molecular characterization of dendritic cell-derived exosomes: Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 1999, 147, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Niel, G.; Raposo, G.; Candalh, C.; Boussac, M.; Hershberg, R.; Cerf-Bensussan, N.; Heyman, M. Intestinal epithelial cells secrete exosome–like vesicles. Gastroenterology 2001, 121, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, T.A.; Lippolis, J.; Nonnecke, B.J.; Sacco, R.E. Bovine milk exosome proteome. J. Proteom. 2012, 75, 1486–1492. [Google Scholar] [CrossRef]
- Chen, T.; Xi, Q.-Y.; Sun, J.-J.; Ye, R.-S.; Cheng, X.; Sun, R.-P.; Wang, S.-B.; Shu, G.; Wang, L.-N.; Zhu, X.-T.; et al. Revelation of mRNAs and proteins in porcine milk exosomes by transcriptomic and proteomic analysis. BMC Vet. Res. 2017, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Van Herwijnen, M.; Zonneveld, M.; Goerdayal, S.; Nolte, E.N.; Garssen, J.; Stahl, B.; Altelaar, A.M.; Redegeld, F.A.; Wauben, M.H. Comprehensive Proteomic Analysis of Human Milk-derived Extracellular Vesicles Unveils a Novel Functional Proteome Distinct from Other Milk Components. Mol. Cell. Proteom. 2016, 15, 3412–3423. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.A.; Zeng, X.; Todd, A.R.; Barnes, L.F.; Winstone, J.M.A.; Trinidad, J.C.; Novotny, M.V.; Jarrold, M.F.; Clemmer, D.E. Charge Detection Mass Spectrometry Measurements of Exosomes and other Extracellular Particles Enriched from Bovine Milk. Anal. Chem. 2020, 92, 3285–3292. [Google Scholar] [CrossRef]
- Benmoussa, A.; Gotti, C.; Bourassa, S.; Gilbert, C.; Provost, P. Identification of protein markers for extracellular vesicle (EV) subsets in cow’s milk. J. Proteom. 2019, 192, 78–88. [Google Scholar] [CrossRef]
- Benmoussa, A.; Ly, S.; Shan, S.T.; Laugier, J.; Boilard, E.; Gilbert, C.; Provost, P. A subset of extracellular vesicles carries the bulk of microRNAs in commercial dairy cow’s milk. J. Extracell. Vesicles 2017, 6, 1401897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryskaliyeva, A.; Krupova, Z.; Henry, C.; Faye, B.; Konuspayeva, G.; Martin, P. Comprehensive proteomic analysis of camel milk-derived extracellular vesicles. Int. J. Biol. Chem. 2019, 12, 93–104. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Zhang, L.; Cai, J.; Zhou, Y.; Liu, H.; Hu, Y.; Chen, W.; Xu, S.; Liu, P.; et al. Identification and Peptidomic Profiling of Exosomes in Preterm Human Milk: Insights into Necrotizing Enterocolitis Prevention. Mol. Nutr. Food Res. 2019, e1801247. [Google Scholar] [CrossRef]
- Samuel, M.; Chisanga, D.; Liem, M.; Keerthikumar, S.; Anand, S.; Ang, C.-S.; Adda, C.; Versteegen, E.; Jois, M.; Mathivanan, S. Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci. Rep. 2017, 7, 5933. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; Meier, S.; Burke, C.R.; Macdonald, K.A.; Roche, J.R.; Almughlliq, F.; Arachchige, B.J.; Reed, S.; et al. Characterization of exosomes from body fluids of dairy cows. J. Anim. Sci. 2017, 95, 3893–3904. [Google Scholar] [CrossRef]
- Donalisio, M.; Cirrincione, S.; Rittà, M.; Lamberti, C.; Civra, A.; Francese, R.; Tonetto, P.; Sottemano, S.; Manfredi, M.; Lorenzato, A.; et al. Extracellular Vesicles in Human Preterm Colostrum Inhibit Infection by Human Cytomegalovirus In Vitro. Microorganisms 2020, 8, 1087. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Takashima, S.; Kamatari, Y.O.; Badr, Y.; Kitamura, Y.; Shimizu, K.; Okada, A.; Inoshima, Y. Proteomic profiling of milk small extracellular vesicles from bovine leukemia virus-infected cattle. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Rahman, M.; Badr, Y.; Kamatari, Y.O.; Kitamura, Y.; Shimizu, K.; Okada, A.; Inoshima, Y. Data on proteomic analysis of milk extracellular vesicles from bovine leukemia virus-infected cattle. Data Brief 2020, 33, 106510. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, S.; Gevi, F.; Pietrucci, D.; Cavinato, L.; Luly, F.R.; Pascucci, L.; Petrini, S.; Ascenzioni, F.; Zolla, L.; Chillemi, G.; et al. Anti-Inflammatory Potential of Cow, Donkey and Goat Milk Extracellular Vesicles as Revealed by Metabolomic Profile. Nutrients 2020, 12, 2908. [Google Scholar] [CrossRef]
- Yang, M.; Song, D.; Cao, X.; Wu, R.; Liu, B.; Ye, W.; Wu, J.; Yue, X. Comparative proteomic analysis of milk-derived exosomes in human and bovine colostrum and mature milk samples by iTRAQ-coupled LC-MS/MS. Food Res. Int. 2017, 92, 17–25. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Sacco, R.E.; Nonnecke, B.J.; Lippolis, J. Bovine milk proteome: Quantitative changes in normal milk exosomes, milk fat globule membranes and whey proteomes resulting from Staphylococcus aureus mastitis. J. Proteom. 2013, 82, 141–154. [Google Scholar] [CrossRef]
- Sedykh, S.E.; Purvinish, L.V.; Monogarov, A.S.; Burkova, E.E.; Grigor’Eva, A.E.; Bulgakov, D.; Dmitrenok, P.S.; Vlassov, V.V.; Ryabchikova, E.I.; Nevinsky, G.A. Purified horse milk exosomes contain an unpredictable small number of major proteins. Biochim. Open 2017, 4, 61–72. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rustam, Y.H.; Reid, G.E. Analytical Challenges and Recent Advances in Mass Spectrometry Based Lipidomics. Anal. Chem. 2017, 90, 374–397. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Cebak, J.E. Lipidomics biomarker studies: Errors, limitations, and the future. Biochem. Biophys. Res. Commun. 2018, 504, 569–575. [Google Scholar] [CrossRef]
- Subra, C.; Laulagnier, K.; Perret, B.; Record, M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 2007, 89, 205–212. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- Yassin, A.M.; Hamid, M.I.A.; Farid, O.A.; Amer, H.; Warda, M. Dromedary milk exosomes as mammary transcriptome nano-vehicle: Their isolation, vesicular and phospholipidomic characterizations. J. Adv. Res. 2016, 7, 749–756. [Google Scholar] [CrossRef] [Green Version]
- Castro-Gómez, P.; Garcia-Serrano, A.; Visioli, F.; Fontecha, J. Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostaglandins Leukot. Essent. Fat. Acids 2015, 101, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Demmelmair, H.; Koletzko, B. Lipids in human milk. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Rodriguez-Palmero, M.; Demmelmair, H.; Fidler, N.; Jensen, R.; Sauerwald, T. Physiological aspects of human milk lipids. Early Hum. Dev. 2001, 65, S3–S18. [Google Scholar] [CrossRef]
- Koletzko, B. Human milk lipids. Ann. Nutr. Metab. 2016, 69, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.H.; Zou, X.; Abed, S.M.; Korma, S.A.; Jin, Q.; Wang, X. Natural phospholipids: Occurrence, biosynthesis, separation, identification, and beneficial health aspects. Crit. Rev. Food Sci. Nutr. 2017, 59, 253–275. [Google Scholar] [CrossRef]
- Boilard, E. Thematic Review Series: Exosomes and Microvesicles: Lipids as Key Components of their Biogenesis and Functions Extracellular vesicles and their content in bioactive lipid mediators: More than a sack of microRNA. J. Lipid Res. 2018, 59, 2037–2046. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.; He, H.; Jia, X.; Chen, S.; Wang, J.; Shi, Y.; Liu, B.; Xiao, W.; Lai, S. Genome-wide microRNA profiling of bovine milk-derived exosomes infected with Staphylococcus aureus. Cell Stress Chaperones 2018, 23, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Vashisht, M.; Golla, N.; Shandilya, S.; Onteru, S.K.; Singh, D. Milk miRNAs encapsulated in exosomes are stable to human digestion and permeable to intestinal barrier in vitro. J. Funct. Foods 2017, 34, 431–439. [Google Scholar] [CrossRef]
- Baier, S.R.; Nguyen, C.; Xie, F.; Wood, J.R.; Zempleni, J. MicroRNAs Are Absorbed in Biologically Meaningful Amounts from Nutritionally Relevant Doses of Cow Milk and Affect Gene Expression in Peripheral Blood Mononuclear Cells, HEK-293 Kidney Cell Cultures, and Mouse Livers. J. Nutr. 2014, 144, 1495–1500. [Google Scholar] [CrossRef]
- Wang, L.; Sadri, M.; Giraud, D.; Zempleni, J. RNase H2-Dependent Polymerase Chain Reaction and Elimination of Confounders in Sample Collection, Storage, and Analysis Strengthen Evidence That microRNAs in Bovine Milk Are Bioavailable in Humans. J. Nutr. 2018, 148, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Chen, T.; Xie, M.; Li, M.; Zeng, B.; Sun, R.; Zhu, Y.; Ye, D.; Wu, J.; Sun, J.; et al. Oral Administration of Bovine and Porcine Milk Exosome Alter miRNAs Profiles in Piglet Serum. Sci. Rep. 2020, 10, 6983. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, A.; Provost, P. Milk MicroRNAs in Health and Disease. Compr. Rev. Food Sci. Food Saf. 2019, 18, 703–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomé-Carneiro, J.; Fernández-Alonso, N.; Tomás-Zapico, C.; Visioli, F.; Iglesias-Gutierrez, E.; Dávalos, A. Breast milk microRNAs harsh journey towards potential effects in infant development and maturation. Lipid encapsulation can help. Pharmacol. Res. 2018, 132, 21–32. [Google Scholar] [CrossRef]
- Cintio, M.; Polacchini, G.; Scarsella, E.; Montanari, T.; Stefanon, B.; Colitti, M. MicroRNA Milk Exosomes: From Cellular Regulator to Genomic Marker. Animals 2020, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Colitti, M.; Sgorlon, S.; Stefanon, B. Exosome cargo in milk as a potential marker of cow health. J. Dairy Res. 2020, 87, 79–83. [Google Scholar] [CrossRef]
- Leiferman, A.; Shu, J.; Upadhyaya, B.; Cui, J.; Zempleni, J. Storage of Extracellular Vesicles in Human Milk, and MicroRNA Profiles in Human Milk Exosomes and Infant Formulas. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.H.; Kaur, S.; Nielsen, L.B.; Størling, J.; Yarani, R.; Roursgaard, M.; Mathiesen, E.R.; Damm, P.; Svare, J.; Mortensen, H.B.; et al. Breast Milk-Derived Extracellular Vesicles Enriched in Exosomes from Mothers with Type 1 Diabetes Contain Aberrant Levels of microRNAs. Front. Immunol. 2019, 10, 2543. [Google Scholar] [CrossRef]
- Kahn, S.; Liao, Y.; Du, X.; Xu, W.; Li, J.; Lönnerdal, B. Exosomal MicroRNAs in Milk from Mothers Delivering Preterm Infants Survive In Vitro Digestion and Are Taken Up by Human Intestinal Cells. Mol. Nutr. Food Res. 2018, 62, e1701050. [Google Scholar] [CrossRef]
- Van Herwijnen, M.J.C.; Driedonks, T.; Snoek, B.; Kroon, A.M.T.; Kleinjan, M.; Jorritsma, R.; Pieterse, C.; Hoen, E.N.M.N.; Wauben, M. Abundantly Present miRNAs in Milk-Derived Extracellular Vesicles Are Conserved Between Mammals. Front. Nutr. 2018, 5, 81. [Google Scholar] [CrossRef]
- Liao, Y.; Du, X.; Yalin, L.; Lönnerdal, B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Simpson, M.R.; Brede, G.; Johansen, J.; Johnsen, R.; Storrø, O.; Sætrom, P.; Øien, T. Human Breast Milk miRNA, Maternal Probiotic Supplementation and Atopic Dermatitis in Offspring. PLoS ONE 2015, 10, e0143496. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-related MicroRNAs are Abundant in Breast Milk Exosomes. Int. J. Biol. Sci. 2012, 8, 118–123. [Google Scholar] [CrossRef]
- Benmoussa, A.; Laugier, J.; Beauparlant, C.J.; Lambert, M.; Droit, A.; Provost, P. Complexity of the microRNA transcriptome of cow milk and milk-derived extracellular vesicles isolated via differential ultracentrifugation. J. Dairy Sci. 2020, 103, 16–29. [Google Scholar] [CrossRef]
- Quan, S.-Y.; Nan, X.-M.; Wang, K.; Zhao, Y.-G.; Jiang, L.-S.; Yao, J.-H.; Xiong, B.-H. Replacement of forage fiber with non-forage fiber sources in dairy cow diets changes milk extracellular vesicle-miRNA expression. Food Funct. 2020, 11, 2154–2162. [Google Scholar] [CrossRef]
- Ma, S.; Tong, C.; Ibeagha-Awemu, E.M.; Zhao, X. Identification and characterization of differentially expressed exosomal microRNAs in bovine milk infected with Staphylococcus aureus. BMC Genom. 2019, 20, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zeng, B.; Chen, T.; Xie, M.-Y.; Luo, J.-Y.; He, J.-J.; Xi, Q.-Y.; Sun, J.-J.; Zhang, Y.-L. Exploration of long noncoding RNA in bovine milk exosomes and their stability during digestion in vitro. J. Dairy Sci. 2019, 102, 6726–6737. [Google Scholar] [CrossRef]
- Izumi, H.; Tsuda, M.; Sato, Y.; Kosaka, N.; Ochiya, T.; Iwamoto, H.; Namba, K.; Takeda, Y. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J. Dairy Sci. 2015, 98, 2920–2933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Aswath, K.; Schroeder, S.G.; Lippolis, J.D.; Reinhardt, T.A.; Sonstegard, T.S.; Sun, J.; Aswath, K.; Schroeder, S.G.; Lippolis, J.D.; et al. MicroRNA expression profiles of bovine milk exosomes in response to Staphylococcus aureus infection. BMC Genom. 2015, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Xi, Q.-Y.; Ye, R.-S.; Cheng, X.; Qi, Q.-E.; Wang, S.-B.; Shu, G.; Wang, L.-N.; Zhu, X.-T.; Jiang, Q.-Y.; et al. Exploration of microRNAs in porcine milk exosomes. BMC Genom. 2014, 15, 100. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Li, M.; Wang, T.; Liang, Y.; Zhong, Z.; Wang, X.; Zhou, Q.; Chen, L.; Lang, Q.; He, Z.; et al. Lactation-Related MicroRNA Expression Profiles of Porcine Breast Milk Exosomes. PLoS ONE 2012, 7, e43691. [Google Scholar] [CrossRef]
- Quan, S.; Nan, X.; Wang, K.; Jiang, L.; Yao, J.; Xiong, B. Characterization of Sheep Milk Extracellular Vesicle-miRNA by Sequencing and Comparison with Cow Milk. Animals 2020, 10, 331. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Xie, Y.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Milk exosome-derived miRNAs from water buffalo are implicated in immune response and metabolism process. BMC Vet. Res. 2020, 16, 1–5. [Google Scholar] [CrossRef]
- Matsuda, A.; Moirangthem, A.; Angom, R.S.; Ishiguro, K.; Driscoll, J.; Yan, I.K.; Mukhopadhyay, D.; Patel, T. Safety of bovine milk derived extracellular vesicles used for delivery of RNA therapeutics in zebrafish and mice. J. Appl. Toxicol. 2019, 40, 706–718. [Google Scholar] [CrossRef]
- Xie, M.-Y.; Hou, L.-J.; Sun, J.-J.; Zeng, B.; Xi, Q.-Y.; Luo, J.-Y.; Chen, T.; Zhang, Y.-L. Porcine Milk Exosome MiRNAs Attenuate LPS-Induced Apoptosis through Inhibiting TLR4/NF-κB and p53 Pathways in Intestinal Epithelial Cells. J. Agric. Food Chem. 2019, 67, 9477–9491. [Google Scholar] [CrossRef]
- Matsuda, A.; Patel, T. Milk-derived Extracellular Vesicles for Therapeutic Delivery of Small Interfering RNAs. In Extracellular RNA: Methods and Protocols, 1st ed.; Patel, T., Walker, J., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1740, pp. 187–197. ISBN 978-149-397-652-2. [Google Scholar]
- Ishikawa, H.; Rahman, M.; Yamauchi, M.; Takashima, S.; Wakihara, Y.; Kamatari, Y.O.; Shimizu, K.; Okada, A.; Inoshima, Y. mRNA Profile in Milk Extracellular Vesicles from Bovine Leukemia Virus-Infected Cattle. Viruses 2020, 12, 669. [Google Scholar] [CrossRef]
- Benmoussa, A.; Diallo, I.; Salem, M.; Michel, S.; Gilbert, C.; Sévigny, J.; Provost, P. Concentrates of two subsets of extracellular vesicles from cow’s milk modulate symptoms and inflammation in experimental colitis. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Reif, S.; Shiff, Y.E.; Golan-Gerstl, R.; Reif, S.; Shiff, Y.E.; Golan-Gerstl, R. Milk-derived exosomes (MDEs) have a different biological effect on normal fetal colon epithelial cells compared to colon tumor cells in a miRNA-dependent manner. J. Transl. Med. 2019, 17, 325. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. MicroRNAs: Milk’s epigenetic regulators. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 427–442. [Google Scholar] [CrossRef]
- Melnik, B.C.; Kakulas, F.; Geddes, D.T.; Hartmann, P.E.; John, S.M.; Carrera-Bastos, P.; Cordain, L.; Schmitz, G. Milk miRNAs: Simple nutrients or systemic functional regulators? Nutr. Metab. 2016, 13, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnik, B.C. Milk—A Nutrient System of Mammalian Evolution Promoting mTORC1-Dependent Translation. Int. J. Mol. Sci. 2015, 16, 17048–17087. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; John, S.M.; Schmitz, G. Milk: An exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy? J. Transl. Med. 2014, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Melnik, B.C.; John, S.M.; Schmitz, G. Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr. J. 2013, 12, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupsco, A.; Prada, D.; Valvi, D.; Hu, L.; Petersen, M.S.; Coull, B.; Grandjean, P.; Weihe, P.; Baccarelli, A.A. Human milk extracellular vesicle miRNA expression and associations with maternal characteristics in a population-based cohort from the Faroe Islands. Sci. Rep. 2021, 11, 5840. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, O.; Rodosthenous, R.; Jara, C.; Brennan, K.J.; Wright, R.; Baccarelli, A.A.; Wright, R.J. Detection of long non-coding RNAs in human breastmilk extracellular vesicles: Implications for early child development. Epigenetics 2016, 11, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Niu, M.; Hao, Z.; Liu, M.; Tong, C.; Zhao, X. Selective packaged circular RNAs in milk extracellular vesicles during Staphylococcus aureus infection may have potential against bacterial infection. RNA Biol. 2020, 18, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Lukasik, A.; Zielenkiewicz, P. In Silico Identification of Plant miRNAs in Mammalian Breast Milk Exosomes—A Small Step Forward? PLoS ONE 2014, 9, e99963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutai, E.; Ramer-Tait, A.E.; Zempleni, J. MicroRNAs in bovine milk exosomes are bioavailable in humans but do not elicit a robust pro-inflammatory cytokine response. ExRNA 2020, 2. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhang, L.; Wu, Y.; Zhou, P. Changes in milk fat globule membrane proteome after pasteurization in human, bovine and caprine species. Food Chem. 2019, 279, 209–215. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, Z.-H.; Sun, L.; Li, P. Fermentation Results in Quantitative Changes in Milk-Derived Exosomes and Different Effects on Cell Growth and Survival. J. Agric. Food Chem. 2017, 65, 1220–1228. [Google Scholar] [CrossRef]
- Kosaka, N.; Izumi, H.; Sekine, K.; Ochiya, T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Maburutse, B.E.; Park, M.-R.; Oh, S.; Kim, Y. Evaluation and Characterization of Milk-derived Microvescicle Isolated from Bovine Colostrum. Food Sci. Anim. Resour. 2017, 37, 654–662. [Google Scholar] [CrossRef] [Green Version]
- Hata, T.; Murakami, K.; Nakatani, H.; Yamamoto, Y.; Matsuda, T.; Aoki, N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem. Biophys. Res. Commun. 2010, 396, 528–533. [Google Scholar] [CrossRef]
- Baddela, V.S.; Nayan, V.; Rani, P.; Onteru, S.K.; Singh, D. Physicochemical Biomolecular Insights into Buffalo Milk-Derived Nanovesicles. Appl. Biochem. Biotechnol. 2015, 178, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Kosaka, N.; Shimizu, T.; Sekine, K.; Ochiya, T.; Takase, M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J. Dairy Sci. 2012, 95, 4831–4841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Yu, S.; Xu, M.; Li, P. Effects of microwave on extracellular vesicles and microRNA in milk. J. Dairy Sci. 2018, 101, 2932–2940. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, S.; Rani, P.; Onteru, S.K.; Singh, D. Small Interfering RNA in Milk Exosomes Is Resistant to Digestion and Crosses the Intestinal Barrier In Vitro. J. Agric. Food Chem. 2017, 65, 9506–9513. [Google Scholar] [CrossRef]
- Wolf, T.; Baier, S.R.; Zempleni, J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. J. Nutr. 2015, 145, 2201–2206. [Google Scholar] [CrossRef] [Green Version]
- Kusuma, R.J.; Manca, S.; Friemel, T.; Sukreet, S.; Nguyen, C.; Zempleni, J. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis. Am. J. Physiol. Cell Physiol. 2016, 310, C800–C807. [Google Scholar] [CrossRef] [Green Version]
- Benmoussa, A.; Lee, C.H.C.; Laffont, B.; Savard, P.; Laugier, J.; Boilard, E.; Gilbert, C.; Fliss, I.; Provost, P. Commercial Dairy Cow Milk microRNAs Resist Digestion under Simulated Gastrointestinal Tract Conditions. J. Nutr. 2016, 146, 2206–2215. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Auerbach, A.; Vyas, G.; Li, A.; Halushka, M.; Witwer, K.W. Uptake of dietary milk miRNAs by adult humans: A validation study. F1000Research 2016, 5, 721. [Google Scholar] [CrossRef]
- Howard, K.M.; Kusuma, R.J.; Baier, S.R.; Friemel, T.; Markham, L.; Vanamala, J.; Zempleni, A.J. Loss of miRNAs during Processing and Storage of Cow’s (Bos taurus) Milk. J. Agric. Food Chem. 2015, 63, 588–592. [Google Scholar] [CrossRef] [Green Version]
- Grossen, P.; Portmann, M.; Koller, E.; Duschmalé, M.; Minz, T.; Sewing, S.; Pandya, N.J.; van Geijtenbeek, S.K.; Ducret, A.; Kusznir, E.-A.; et al. Evaluation of bovine milk extracellular vesicles for the delivery of locked nucleic acid antisense oligonucleotides. Eur. J. Pharm. Biopharm. 2021, 158, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Lässer, C.; Alikhani, V.S.; Ekström, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, M.I.; Martín-Duque, P.; Desco, M.; Salinas, B. Radioactive Labeling of Milk-Derived Exosomes with 99mTc and In Vivo Tracking by SPECT Imaging. Nanomaterials 2020, 10, 1062. [Google Scholar] [CrossRef] [PubMed]
- Kleinjan, M.; van Herwijnen, M.J.; Libregts, S.F.; van Neerven, R.J.; Feitsma, A.L.; Wauben, M.H. Regular Industrial Processing of Bovine Milk Impacts the Integrity and Molecular Composition of Extracellular Vesicles. J. Nutr. 2021, 151, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.S.; Gadegaard, I.S.E.; Arnspang, E.C.; Blans, K.; Nejsum, L.N.; Rasmussen, J.T. Specific and Non-Invasive Fluorescent Labelling of Extracellular Vesicles for Evaluation of Intracellular Processing by Intestinal Epithelial Cells. Biomedicines 2020, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, B.; Buschmann, D.; Paul, V.; Pfaffl, M.W. Postprandial transfer of colostral extracellular vesicles and their protein and miRNA cargo in neonatal calves. PLoS ONE 2020, 15, e0229606. [Google Scholar] [CrossRef] [PubMed]
- Sadri, M.; Shu, J.; Kachman, S.D.; Cui, J.; Zempleni, J. Milk exosomes and miRNA cross the placenta and promote embryo survival in mice. Reproduction 2020, 160, 501–509. [Google Scholar] [CrossRef]
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.; Zempleni, J. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, M.; González-Arjona, M.; Santos-Coquillat, A.; Vaquero, J.; Vázquez-Ogando, E.; de Molina, A.; Peinado, H.; Desco, M.; Salinas, B. Covalently Labeled Fluorescent Exosomes for In Vitro and In Vivo Applications. Biomedicines 2021, 9, 81. [Google Scholar] [CrossRef]
- Tong, L.; Hao, H.; Zhang, X.; Zhang, Z.; Lv, Y.; Zhang, L.; Yi, H. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Alters the Gut Microbiota and Enhances Intestinal Immunity in Mice. Mol. Nutr. Food Res. 2020, 64, e1901251. [Google Scholar] [CrossRef]
- Vashisht, M.; Rani, P.; Onteru, S.K.; Singh, D. Curcumin Encapsulated in Milk Exosomes Resists Human Digestion and Possesses Enhanced Intestinal Permeability in Vitro. Appl. Biochem. Biotechnol. 2017, 183, 993–1007. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.; Kyakulaga, A.-H.; Wilcher, S.A.; Gupta, R.C. Milk exosomes—Natural nanoparticles for siRNA delivery. Cancer Lett. 2019, 449, 186–195. [Google Scholar] [CrossRef]
- Mudd, A.M.; Gu, T.; Munagala, R.; Jeyabalan, J.; Egilmez, N.K.; Gupta, R.C. Chemoprevention of Colorectal Cancer by Anthocyanidins and Mitigation of Metabolic Shifts Induced by Dysbiosis of the Gut Microbiome. Cancer Prev. Res. 2019, 13, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Betker, J.L.; Angle, B.M.; Graner, M.W.; Anchordoquy, T.J. The Potential of Exosomes from Cow Milk for Oral Delivery. J. Pharm. Sci. 2019, 108, 1496–1505. [Google Scholar] [CrossRef]
- Shandilya, S.; Rani, P.; Onteru, S.K.; Singh, D. Natural ligand-receptor mediated loading of siRNA in milk derived exosomes. J. Biotechnol. 2020, 318, 1–9. [Google Scholar] [CrossRef]
- Aqil, F.; Kausar, H.; Agrawal, A.; Jeyabalan, J.; Kyakulaga, A.-H.; Munagala, R.; Gupta, R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 2016, 101, 12–21. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Heires, A.J.; Zempleni, J.; Swanson, B.J.; Wichman, C.; Romberger, D.J. Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J. Nutr. Biochem. 2019, 64, 110–120. [Google Scholar] [CrossRef]
- Pieters, B.C.H.; Arntz, O.J.; Bennink, M.B.; Broeren, M.; van Caam, A.; Koenders, M.I.; Van Lent, P.L.E.M.; Berg, W.B.V.D.; De Vries, M.; Van Der Kraan, P.M.; et al. Commercial Cow Milk Contains Physically Stable Extracellular Vesicles Expressing Immunoregulatory TGF-β. PLoS ONE 2015, 10, e0121123. [Google Scholar] [CrossRef]
- Maji, S.; Yan, I.K.; Parasramka, M.; Mohankumar, S.; Matsuda, A.; Patel, T. In vitrotoxicology studies of extracellular vesicles. J. Appl. Toxicol. 2016, 37, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Näslund, T.I.; Proulx, D.P.; Paredes, P.T.; Vallhov, H.; Sandberg, J.; Gabrielsson, S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS 2014, 28, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Matic, S.; D’Souza, D.H.; Wu, T.; Pangloli, P.; Dia, V.P. Bovine Milk Exosomes Affect Proliferation and Protect Macrophages against Cisplatin-Induced Cytotoxicity. Immunol. Investig. 2020, 49, 711–725. [Google Scholar] [CrossRef]
- Stremmel, W.; Weiskirchen, R.; Melnik, B.C. Milk Exosomes Prevent Intestinal Inflammation in a Genetic Mouse Model of Ulcerative Colitis: A Pilot Experiment. Inflamm. Intest. Dis. 2020, 5, 117–123. [Google Scholar] [CrossRef]
- Arntz, O.J.; Pieters, B.C.; Oliveira, M.; Broeren, M.; Bennink, M.B.; De Vries, M.; Van Lent, P.L.; Koenders, M.I.; Berg, W.B.V.D.; Van Der Kraan, P.M.; et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol. Nutr. Food Res. 2015, 59, 1701–1712. [Google Scholar] [CrossRef]
- Miyake, H.; Lee, C.; Chusilp, S.; Bhalla, M.; Li, B.; Pitino, M.; Seo, S.; O’Connor, D.; Pierro, A. Human breast milk exosomes attenuate intestinal damage. Pediatr. Surg. Int. 2019, 36, 155–163. [Google Scholar] [CrossRef]
- Martin, C.; Patel, M.; Williams, S.; Arora, H.; Sims, B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. 2018, 24, 278–284. [Google Scholar] [CrossRef]
- Reif, S.; Elbaum-Shiff, Y.; Koroukhov, N.; Shilo, I.; Musseri, M.; Golan-Gerstl, R. Cow and Human Milk-Derived Exosomes Ameliorate Colitis in DSS Murine Model. Nutrients 2020, 12, 2589. [Google Scholar] [CrossRef]
- Li, B.; Hock, A.; Wu, R.Y.; Minich, A.; Botts, S.; Lee, C.; Antounians, L.; Miyake, H.; Koike, Y.; Chen, Y.; et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE 2019, 14, e0211431. [Google Scholar] [CrossRef]
- Hock, A.; Miyake, H.; Li, B.; Lee, C.; Ermini, L.; Koike, Y.; Chen, Y.; Määttänen, P.; Zani, A.; Pierro, A. Breast milk-derived exosomes promote intestinal epithelial cell growth. J. Pediatr. Surg. 2017, 52, 755–759. [Google Scholar] [CrossRef]
- Qin, W.; Tsukasaki, Y.; Dasgupta, S.; Mukhopadhyay, N.; Ikebe, M.; Sauter, E.R. Exosomes in Human Breast Milk Promote EMT. Clin. Cancer Res. 2016, 22, 4517–4524. [Google Scholar] [CrossRef] [Green Version]
- Leiferman, A.; Shu, J.; Grove, R.; Cui, J.; Adamec, J.; Zempleni, J. A diet defined by its content of bovine milk exosomes and their RNA cargos has moderate effects on gene expression, amino acid profiles and grip strength in skeletal muscle in C57BL/6 mice. J. Nutr. Biochem. 2018, 59, 123–128. [Google Scholar] [CrossRef]
- Mobley, C.B.; Mumford, P.; McCarthy, J.J.; Miller, M.E.; Young, K.C.; Martin, J.S.; Beck, D.T.; Lockwood, C.; Roberts, M.D. Whey protein-derived exosomes increase protein synthesis and hypertrophy in C2C12 myotubes. J. Dairy Sci. 2017, 100, 48–64. [Google Scholar] [CrossRef] [Green Version]
- Paredes, P.T.; Gutzeit, C.; Johansson, S.; Admyre, C.; Stenius, F.; Alm, J.; Scheynius, A.; Gabrielsson, S. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy 2014, 69, 463–471. [Google Scholar] [CrossRef]
- Oliveira, M.; Di Ceglie, I.; Arntz, O.J.; Berg, W.B.V.D.; Hoogen, F.H.J.V.D.; Ferreira, A.; Van Lent, P.L.; Van De Loo, F.A. Milk-Derived Nanoparticle Fraction Promotes the Formation of Small Osteoclasts But Reduces Bone Resorption. J. Cell Physiol. 2016, 232, 225–233. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Pieters, B.C.H.; Guimarães, P.B.; Duffles, L.F.; Heredia, J.E.; Silveira, A.L.M.; Oliveira, A.C.C.; Teixeira, M.M.; Ferreira, A.V.M.; Silva, T.A.; et al. Bovine Milk Extracellular Vesicles Are Osteoprotective by Increasing Osteocyte Numbers and Targeting RANKL/OPG System in Experimental Models of Bone Loss. Front. Bioeng. Biotechnol. 2020, 8, 891. [Google Scholar] [CrossRef] [PubMed]
- Golan-Gerstl, R.; Shiff, Y.E.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and biological function of milk-derived miRNAs. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhao, Z.-H.; Xu, X.; Li, M.; Li, P. Characterization of three different types of extracellular vesicles and their impact on bacterial growth. Food Chem. 2019, 272, 372–378. [Google Scholar] [CrossRef]
- Badawy, A.A.; El-Magd, M.A.; AlSadrah, S. Therapeutic Effect of Camel Milk and Its Exosomes on MCF7 Cells In Vitro and In Vivo. Integr. Cancer Ther. 2018, 17, 1235–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2017, 20, 1–7. [Google Scholar] [CrossRef]
- Civra, A.; Francese, R.; Donalisio, M.; Tonetto, P.; Coscia, A.; Sottemano, S.; Balestrini, R.; Faccio, A.; Cavallarin, L.; Moro, G.E.; et al. Human Colostrum and Derived Extracellular Vesicles Prevent Infection by Human Rotavirus and Respiratory Syncytial Virus in Vitro. J. Hum. Lact. 2021, 37, 122–134. [Google Scholar] [CrossRef]
- Francese, R.; Civra, A.; Donalisio, M.; Volpi, N.; Capitani, F.; Sottemano, S.; Tonetto, P.; Coscia, A.; Maiocco, G.; Moro, G.E.; et al. Anti-Zika virus and anti-Usutu virus activity of human milk and its components. PLoS Negl. Trop. Dis. 2020, 14, e0008713. [Google Scholar] [CrossRef]
- Hu, Y.; Hell, L.; Kendlbacher, R.A.; Hajji, N.; Hau, C.; van Dam, A.; Berckmans, R.J.; Wisgrill, L.; Ay, C.; Pabinger, I.; et al. Human milk triggers coagulation via tissue factor–exposing extracellular vesicles. Blood Adv. 2020, 4, 6274–6282. [Google Scholar] [CrossRef] [PubMed]
- Pisano, C.; Galley, J.; Elbahrawy, M.; Wang, Y.; Farrell, A.; Brigstock, D.; Besner, G.E. Human Breast Milk-Derived Extracellular Vesicles in the Protection Against Experimental Necrotizing Enterocolitis. J. Pediatr. Surg. 2020, 55, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Ascanius, S.; Hansen, M.; Ostenfeld, M.; Rasmussen, J. Milk-Derived Extracellular Vesicles Suppress Inflammatory Cytokine Expression and Nuclear Factor-κB Activation in Lipopolysaccharide-Stimulated Macrophages. Dairy 2021, 2, 165–178. [Google Scholar] [CrossRef]
- Go, G.; Jeon, J.; Lee, G.; Lee, J.H.; Lee, S.H. Bovine milk extracellular vesicles induce the proliferation and differentiation of osteoblasts and promote osteogenesis in rats. J. Food Biochem. 2021, 45, e13705. [Google Scholar] [CrossRef]
- Maghraby, M.K.; Li, B.; Chi, L.; Ling, C.; Benmoussa, A.; Provost, P.; Postmus, A.C.; Abdi, A.; Pierro, A.; Bourdon, C.; et al. Extracellular vesicles isolated from milk can improve gut barrier dysfunction induced by malnutrition. Sci. Rep. 2021, 11, 7635. [Google Scholar] [CrossRef] [PubMed]
- Carobolante, G.; Mantaj, J.; Ferrari, E.; Vllasaliu, D. Cow Milk and Intestinal Epithelial Cell-Derived Extracellular Vesicles as Systems for Enhancing Oral Drug Delivery. Pharmaceutics 2020, 12, 226. [Google Scholar] [CrossRef] [Green Version]
- Parry, H.A.; Mobley, C.B.; Mumford, P.; Romero, M.A.; Haun, C.T.; Zhang, Y.; Roberson, P.A.; Zempleni, J.; Ferrando, A.A.; Vechetti, I.J.J.; et al. Bovine Milk Extracellular Vesicles (EVs) Modification Elicits Skeletal Muscle Growth in Rats. Front. Physiol. 2019, 10, 436. [Google Scholar] [CrossRef]
- Oliveira, M.; Arntz, O.J.; Davidson, E.N.B.; van Lent, P.L.; Koenders, M.I.; van der Kraan, P.M.; Berg, W.B.V.D.; Ferreira, A.; van de Loo, F.A. Milk extracellular vesicles accelerate osteoblastogenesis but impair bone matrix formation. J. Nutr. Biochem. 2016, 30, 74–84. [Google Scholar] [CrossRef]
- Del Pozo-Acebo, L.; López de las Hazas, M.-C.; Tomé-Carneiro, J.; Gil-Cabrerizo, P.; San-Cristobal, R.; Busto, R.; García-Ruiz, A.; Dávalos, A. Bovine milk-derived exosomes as a drug delivery vehicle for miRNA-based therapy. Int. J. Mol. Sci. 2021, 22, 1105. [Google Scholar] [CrossRef]
- Komine-Aizawa, S.; Ito, S.; Aizawa, S.; Namiki, T.; Hayakawa, S. Cow milk exosomes activate NK cells and γδT cells in human PBMCs in vitro. Immunol. Med. 2020, 43, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, M.K.H.A. Using Milk-Derived Exosomes to Repair Malnutrition-Induced Gut Barrier Dysfunction in Mice; University of Toronto: Toronto, ON, Canada, 2020. [Google Scholar]
- Wang, L.; Shi, Z.; Wang, X.; Mu, S.; Xu, X.; Shen, L.; Li, P. Protective effects of bovine milk exosomes against oxidative stress in IEC-6 cells. Eur. J. Nutr. 2020, 60, 317–327. [Google Scholar] [CrossRef]
- Yun, B.; Maburutse, B.; Kang, M.; Park, M.; Park, D.; Kim, Y.; Oh, S. Short communication: Dietary bovine milk–derived exosomes improve bone health in an osteoporosis-induced mouse model. J. Dairy Sci. 2020, 103, 7752–7760. [Google Scholar] [CrossRef]
- Wu, D.; Kittana, H.; Shu, J.; Kachman, S.D.; Cui, J.; Ramer-Tait, A.E.; Zempleni, J. Dietary depletion of milk exosomes and their microRNA cargos elicits a depletion of miR-200a-3p and elevated intestinal inflammation and chemokine (C-X-C motif) ligand 9 expression in Mdr1a−/− mice. Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef]
- Gao, H.; Guo, H.; Zhang, H.; Xie, X.; Wen, P.; Ren, F. Yak-milk-derived exosomes promote proliferation of intestinal epithelial cells in an hypoxic environment. J. Dairy Sci. 2019, 102, 985–996. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.-Y.; Chen, T.; Xi, Q.-Y.; Hou, L.-J.; Luo, J.-Y.; Zeng, B.; Li, M.; Sun, J.-J.; Zhang, Y.-L. Porcine milk exosome miRNAs protect intestinal epithelial cells against deoxynivalenol-induced damage. Biochem. Pharmacol. 2020, 175, 113898. [Google Scholar] [CrossRef]
- Chen, T.; Xie, M.-Y.; Sun, J.-J.; Ye, R.-S.; Cheng, X.; Sun, R.-P.; Wei, L.-M.; Li, M.; Lin, D.-L.; Jiang, Q.-Y.; et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci. Rep. 2016, 6, 33862. [Google Scholar] [CrossRef]
- Pietrzak-Fiećko, R.; Kamelska-Sadowska, A.M. The Comparison of Nutritional Value of Human Milk with Other Mammals’ Milk. Nutrients 2020, 12, 1404. [Google Scholar] [CrossRef]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, Structure, and Digestive Dynamics of Milk from Different Species—A Review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef]
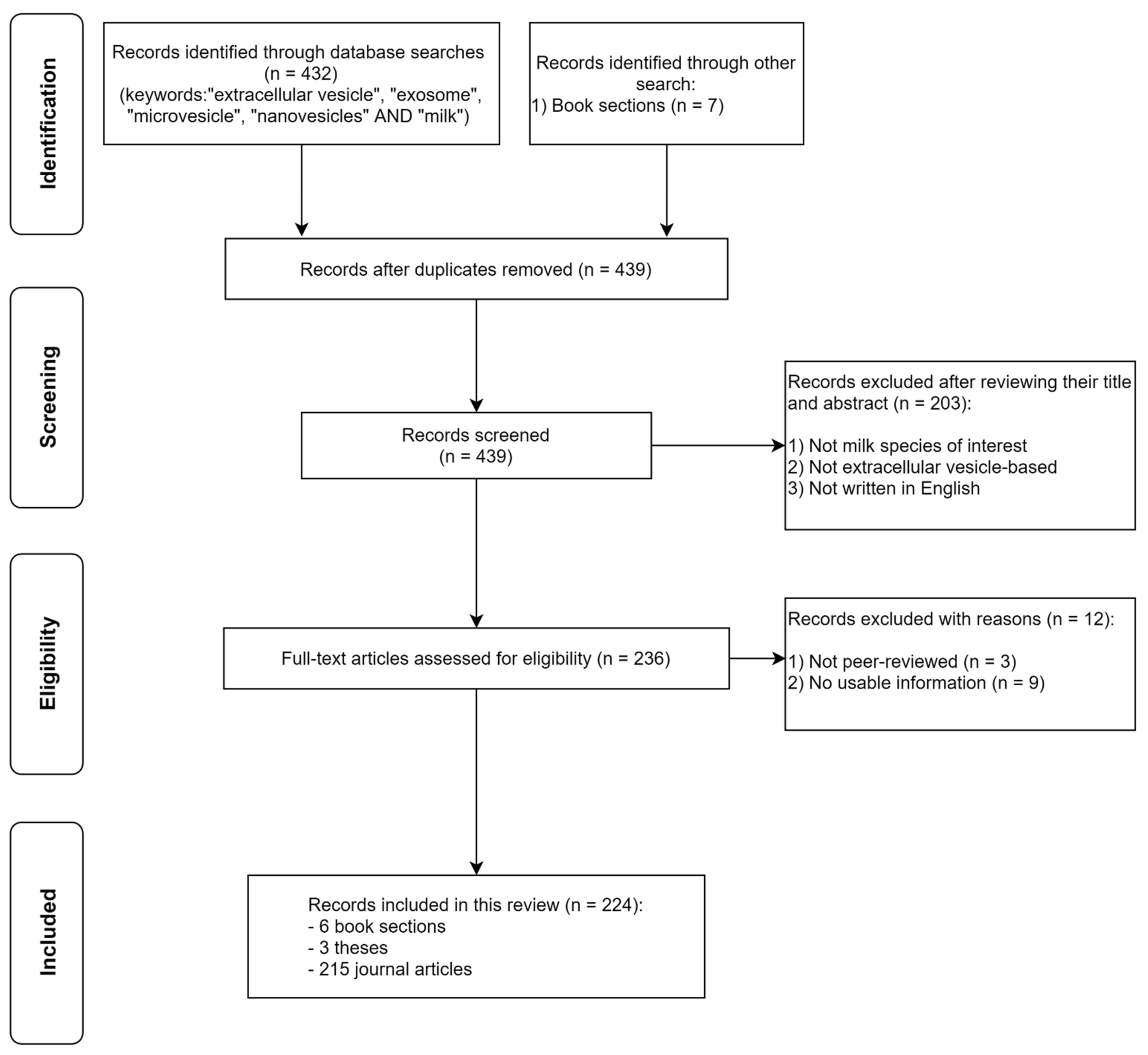
| Species | Energy (kJ) | Carbohydrate (g) | Fat (g) | Protein (g) | Water (g) | Ref. |
|---|---|---|---|---|---|---|
| Buffalo | 473 (66) | 4.9 (0.4) | 8.3 (1.4) | 4.7 (1.3) | 80.9 (2.2) | [19,20,21] |
| Camel | 273 (42) | 4.2 (1.6) | 3.8 (0.4) | 3.6 (1.3) | 87.6 (2.2) | [19,21,22] |
| Cow (≥3% fat) | 277 (22) | 4.7 (0.5) | 3.8 (0.5) | 3.3 (0.2) | 87.3 (0.7) | [19,20,22,23,24,25,26,27] |
| Cow (1–2.9% fat) | 196 (14) | 4.9 (0.1) | 1.5 (0.4) | 3.4 (0.2) | 89.6 (0.3) | [19,22,23,24,25,26,27] |
| Cow (<1% fat) | 151 (7) | 4.9 (0.2) | 0.2 (0.2) | 3.6 (0.2) | 90.3 (0.6) | [19,22,23,25,26,27] |
| Donkey | 175 | 6.1 | 1.0 | 2.0 | 90.4 | [19] |
| Goat (≥3% fat) | 288 (39) | 4.6 (0.3) | 4.0 (0.7) | 3.4 (0.4) | 87.5 (1.5) | [20,22,23,24,25,26] |
| Goat (<3% fat) | 212 | 3.9 | 2.4 | 2.7 | 90.2 | [24] |
| Horse | 177 | 5.4 | 1.1 | 2.1 | 91.0 | [28] |
| Sheep | 406 (16) | 4.9 (0.2) | 6.2 (0.5) | 5.6 (0.3) | 82.7 (0.6) | [22,23,26] |
| Human | ||||||
| Colostrum | 242 (7) | 6.8 (0.3) | 2.6 (0.0) | 2.0 (0.1) | 88.2 (0.0) | [22,23,29] |
| Transitional | 267 (18) | 6.7 (0.2) | 3.4 (0.5) | 1.5 (0.1) | 87.1 (0.6) | [19,23,26] |
| Mature | 293 (9) | 7.3 (0.7) | 4.2 (0.2) | 1.2 (0.1) | 87.3 (0.3) | [19,22,23,25,27] |
| Authors | Scope of Review | Ref. |
|---|---|---|
| Galley et al. | Update on the therapeutic potential of human milk-derived EVs in disease, with an emphasis on necrotising enterocolitis. | [11] |
| Sanwlani et al. | Discussed the mediator role of milk-derived EV crosstalk from inter-cellular to cross-species and highlighted the emerging therapeutic potential of milk-derived EVs. | [12] |
| Melnik et al. | Reviewed epidemiological and translational evidence on how dairy milk-derived exosomes (along with their cargo) contribute to the pathogenesis of common Western diseases. | [13] |
| Munir et al. | Highlighted the role of food-derived exosomes on human physiological and pathological events, as well as their potential as a therapeutic agent. | [14] |
| Zempleni et al. | Discussed the bioavailability and the distribution of milk-derived exosomes and their cargo (emphasis on miRNA). | [63] |
| de la Torre et al. | Summarised the general biophysical features and roles in health and disease of EVs. The authors also focused on human breast milk-derived exosomes in maternal and infant health, based on an in-depth discussion on two proteomic datasets of human breast milk exosome studies. | [16] |
| Le Doare et al. | Discussed the role of human milk microbiota, milk oligosaccharides, and EVs in the development of the infant gut microbiome and immune system. | [17] |
| Foster et al. | Summarised the knowledge about EVs derived from human biofluids, with emphasis on the human reproductive system. | [18] |
| Species | Methodology | Findings | Ref. |
|---|---|---|---|
| Extracellular Vesicle | |||
| Human | DC + PR | EV isolation from human milk via precipitation using ExoQuick. | [105,106] |
| Human | DC + top-down DG-UC, DC + bottom-up DG-UC | DC + top-down DG-UC was efficient and reproducible with a heterogeneous population of EVs (sizes and types). | [107] |
| Cow | UC, SEC, PR, membrane affinity column, PS-affinity isolation | SEC-based qEV column (Izon Science) yielded high purity (high EV count per mg protein) and a large amount of RNA with minimal operation time. | [108] |
| Cow | DC + UC, DC + EDTA + UC, DC + DG-UC | DC + DG-UC yielded the highest abundance of miRNA with EV surface protein markers. | [109] |
| Cow | UC, HCl or AA | EV concentration was significantly higher for samples treated with acidification, suggesting efficient removal of casein. However, acidification was reported to partially degrade EV surface proteins (i.e., CD9 and CD81). TEM images revealed a rough surface of EVs isolated with acids. | [110] |
| Cow | AA+UC, C + UC | AA+UC method yielded lower protein content, but EV protein markers (CD81, Rab5B, TSG101, and Hsc70) were reported to be present in high abundance. Proteome analysis revealed C/UC EV fraction contains whey proteins such as casein, albumin, lactoferrin, and lactoglobulin. | [111] |
| Cow | Total particles and Annexin V+ particles measured using flow cytometry (Canto II and Cytoflex) and NTA (NanoSight) | Significant correlation of total particle counts using Cytoflex and NanoSight and for Annexin V+ particles using Canto II and Cytoflex. | [112] |
| Cow + HCT 116 cell line + Ascaris suum | AFM-based force spectroscopy (FS) | Demonstrated an AFM-based characterisation strategy with the ability to discriminate EVs from contaminants. | [113] |
| Exosome | |||
| Human | Novel solid-phase extraction in tip-based format | Demonstrated successful recovery of spiked lyophilised human urine exosomes from 3 different matrices (mock urine, reconstituted non-fat milk, and foetal bovine serum). | [114] |
| Cow | UC, IP | IP had a better efficiency in removing casein and reduced operator time. TEM revealed precipitated exosomes had rough surfaces. Other features of exosomes isolated were not significantly different. | [115] |
| Cow | DC + DG-UC, DC + SEC | Increased yield and better purity of intact exosomes with DC + SEC method. | [116] |
| Cow | PR, UC + PR, UC + DG-UC, Filtration + UC | PR alone and Filtration + UC unsuitable due to the species difference. UC + PR was useful for rapid isolation with increased recovery. UC + DG-UC suitable for efficient purification with native form intact. | [117] |
| Human + Cow | DC + SEC | Evaluation of Vaswani et al. [116] on human milk. The enrichment profile of exosomes was similar to that obtained in cow milk in their previous study, suggesting the method was suitable for use on human milk. | [118] |
| Human + Cow | UC (milk serum) + SEC, C+ UC (fluff layer) + SEC | Isolation and characterisation of EVs from both milks compared to conventional UC. | [119] |
| Species | Technique | Findings | Ref. |
|---|---|---|---|
| Extracellular Vesicle | |||
| Human | μLC-MS/MS | Identified 258 EV membrane surface proteins (surfaceome) that contributed to antiviral activity. | [138] |
| Human | nLC-MS/MS, LC-MS/MS | Identified 1963 proteins (198 novel). Construction of human milk proteome (n = 39 individual studies) found 2698 unique proteins (633 previously reported in EVs). | [130] |
| Human | nLC-MS/MS | Identified 73 proteins and the presence of several exosomal protein markers. | [60] |
| Cow | CDMS vs. nLC-MS/MS | Detected 57,350 particles in 8 distinct subpopulations (2D Gaussian model). nLC-MS/MS data corroborated exosome enrichment in CDMS samples and identified 162 proteins and 43 exosome-specific proteins. | [131] |
| Cow | nLC-MS/MS | Identified 1330 proteins (118 unique to infection) in bovine leukaemia virus (BLV)-infected cattle. Presented 3 proteomic datasets of milk-derived EVs from healthy and BLV-infected cattle. | [139,140] |
| Cow | nLC-MS/MS | Identified 1899 proteins (20 and 41 specific to 35 K and 100 K pellets, respectively). | [132] |
| Cow | nLC-MS/MS | A novel subset of EVs with unique proteins and other cargoes. | [133] |
| Camel | nLC-MS/MS | Identified 1010 functional groups of proteins. Total of 890 proteins in all 3 species, with 5 specific to C. dromedaries, 31 to C. bacterianus, and 12 to hybrid camels. | [134] |
| Cow + donkey + goat | UHPLC-HRMS | Metabolomic analysis of 5 different pools of fractions obtained from differential centrifugation from 3 different species. | [141] |
| Exosome | |||
| Human | iTRAQ-labelled, nLC-MS/MS | Total of 70 peptides from 28 proteins in preterm milk exosomes differentially expressed compared to full-term milk exosomes, with 47 upregulated and 23 downregulated. | [135] |
| Human + Cow | iTRAQ-labelled, nLC-MS/MS | Total of 920 proteins identified with 575 proteins differentially expressed between colostrum and mature milk in both species. | [142] |
| Cow | nLC-MS/MS | Total of 9430 proteins identified, with 1264, 1404, 963, and 1306 unique proteins (24, 48, and 72 h colostrum and mature milk, respectively). | [136] |
| Cow | μLC-MS/MS, 2D LC-MS | Insufficient exosomes from saliva and urine for analyses. Validation of TSG101 protein milk and plasma exosomes. Total of 86 proteins unique to milk exosomes and 37 proteins unique to plasma exosomes identified. | [137] |
| Cow | iTRAQ-labelled, nLC-MS/MS | Total of 2971 proteins identified, of which 1490, 302, and 334 were unique to exosomes, whey, and MFGMs, respectively. Total of 90 exosome proteins were differentially regulated by mastitis. | [143] |
| Cow | iTRAQ labelled, nLC-MS/MS | Total of 2107 proteins identified. Major MFGM proteins were abundant in exosomes but only represented 0.4% to 1.2% of the total exosomal proteome compared to 15% to 28% of that of the MFGM proteome. | [128] |
| Pig | Nlc-MS/MS | Total of 2313 peptides from 639 proteins, with 68 novel proteins identified. | [129] |
| Horse | MALDI-ToF | Identification of exosome-associated proteins, CD81 and CD63, in horse milk. | [144] |
| Species | Findings | Ref. |
|---|---|---|
| Extracellular vesicle | ||
| Human | Stability and uptake of natural and synthetic EVs loaded with locked nucleic acid anti-sense oligonucleotides in vitro (PHH, NCI-H460 cell line, and hPSC) and in vivo (mice). | [216] |
| Cow | The impact of industrial processing on milk EVs’ structural integrity and molecular composition. | [219] |
| Cow | Cellular internalisation of EVs in vitro (hPAEC and NRCM). | [109] |
| Cow | Development of non-invasive fluorescent labelling of EVs in vitro (Caco-2 cell line), demonstrating internalisation and co-localisation of labelled EVs. | [220] |
| Cow | Time-dependent uptake of colostral miRNA, EV proteins, and isomiRs after feeding in vivo (calves). | [221] |
| Cow | Demonstrated that microwaving, but not autoclaving, agitation, or freezing, reduced miR-220c abundance. | [207] |
| Exosome | ||
| Human | Resistance of exosomes isolated from preterm human milk to in vitro digestion and internalisation in vitro (HIEC). | [169] |
| Human | Exosomal protein markers resist degradation by in vitro digestion, pH 4.5, and the uptake of digested and undigested exosomes, based on immunofluorescence imaging of exosomal protein markers in vitro (HIEC). | [171] |
| Human | Resistance of miRNA to degradation caused by incubation at 26 °C over 24 h, six freeze–thaw cycles at −20 °C, treatment with RNase A and RNase T1, and incubation at 100 °C for 10 min. | [173] |
| Human | Demonstrated the uptake of RNA ex vivo (macrophages). | [217] |
| Human + Cow | Storage at 4 °C substantially reduced the exosome content, especially miRNA, of human milk over time, and the infant formulae tested had no detectable miRNA. | [167] |
| Cow | Assessed the accumulation and effects of milk exosomes and miRNA cargoes on embryo development in C57BL/6 mice. | [222] |
| Cow | Resistance of lncRNA to degradation by in vitro digestion. | [177] |
| Cow | Resistance of paclitaxel (chemotherapeutic), encapsulated in these exosomes, to degradation and loss of efficacy from long-term storage at −80 °C for 4 weeks. | [213] |
| Cow | Resistance of 5 miRNAs to degradation by an in vitro digestion method and in vitro internalisation of exosomes. | [159] |
| Cow | Uptake of exosomes and exosome-encapsulated siRNA (both digested and undigested) in vitro (Caco-2 cell line). | [208] |
| Cow | Fermentation of milk exosomes with probiotic Streptococcus thermophiles, Lactobacilli, and Bifidobacteria reduces miR-29b and miR-21 abundance and total protein concentration. | [201] |
| Cow | Challenged the findings from a previous study [160] regarding the dietary transfer of cow milk-derived miRNA in humans. | [214] |
| Cow | Demonstrated that miR-223 and miR-125b persist in high abundance after simulated in vitro digestion (TNO TIM-1 model). Authors found that exosomes may not be the only carrier of these miRNAs in milk. | [211] |
| Cow | Uptake and bioavailability of fluorescent-labelled exosomes and their miRNA cargoes via endocytosis in vivo (C57BL/6 mice) and in vitro (HUVEC). | [210] |
| Cow | Resistance of native miRNA and anticancer compounds encapsulated in these exosomes to degradation from long-term storage at −80 °C for 6 months. | [212] |
| Cow | Uptake of miRNA in differentiated and undifferentiated THP-1 cells. | [178] |
| Cow | Uptake and transport of miRNA by endocytosis in vitro (Caco-2 and IEC-6 cell lines). | [209] |
| Cow | Uptake of miR-29b and miR-200c in a randomised crossover feeding study, in C57BL/6J mice (± miRNA depletion), and human peripheral blood mononuclear cells (PBMCs). | [160] |
| Cow + Pig + Mice | Cross-species biodistribution profile of miRNAs in mice and pig model. | [223] |
| Goat + cancer cell lines | A novel approach of covalently labelled exosomes with commercial fluorophores in vitro (U87 and B16F10 cell lines) and in vivo (C57BL/6 mice). | [224] |
| Goat | Uptake, bioavailability, and tissue distribution of radiolabelled (reduced technetium, 99mTc (IV)) exosomes using non-invasive single-photon emission computed tomography imaging in Balb/C mice. | [218] |
| Microvesicles/Nanovesicles/Other | ||
| Human | Presence of immune-related miRNA in human milk, two of which were present in exosomes. miR-21 and miR-181a were resistant to degradation by RNase, pH 1, and freeze–thaw, indicating an extracellular protective mechanism. | [202] |
| Cow | Pasteurisation and homogenisation, but not 4 °C storage, substantially reduce the abundance of miR-200c and miR29b in four types of milk tested. Somatic cells in the milk accounted for <1% of the abundance of these miRNAs in milk, consistent with these miRNAs packaged in extracellular structures such as EVs. | [215] |
| Cow | Presence of mRNA and miRNA which were resistant to degradation by RNase, pH 2, incubation at 37 °C, but not Triton X-100, indicating an extracellular protective mechanism. | [206] |
| Cow | Presence of mRNA and miRNA in both samples. These RNAs were resistant to degradation by pH 2, indicating an extracellular protective mechanism. | [204] |
| Buffalo | Demonstrated that 4 °C storage and multiple freeze–thaws reduced the abundance of miR-21 and miR-500. | [205] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, S.L.; Blenkiron, C.; Haines, S.; Acevedo-Fani, A.; Leite, J.A.S.; Zempleni, J.; Anderson, R.C.; McCann, M.J. Ruminant Milk-Derived Extracellular Vesicles: A Nutritional and Therapeutic Opportunity? Nutrients 2021, 13, 2505. https://doi.org/10.3390/nu13082505
Ong SL, Blenkiron C, Haines S, Acevedo-Fani A, Leite JAS, Zempleni J, Anderson RC, McCann MJ. Ruminant Milk-Derived Extracellular Vesicles: A Nutritional and Therapeutic Opportunity? Nutrients. 2021; 13(8):2505. https://doi.org/10.3390/nu13082505
Chicago/Turabian StyleOng, Siew Ling, Cherie Blenkiron, Stephen Haines, Alejandra Acevedo-Fani, Juliana A. S. Leite, Janos Zempleni, Rachel C. Anderson, and Mark J. McCann. 2021. "Ruminant Milk-Derived Extracellular Vesicles: A Nutritional and Therapeutic Opportunity?" Nutrients 13, no. 8: 2505. https://doi.org/10.3390/nu13082505
APA StyleOng, S. L., Blenkiron, C., Haines, S., Acevedo-Fani, A., Leite, J. A. S., Zempleni, J., Anderson, R. C., & McCann, M. J. (2021). Ruminant Milk-Derived Extracellular Vesicles: A Nutritional and Therapeutic Opportunity? Nutrients, 13(8), 2505. https://doi.org/10.3390/nu13082505







