Hsp70 in Liquid Biopsies—A Tumor-Specific Biomarker for Detection and Response Monitoring in Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Uniprot Analysis
2.2. Recombinant Hsp70
2.3. Microscale Thermophoresis-Based Affinity Measurements
2.4. Western Blot and Dot Blot Analysis
2.5. Peptide SPOT Synthesis and Analysis
2.6. Cell Culture
2.7. Flow Cytometry
2.8. Hsp70 Containing Artificial Lipid Vesicles
2.9. Collection of Human Plasma and Serum Samples
2.10. Biotinylation of the Detection Antibody
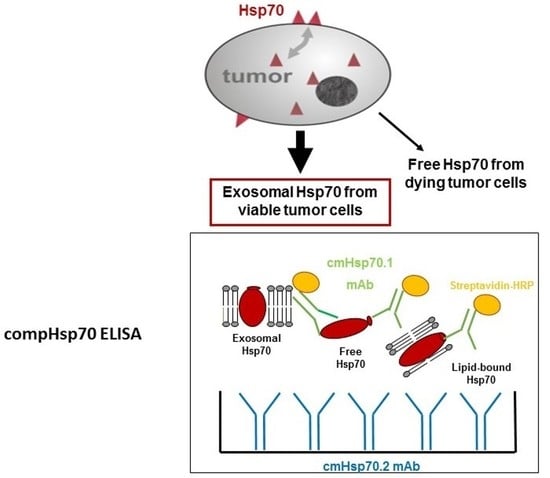
2.11. compHsp70 Sandwich ELISA
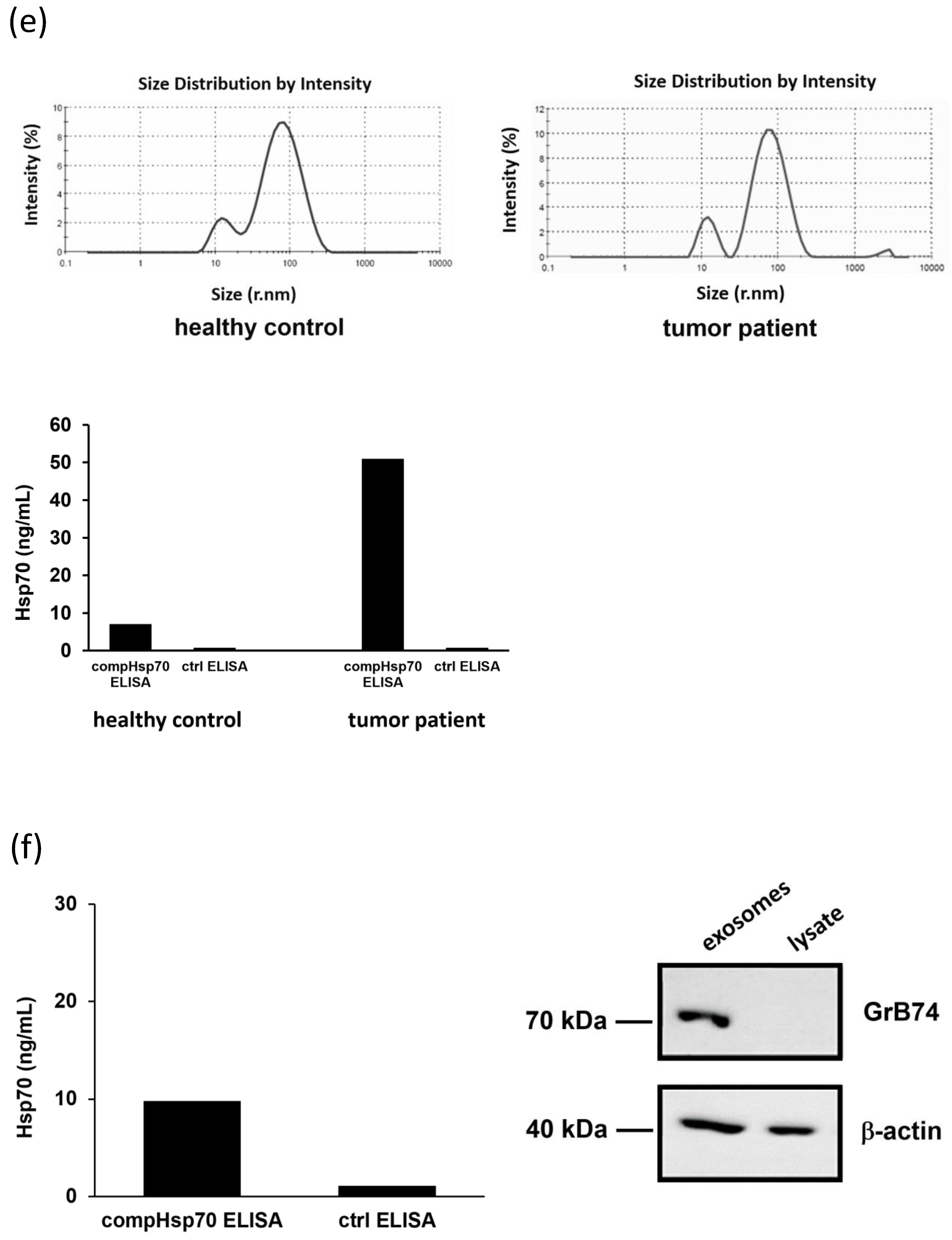
2.12. Isolation of Exosomes from EDTA Blood and Supernatants of Tumor Cells
2.13. Validation of the compHsp70 ELISA
2.14. Statistical Tests
3. Results
3.1. Epitope Mapping of the cmHsp70.1 and cmHsp70.2 Monoclonal Antibodies (mAbs)
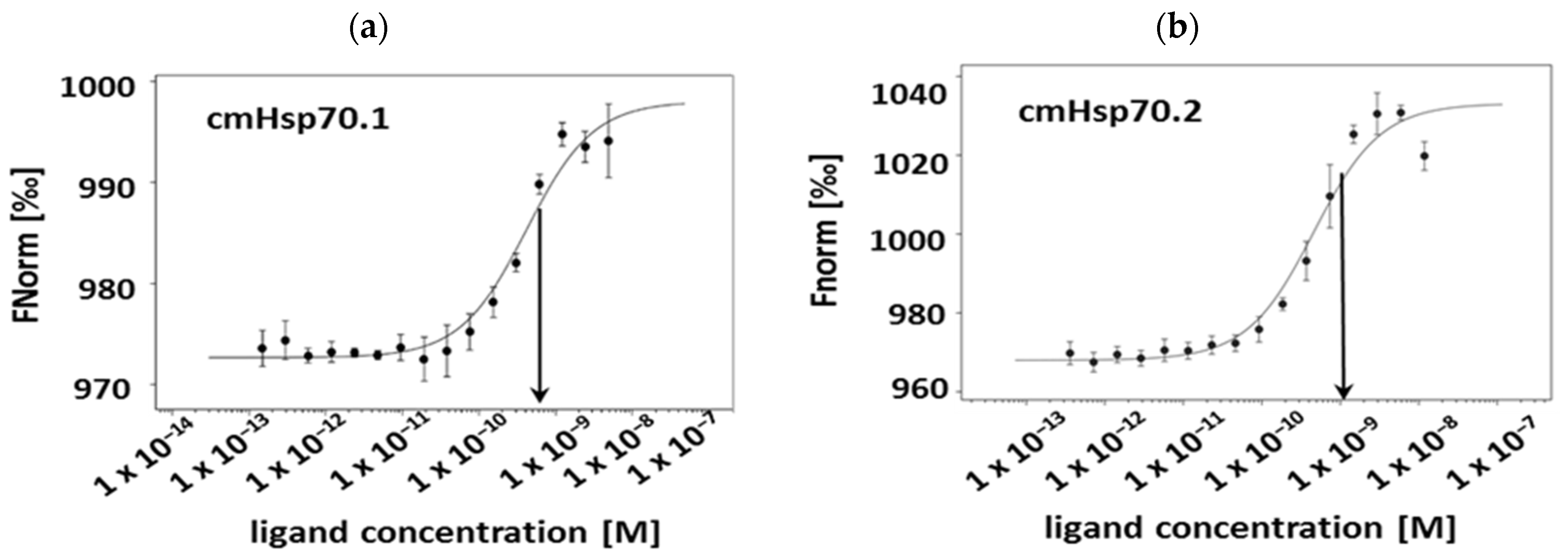
3.2. Affinities of cmHsp70.1 and cmHsp70.2 mAbs to Recombinant Hsp70 Protein
3.3. Binding Characteristics and Specificities of Hsp70 Antibodies to Recombinant Hsp70 Protein, Tumor Cell Lysates and Lipid-Bound Hsp70 Protein
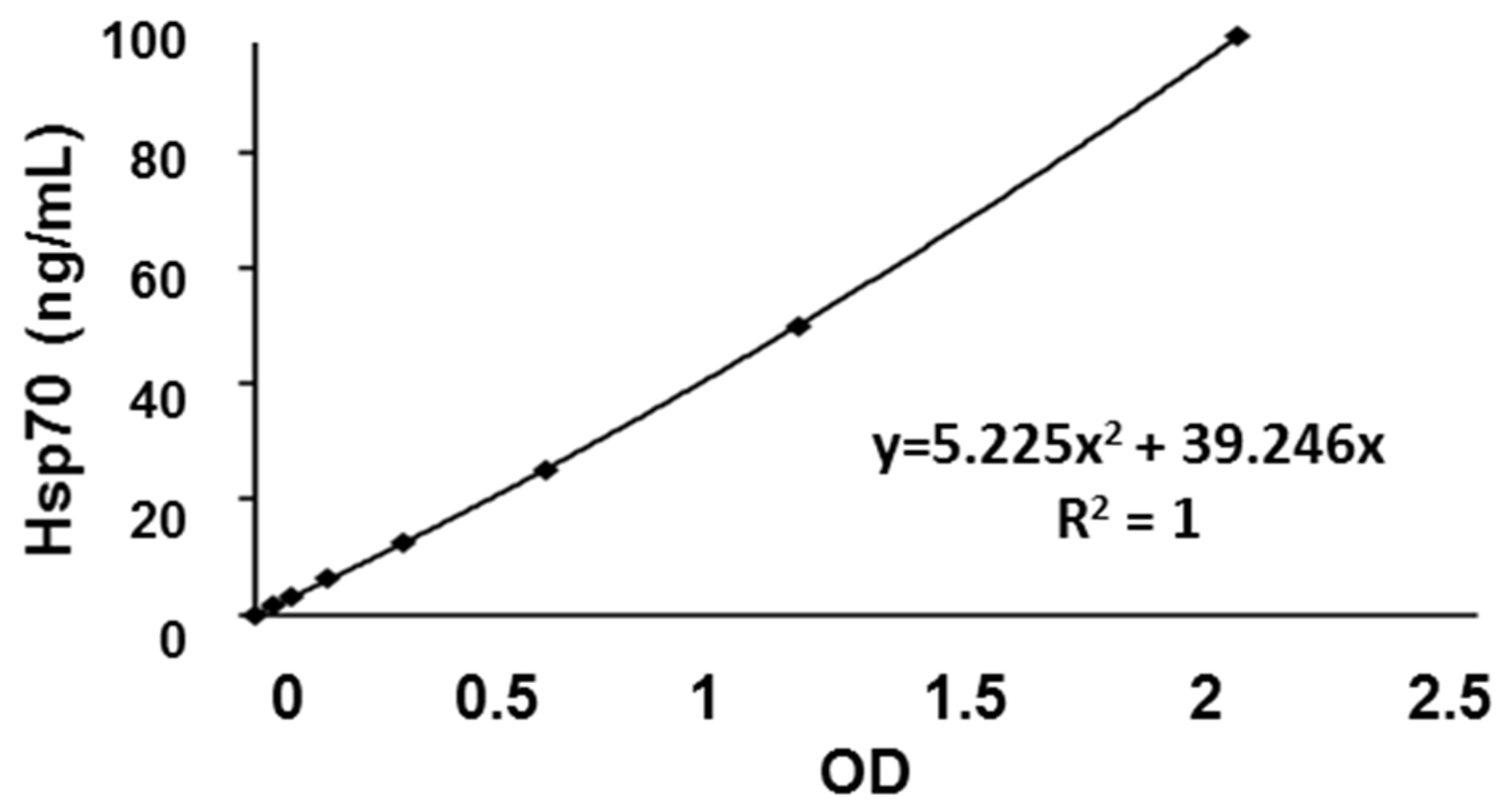
3.4. Calibration Curve and Inter- and Intra-Assay Precision of the compHsp70 ELISA Using cmHsp70.1 and cmHsp70.2 mAbs
3.5. Recovery of Spiked Liposomal Hsp70 Using cmHsp70.1 and Ctrl Hsp70A mAbs as Detection Antibodies
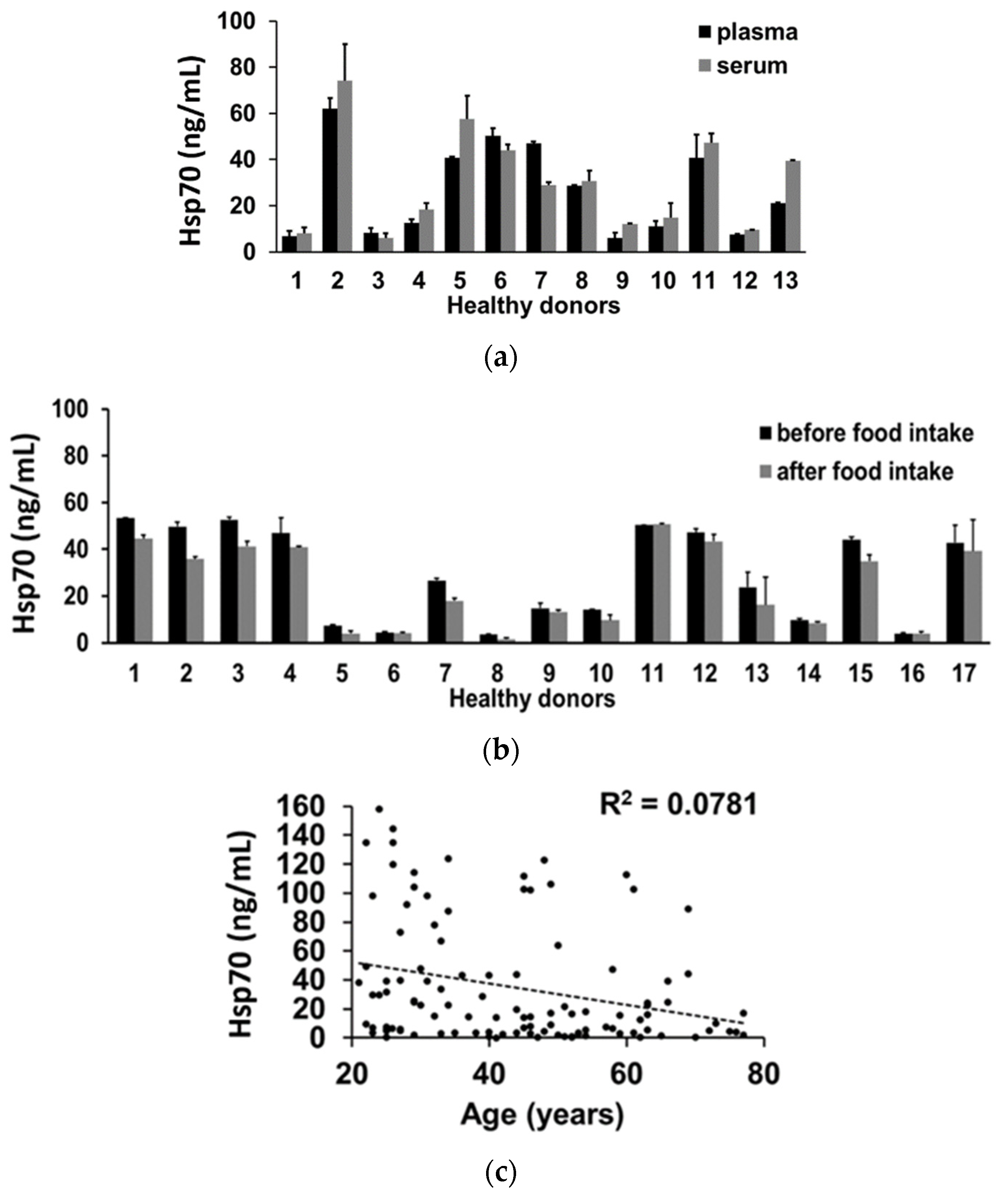
3.6. Impact of Interference Factors on Hsp70 Levels in the Blood Determined by the compHsp70 ELISA
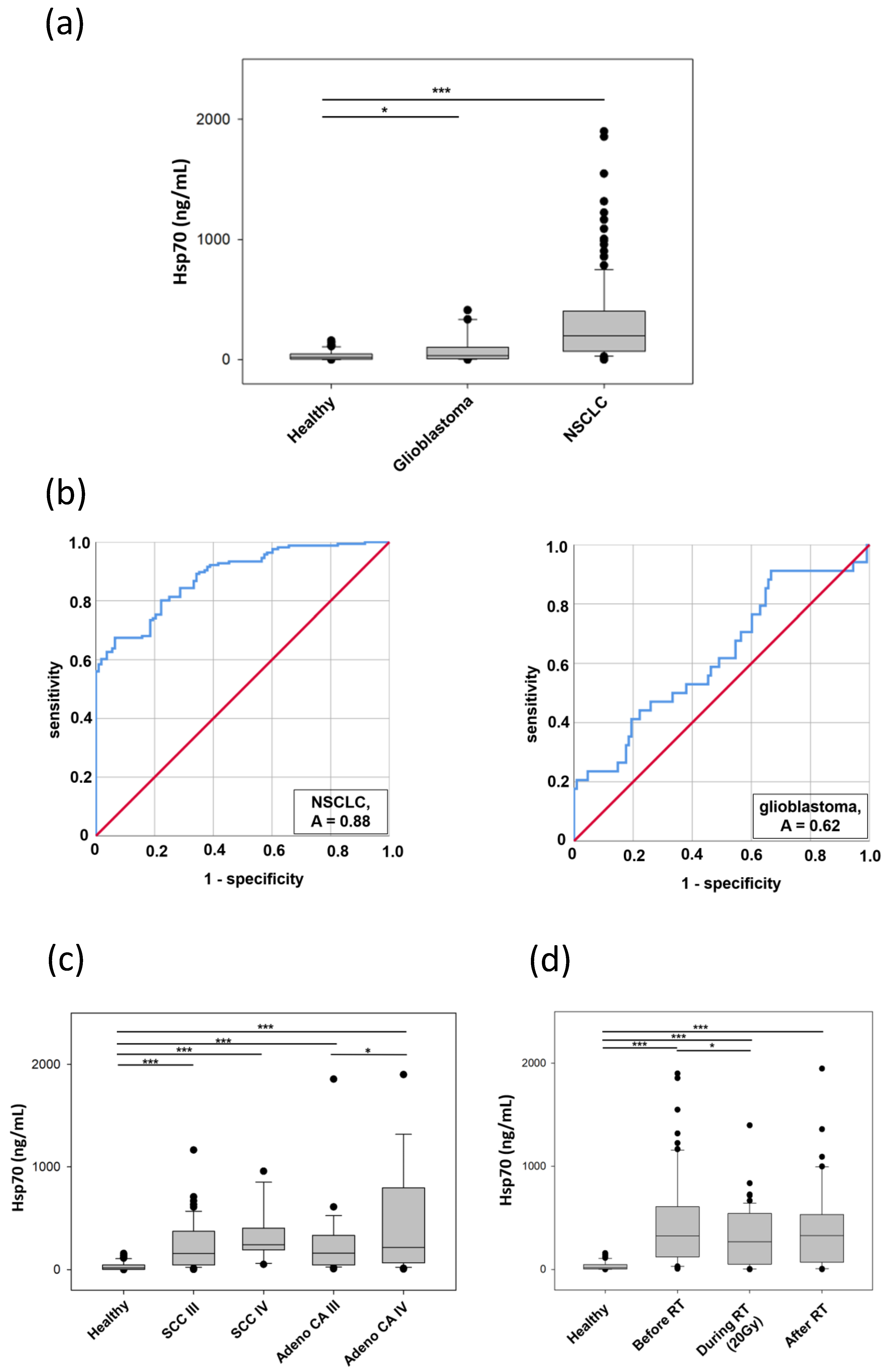
3.7. Comparative Analysis of Hsp70 Concentrations in the Blood and Exosomes of Cancer Patients and Healthy Donors
| Parameters | Healthy Donors | NSCLC | HGG | |
|---|---|---|---|---|
| Number (n) | 108 | 166 | 34 | |
| Gender (m/f) | 51/57 | 109/51 | 28/6 | |
| Age | Mean | 43 | 67 | 59 |
| Range | 21–77 | 41–91 | 21–84 | |
| SD | 16 | 10 | 14 | |
| Median | 44 | 67 | 60 | |
| compHsp70 ELISA | Mean Hsp70 (ng/mL) | 35.06 | 332.19 | 91.82 |
| SEM | 3.99 | 37.90 | 21.28 | |
| ROC | AUC (CI 95%) | 0.88 | 0.62 | |
| p-value | <0.00001 | 0.03 | ||
| Sensitivity (%) | 68 | 91 | ||
| Specificity (%) | 94 | 33 | ||
| Threshold (ng/mL) | 114 | 6 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Allgäuer, M.; Appold, S.; Dieckmann, K.; Ernst, I.; Ganswindt, U.; Holy, R.; Nestle, U.; Nevinny-Stickel, M.; Semrau, S.; et al. Safety and Efficacy of Stereotactic Body Radiotherapy for Stage I Non–Small-Cell Lung Cancer in Routine Clinical Practice: A Patterns-of-Care and Outcome Analysis. J. Thorac. Oncol. 2013, 8, 1050–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberije, C.; De Ruysscher, D.; Houben, R.; van de Heuvel, M.; Uyterlinde, W.; Deasy, J.; Belderbos, J.; Dingemans, A.-M.C.; Rimner, A.; Din, S.; et al. A Validated Prediction Model for Overall Survival from Stage III Non-Small Cell Lung Cancer: Toward Survival Prediction for Individual Patients. Int. J. Radiat. Oncol. 2015, 92, 935–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, C.Y.; Antonia, S.J. Tumor Immunology and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Tuberc. Respir. Dis. 2018, 81, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Hayer-Hartl, M. Molecular Chaperones in the Cytosol: From Nascent Chain to Folded Protein. Science 2002, 295, 1852–1858. [Google Scholar] [CrossRef] [Green Version]
- Lindquist, S.; Craig, E.A. The Heat-Shock Proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Schmitt, E.; Gehrmann, M.; Brunet, M.; Multhoff, G.; Garrido, C. Intracellular and extracellular functions of heat shock proteins: Repercussions in cancer therapy. J. Leukoc. Biol. 2006, 81, 15–27. [Google Scholar] [CrossRef]
- Lund, P.A. The roles of molecular chaperones in vivo. Essays Biochem. 1995, 29, 113–123. [Google Scholar]
- Hartl, F.U. Molecular chaperones in cellular protein folding. Nat. Cell Biol. 1996, 381, 571–580. [Google Scholar] [CrossRef]
- Hunt, C.; Morimoto, R.I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc. Natl. Acad. Sci. USA 1985, 82, 6455–6459. [Google Scholar] [CrossRef] [Green Version]
- Mirault, M.; Southgate, R.; Delwart, E. Regulation of heat-shock genes: A DNA sequence upstream of Drosophila hsp70 genes is essential for their induction in monkey cells. EMBO J. 1982, 1, 1279–1285. [Google Scholar] [CrossRef]
- Pelham, H. Hsp70 accelerates the recovery of nucleolar morphology after heat shock. EMBO J. 1984, 3, 3095–3100. [Google Scholar] [CrossRef] [PubMed]
- Pelham, H.; Lewis, M.; Lindquist, S.L. Expression of a Drosophila heat shock protein in mammalian cells: Transient association with nucleoli after heat shock. Philos. Trans. R. Soc. B Biol. Sci. 1984, 307, 301–307. [Google Scholar] [CrossRef]
- Suzuki, K.; Sawa, Y.; Kaneda, Y.; Ichikawa, H.; Shirakura, R.; Matsuda, H. In vivo gene transfection with heat shock protein 70 enhances myocardial tolerance to ischemia-reperfusion injury in rat. J. Clin. Investig. 1997, 99, 1645–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäättelä, M. Escaping Cell Death: Survival Proteins in Cancer. Exp. Cell Res. 1999, 248, 30–43. [Google Scholar] [CrossRef]
- Multhoff, G.; Botzler, C.; Wiesnet, M.; Müller, E.; Meier, T.; Wilmanns, W.; Issels, R. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int. J. Cancer 1995, 61, 272–279. [Google Scholar] [CrossRef]
- Shin, B.K.; Wang, H.; Yim, A.M.; Le Naour, F.; Brichory, F.; Jang, J.H.; Zhao, R.; Puravs, E.; Tra, J.; Michael, C.W.; et al. Global Profiling of the Cell Surface Proteome of Cancer Cells Uncovers an Abundance of Proteins with Chaperone Function. J. Biol. Chem. 2003, 278, 7607–7616. [Google Scholar] [CrossRef] [Green Version]
- Gehrmann, M.; Liebisch, G.; Schmitz, G.; Anderson, R.; Steinem, C.; De Maio, A.; Pockley, A.; Multhoff, G. Tumor-Specific Hsp70 Plasma Membrane Localization Is Enabled by the Glycosphingolipid Gb3. PLoS ONE 2008, 3, e1925. [Google Scholar] [CrossRef] [Green Version]
- Gehrmann, M.; Pfister, K.; Hutzler, P.; Gastpar, R.; Margulis, B.; Multhoff, G. Effects of Antineoplastic Agents on Cytoplasmic and Membrane-Bound Heat Shock Protein 70 (Hsp70) Levels. Biol. Chem. 2002, 383, 1715–1725. [Google Scholar] [CrossRef]
- Armijo, G.; Okerblom, J.; Cauvi, D.M.; Lopez, V.; Schlamadinger, D.E.; Kim, J.; Arispe, N.; De Maio, A. Interaction of heat shock protein 70 with membranes depends on the lipid environment. Cell Stress Chaperones 2014, 19, 877–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamprecht, C.; Gehrmann, M.; Madl, J.; Römer, W.; Multhoff, G.; Ebner, A. Molecular AFM imaging of Hsp70-1A association with dipalmitoyl phosphatidylserine reveals membrane blebbing in the presence of cholesterol. Cell Stress Chaperones 2018, 23, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Khaleque, A.; Sawyer, D.B.; Ciocca, D.R. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem. Sci. 2006, 31, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Hantschel, M.; Pfister, K.; Jordan, A.; Scholz, R.; Andreesen, R.; Schmitz, G.; Schmetzer, H.; Hiddemann, W.; Multhoff, G. Hsp70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones 2000, 5, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Pfister, K.; Radons, J.; Busch, R.; Tidball, J.G.; Pfeifer, M.; Freitag, L.; Feldmann, H.-J.; Milani, V.; Issels, R.; Multhoff, G. Patient survival by Hsp70 membrane phenotype. Cancer 2007, 110, 926–935. [Google Scholar] [CrossRef]
- Murakami, N.; Kühnel, A.; Schmid, T.; Ilicic, K.; Stangl, S.; Braun, I.S.; Gehrmann, M.; Molls, M.; Itami, J.; Multhoff, G. Role of membrane Hsp70 in radiation sensitivity of tumor cells. Radiat. Oncol. 2015, 10, 149. [Google Scholar] [CrossRef] [Green Version]
- Ishaq, M.; Ojha, R.; Sharma, K.; Sharma, G.; Singh, S.K.; Majumdar, S. Functional inhibition of Hsp70 by Pifithrin-μ switches Gambogic acid induced caspase dependent cell death to caspase independent cell death in human bladder cancer cells. Biochim. Biophys. Acta 2016, 1863, 2560–2573. [Google Scholar] [CrossRef]
- Botzler, C.; Schmidt, J.; Luz, A.; Jennen, L.; Issels, R.; Multhoff, G. Differential Hsp70 plasma-membrane expression on primary human tumors and metastases in mice with severe combined immunodeficiency. Int. J. Cancer 1998, 77, 942–948. [Google Scholar] [CrossRef]
- Vega, V.L.; Rodríguez-Silva, M.; Frey, T.; Gehrmann, M.; Diaz, J.C.; Steinem, C.; Multhoff, G.; Arispe, N.; De Maio, A. Hsp70 Translocates into the Plasma Membrane after Stress and Is Released into the Extracellular Environment in a Membrane-Associated Form that Activates Macrophages. J. Immunol. 2008, 180, 4299–4307. [Google Scholar] [CrossRef] [Green Version]
- Broquet, A.; Thomas, G.; Masliah, J.; Trugnan, G.; Bachelet, M. Expression of the Molecular Chaperone Hsp70 in Detergent-resistant Microdomains Correlates with Its Membrane Delivery and Release. J. Biol. Chem. 2003, 278, 21601–21606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat Shock Protein 70 Surface-Positive Tumor Exosomes Stimulate Migratory and Cytolytic Activity of Natural Killer Cells. Cancer Res. 2005, 65, 5238–5247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharaziha, P.; Ceder, S.; Li, Q.; Panaretakis, T. Tumor cell-derived exosomes: A message in a bottle. Biochim. Biophys. Acta 2012, 1826, 103–111. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, M.; Cervello, M.; Montalto, G.; Cappello, F.; Gulino, A.; Knape, C.; Specht, H.M.; Multhoff, G. Heat Shock Protein 70 Serum Levels Differ Significantly in Patients with Chronic Hepatitis, Liver Cirrhosis, and Hepatocellular Carcinoma. Front. Immunol. 2014, 5, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Maio, A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: A form of communication during injury, infection, and cell damage. Cell Stress Chaperones 2010, 16, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef]
- Shevtsov, M.; Balogi, Z.; Khachatryan, W.; Gao, H.; Vígh, L.; Multhoff, G. Membrane-Associated Heat Shock Proteins in Oncology: From Basic Research to New Theranostic Targets. Cells 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Smulders, L.; Daniels, A.J.; Plescia, C.B.; Berger, D.; Stahelin, R.V.; Nikolaidis, N. Characterization of the Relationship between the Chaperone and Lipid-Binding Functions of the 70-kDa Heat-Shock Protein, HspA1A. Int. J. Mol. Sci. 2020, 21, 5995. [Google Scholar] [CrossRef]
- Balogi, Z.; Multhoff, G.; Jensen, T.K.; Lloyd-Evans, E.; Yamashima, T.; Jäättelä, M.; Harwood, J.L.; Vígh, L. Hsp70 interactions with membrane lipids regulate cellular functions in health and disease. Prog. Lipid Res. 2019, 74, 18–30. [Google Scholar] [CrossRef]
- Stangl, S.; Gehrmann, M.; Riegger, J.; Kuhs, K.; Riederer, I.; Sievert, W.; Hube, K.; Mocikat, R.; Dressel, R.; Kremmer, E.; et al. Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc. Natl. Acad. Sci. USA 2011, 108, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Multhoff, G.; Hightower, L.E. Distinguishing integral and receptor-bound heat shock protein 70 (Hsp70) on the cell surface by Hsp70-specific antibodies. Cell Stress Chaperones 2010, 16, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Jerabek-Willemsen, M.; Wienken, C.J.; Braun, D.; Baaske, P.; Duhr, S. Molecular Interaction Studies Using Microscale Thermophoresis. ASSAY Drug Dev. Technol. 2011, 9, 342–353. [Google Scholar] [CrossRef] [Green Version]
- Wienken, C.J.; Baaske, P.; Rothbauer, U.; Braun, D.; Duhr, S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 2010, 1, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, R. The SPOT-synthesis technique: Synthetic peptide arrays on membrane supports—principles and applications. J. Immunol. Methods 2002, 267, 13–26. [Google Scholar] [CrossRef]
- Zander, H.; Reineke, U.; Schneider-Mergener, J.; Skerra, A. Epitope mapping of the neuronal growth inhibitor Nogo-A for the Nogo receptor and the cognate monoclonal antibody IN-1 by means of the SPOT technique. J. Mol. Recognit. 2007, 20, 185–196. [Google Scholar] [CrossRef]
- Armbruster, D.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29, S49–S52. [Google Scholar] [PubMed]
- Schaefer, C.; Rost, B. Predict impact of single amino acid change upon protein structure. BMC Genom. 2012, 13, S4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Crosbie, P.A.; Shah, R.; Summers, Y.; Dive, C.; Blackhall, F. Prognostic and predictive biomarkers in early stage NSCLC: CTCs and serum/plasma markers. Transl. Lung Cancer Res. 2013, 2, 382–397. [Google Scholar] [CrossRef]
- Rolfo, C.; Castiglia, M.; Hong, D.; Alessandro, R.; Mertens, I.; Baggerman, G.; Zwaenepoel, K.; Gil-Bazo, I.; Passiglia, F.; Carreca, A.P.; et al. Liquid biopsies in lung cancer: The new ambrosia of researchers. Biochim. Biophys. Acta 2014, 1846, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulpa, J.; Wojcik, E.; Reinfuss, M.; Kołodziejski, L. Carcinoembryonic Antigen, Squamous Cell Carcinoma Antigen, CYFRA 21-1, and Neuron-specific Enolase in Squamous Cell Lung Cancer Patients. Clin. Chem. 2002, 48, 1931–1937. [Google Scholar] [CrossRef]
- Pujol, J.-L.; Boher, J.-M.; Grenier, J.; Quantin, X. Cyfra 21-1, neuron specific enolase and prognosis of non-small cell lung cancer: Prospective study in 621 patients. Lung Cancer 2001, 31, 221–231. [Google Scholar] [CrossRef]
- Kozu, Y.; Maniwa, T.; Takahashi, S.; Isaka, M.; Ohde, Y.; Nakajima, T. Prognostic significance of postoperative serum carcinoembryonic antigen levels in patients with completely resected pathological-stage I non-small cell lung cancer. J. Cardiothorac. Surg. 2013, 8, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, M.; Shimizu, T.; Ayabe, T.; Onitsuka, T. Maximum SUV on positron emission tomography and serum CEA level as prognostic factors after curative resection for non-small cell lung cancer. Asia Pac. J. Clin. Oncol. 2012, 8, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.-L.; Molinier, O.; Ebert, W.; Daurès, J.-P.; Barlesi, F.; Buccheri, G.; Paesmans, M.; Quoix, E.; Moro-Sibilot, D.; Szturmowicz, M.; et al. CYFRA 21-1 is a prognostic determinant in non-small-cell lung cancer: Results of a meta-analysis in 2063 patients. Br. J. Cancer 2004, 90, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Filella, X.; Augé, J.; Fuentes, R.; Bover, I.; Rifa, J.; Moreno, V.; Canals, E.; Viñolas, N.; Marquez, A.; et al. Tumor Markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in Patients with Non-Small Cell Lung Cancer as an Aid in Histological Diagnosis and Prognosis. Tumor Biol. 2003, 24, 209–218. [Google Scholar] [CrossRef]
- Chen, H.; Fu, F.; Zhao, Y.; Wu, H.; Hu, H.; Sun, Y.; Zhang, Y.; Xiang, J.; Zhang, Y. The Prognostic Value of Preoperative Serum Tumor Markers in Non-Small Cell Lung Cancer Varies with Radiological Features and Histological Types. Front. Oncol. 2021, 11, e645159. [Google Scholar] [CrossRef]
- Andre, F.; Schartz, N.E.; Movassagh, M.; Flament, C.; Pautier, P.; Morice, P.; Pomel, C.; Lhomme, C.; Escudier, B.; Le Chevalier, T.; et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002, 360, 295–305. [Google Scholar] [CrossRef]
- Wyciszkiewicz, A.; Kalinowska-Łyszczarz, A.; Nowakowski, B.; Kaźmierczak, K.; Osztynowicz, K.; Michalak, S. Expression of small heat shock proteins in exosomes from patients with gynecologic cancers. Sci. Rep. 2019, 9, 9817. [Google Scholar] [CrossRef] [Green Version]
- McCready, J.; Sims, J.D.; Chan, D.; Jay, D.G. Secretion of extracellular hsp90α via exosomes increases cancer cell motility: A role for plasminogen activation. BMC Cancer 2010, 10, 294. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, S.; Zhu, R.; Li, H.; Han, Q.; Zhao, R.C. Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFκB-TLR signaling pathway. J. Hematol. Oncol. 2016, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Chen, Y.; Wang, S.; Yu, L.; Shen, Y.; Zhong, H.; Yang, Y. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology 2018, 154, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Lv, L.-H.; Wan, Y.-L.; Lin, Y.; Zhang, W.; Yang, M.; Li, G.-L.; Lin, H.-M.; Shang, C.-Z.; Chen, Y.-J.; Min, J. Anticancer Drugs Cause Release of Exosomes with Heat Shock Proteins from Human Hepatocellular Carcinoma Cells That Elicit Effective Natural Killer Cell Antitumor Responses in Vitro. J. Biol. Chem. 2012, 287, 15874–15885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yukawa, H.; Suzuki, K.; Aoki, K.; Arimoto, T.; Yasui, T.; Kaji, N.; Ishikawa, T.; Ochiya, T.; Baba, Y. Imaging of angiogenesis of human umbilical vein endothelial cells by uptake of exosomes secreted from hepatocellular carcinoma cells. Sci. Rep. 2018, 8, 6765. [Google Scholar] [CrossRef]
- Gunther, S.; Ostheimer, C.; Stangl, S.; Specht, H.M.; Mozes, P.; Jesinghaus, M.; Vordermark, D.; Combs, S.; Peltz, F.; Jung, M.P.; et al. Correlation of Hsp70 Serum Levels with Gross Tumor Volume and Composition of Lymphocyte Subpopulations in Patients with Squamous Cell and Adeno Non-Small Cell Lung Cancer. Front. Immunol. 2015, 6, 556. [Google Scholar] [CrossRef] [Green Version]
- Chanteloup, G.; Cordonnier, M.; Isambert, N.; Bertaut, A.; Hervieu, A.; Hennequin, A.; Luu, M.; Zanetta, S.; Coudert, B.; Bengrine, L.; et al. Monitoring HSP70 exosomes in cancer patients’ follow up: A clinical prospective pilot study. J. Extracell. Vesicles 2020, 9, 1766192. [Google Scholar] [CrossRef] [PubMed]
- Ostheimer, C.; Gunther, S.; Bache, M.; Vordermark, D.; Multhoff, G. Dynamics of Heat Shock Protein 70 Serum Levels as a Predictor of Clinical Response in Non-Small-Cell Lung Cancer and Correlation with the Hypoxia-Related Marker Osteopontin. Front. Immunol. 2017, 8, 1305. [Google Scholar] [CrossRef] [PubMed]
- Clark, O.; Yen, K.; Mellinghoff, I.K. Molecular Pathways: Isocitrate Dehydrogenase Mutations in Cancer. Clin. Cancer Res. 2016, 22, 1837–1842. [Google Scholar] [CrossRef] [Green Version]
- Konteatis, Z.; Artin, E.; Nicolay, B.; Straley, K.; Padyana, A.K.; Jin, L.; Chen, Y.; Narayaraswamy, R.; Tong, S.; Wang, F.; et al. Vorasidenib (AG-881): A First-in-Class, Brain-Penetrant Dual Inhibitor of Mutant IDH1 and 2 for Treatment of Glioma. ACS Med. Chem. Lett. 2020, 11, 101–107. [Google Scholar] [CrossRef]
- Tejera, D.; Kushnirsky, M.; Gultekin, S.H.; Lu, M.; Steelman, L.; de La Fuente, M.I. Ivosidenib, an IDH1 inhibitor, in a patient with recurrent, IDH1-mutant glioblastoma: A case report from a Phase I study. CNS Oncol. 2020, 9, CNS62. [Google Scholar] [CrossRef]
- Platten, M.; Bunse, L.; Wick, A.; Bunse, T.; Le Cornet, L.; Harting, I.; Sahm, F.; Sanghvi, K.; Tan, C.L.; Poschke, I.; et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nat. Cell Biol. 2021, 592, 463–468. [Google Scholar] [CrossRef]
- Cordonnier, M.; Chanteloup, G.; Isambert, N.; Seigneuric, R.; Fumoleau, P.; Garrido, C.; Gobbo, J. Exosomes in cancer theranostic: Diamonds in the rough. Cell Adhes. Migr. 2017, 11, 151–163. [Google Scholar] [CrossRef]
- Multhoff, G.; Seier, S.; Stangl, S.; Sievert, W.; Shevtsov, M.; Werner, C.; Pockley, A.G.; Blankenstein, C.; Hildebrandt, M.; Offner, R.; et al. Targeted Natural Killer Cell–Based Adoptive Immunotherapy for the Treatment of Patients with NSCLC after Radiochemotherapy: A Randomized Phase II Clinical Trial. Clin. Cancer Res. 2020, 26, 5368–5379. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danxi, L.; Duncan, R.F. Transient Acquired Thermotolerance in Drosophila, Correlated with Rapid Degradation of Hsp70 During Recovery. Eur. J. Biochem. 1995, 231, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Shimizu, T.; Ayabe, T.; Yonei, A.; Onitsuka, T. Prognostic significance of tumour marker index based on preoperative CEA and CYFRA 21-1 in non-small cell lung cancer. Anticancer. Res. 2010, 30, 3099–3102. [Google Scholar] [PubMed]


| Species | UniProt ID HSP70 Names | Total aa | Epitope of cmHsp70.1 (8-mer) aa Sequence | Epitope of cmHsp70.2 (10-mer) aa Sequence |
|---|---|---|---|---|
| Human | P11142 HSPA8, Hsc70 | 646 | N L L G K F E L 454–461 | A G G M P G G M P G 614–623 |
| Human | P0DMV8 HSPA1A, Hsp70 | 641 | N L L G R F E L 454–461 | A G G P G P G G F G 614–623 |
| Mouse | Q61696 HSPA1A, Hsp70 | 641 | N L L G R F E L 454–461 | A G A P G A G G F G 614–623 |
| Rat | P0DMW0 HSPA1A, Hsp70 | 641 | N L L G R F E L 454–461 | A G A P G A G G F G 614–623 |
| Dog | Q7YQC6 HSPA1A, Hsp70 | 641 | N L L G R F E L 454–461 | A G G P G A G G F G 614–623 |
| Bovine | Q27975 HSPA1A, Hsp70 | 641 | N L L G R F E L 454–461 | A G G P G A G G F G 614–623 |
| Horse | F7DW69 HSPA1A, Hsp70 | 641 | N L L G R F E L 454–461 | A G G P G A G G F G 614–623 |
| Pig | P34930 HSPA1A, Hsp70 | 641 | N L L G R F E L 454–461 | A G G P G P G G F G 614–623 |
| Cat | M3W8G1 HSPA2 *, Hsp70.2 | 639 | N L L G K F D L 457–463 | Q G G P G G G G S G 615–624 |
| Zebrafish | B0S610 HSPA1A, Hsp70 | 643 | N L L G K F E L 456–463 | Q G G M P A G G C G 614–623 |
| Parameters | Performance | Mean Values |
|---|---|---|
| Linear range (ng/mL) | 1.56–25.00 | |
| Intra-assay precision (% CV) | 0.02–12.50 | 3.82% |
| Inter-assay precision (% CV) | 0.38–7.34 | 3.64% |
| Recovery of Hsp70 (%) buffer (2.5 ng)/plasma (15 ng) | 105 ± 1/92 ± 9 | |
| Limit of Detection (LoD, ng/mL) | 4.37 |
| Histology | Stage | Number of Cases (n) | compHsp70 ELISA Hsp70 (ng/mL) |
|---|---|---|---|
| Squamous cell carcinoma | I | 2 | |
| Squamous cell carcinoma | II | 4 | |
| Squamous cell carcinoma | III | 59 | 234.4 ± 29.2 |
| Squamous cell carcinoma | IV | 12 | 321.2 ± 68.8 |
| Squamous cell carcinoma | Not specified | 5 | 249.9 ± 64.4 |
| Adeno carcinoma | I | 1 | |
| Adeno carcinoma | II | 1 | |
| Adeno carcinoma | III | 24 | 260.3 ± 75.3 |
| Adeno carcinoma | IV | 29 | 561.3 ± 173.4 * |
| Adeno carcinoma | Not specified | 2 | |
| Not otherwise specified | Not specified | 27 | 336.1 ± 65.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, C.; Stangl, S.; Salvermoser, L.; Schwab, M.; Shevtsov, M.; Xanthopoulos, A.; Wang, F.; Dezfouli, A.B.; Thölke, D.; Ostheimer, C.; et al. Hsp70 in Liquid Biopsies—A Tumor-Specific Biomarker for Detection and Response Monitoring in Cancer. Cancers 2021, 13, 3706. https://doi.org/10.3390/cancers13153706
Werner C, Stangl S, Salvermoser L, Schwab M, Shevtsov M, Xanthopoulos A, Wang F, Dezfouli AB, Thölke D, Ostheimer C, et al. Hsp70 in Liquid Biopsies—A Tumor-Specific Biomarker for Detection and Response Monitoring in Cancer. Cancers. 2021; 13(15):3706. https://doi.org/10.3390/cancers13153706
Chicago/Turabian StyleWerner, Caroline, Stefan Stangl, Lukas Salvermoser, Melissa Schwab, Maxim Shevtsov, Alexia Xanthopoulos, Fei Wang, Ali Bashiri Dezfouli, Dennis Thölke, Christian Ostheimer, and et al. 2021. "Hsp70 in Liquid Biopsies—A Tumor-Specific Biomarker for Detection and Response Monitoring in Cancer" Cancers 13, no. 15: 3706. https://doi.org/10.3390/cancers13153706
APA StyleWerner, C., Stangl, S., Salvermoser, L., Schwab, M., Shevtsov, M., Xanthopoulos, A., Wang, F., Dezfouli, A. B., Thölke, D., Ostheimer, C., Medenwald, D., Windberg, M., Bache, M., Schlapschy, M., Skerra, A., & Multhoff, G. (2021). Hsp70 in Liquid Biopsies—A Tumor-Specific Biomarker for Detection and Response Monitoring in Cancer. Cancers, 13(15), 3706. https://doi.org/10.3390/cancers13153706