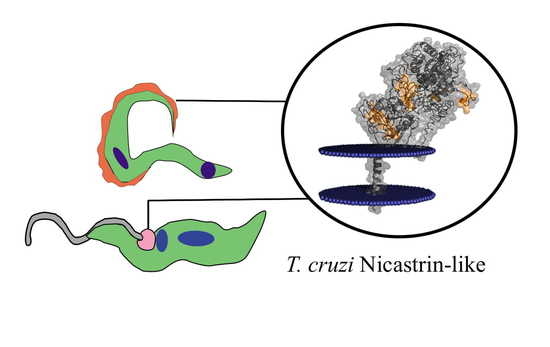
Nicastrin-Like, a Novel Transmembrane Protein from Trypanosoma cruzi Associated to the Flagellar Pocket
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Parasites and Cell Culture
2.3. Synthesis of the Cellulose-Membrane-Bound Peptide Array
2.4. Screening of SPOT Membranes
2.5. Scanning and Measurement of Spot Signal Intensities
2.6. Peptide Synthesis, BSA Conjugation and Polyclonal Antibodies Production
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Preparation of Cell Extract
2.9. SDS-PAGE and Western Blotting
2.10. Identification of Glycoconjugates
2.11. Immunofluorescence Microscopy
2.12. T. cruzi–Host Cell Interaction
2.13. Phylogeny
2.14. Bioinformatics Tools
2.15. Animal, Human Sera and Ethics Statement
2.16. Statistical Analysis
3. Results
3.1. Identification of the Immunodominant IgG Epitopes in T. cruzi Nicastrin
3.2. Antigenicity of the Synthetic Peptides and Specificity of the Antisera
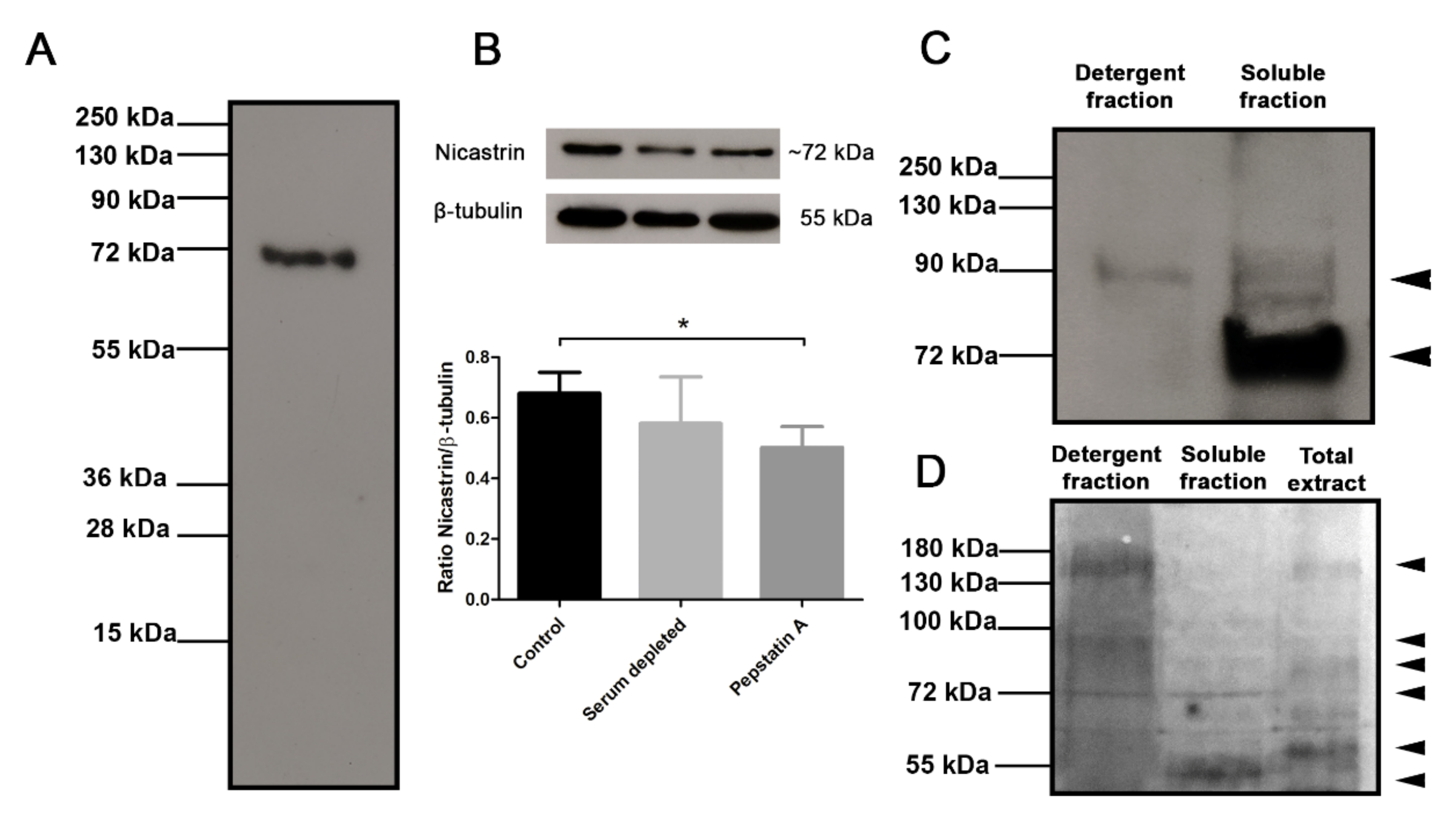
3.3. Subcellular Localization of the Tc-NICT
3.4. Nicastrin Participates in Cell Binding
3.5. Cross-Immunity
3.6. Structure and Topology of T. cruzi NICT-Like
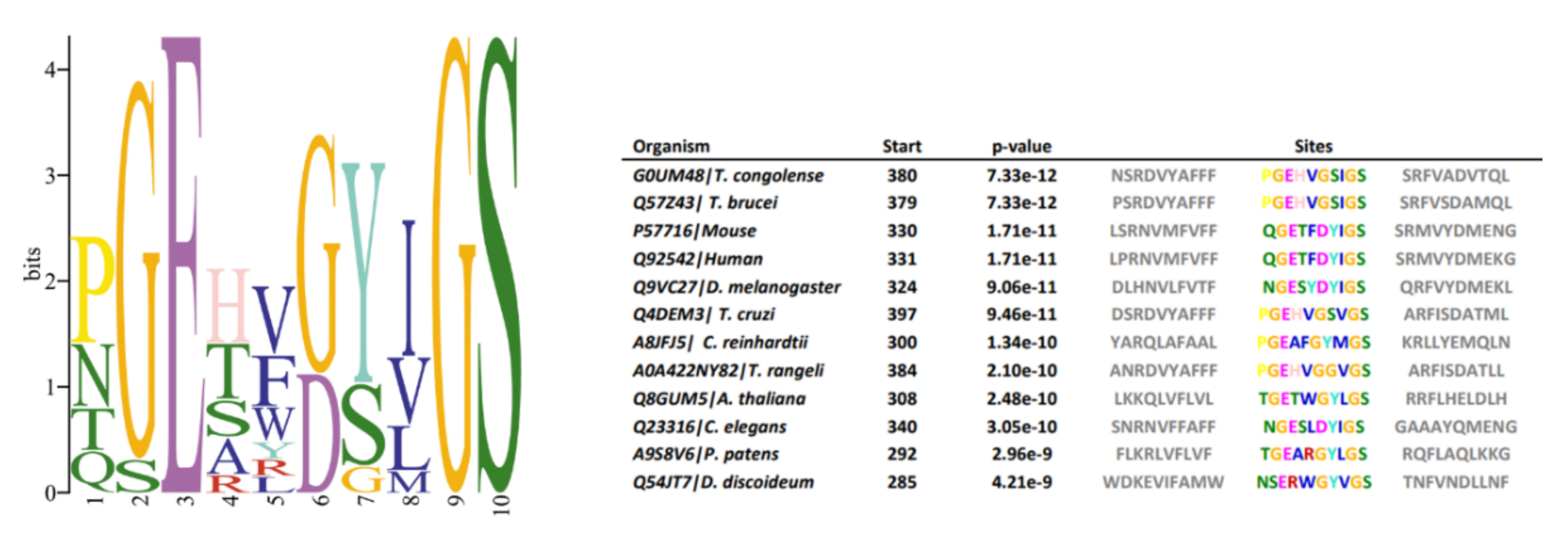
3.7. Conserved Motifs
3.8. Glycosylation
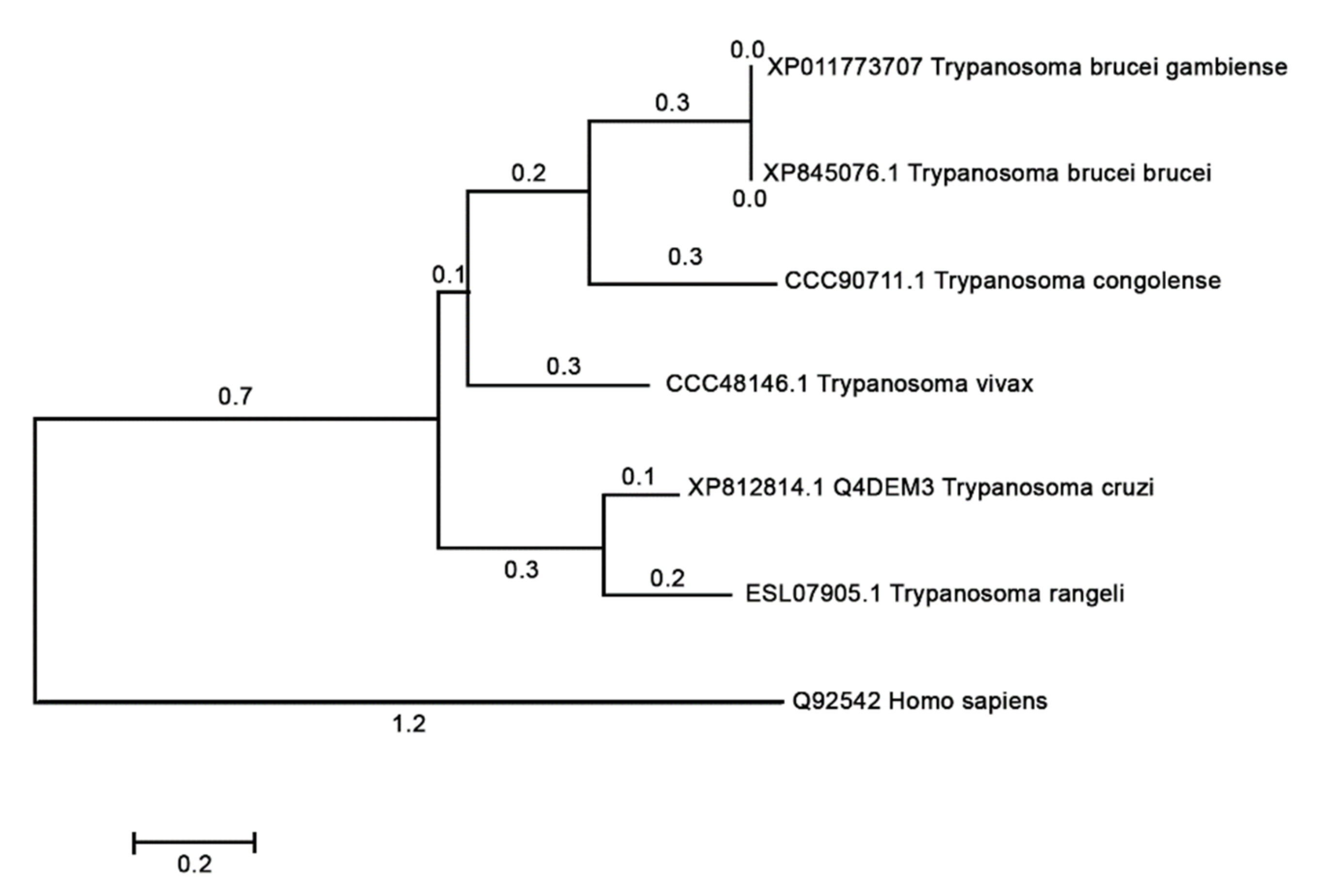
3.9. Phylogenies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, F.; Yu, G.; Arawaka, S.; Nishimura, M.; Kawarai, T.; Yu, H.; Tandon, A.; Supala, A.; Song, Y.Q.; Rogaeva, E.; et al. Nicastrin binds to membrane-tethered Notch. Nat. Cell Biol. 2001, 3, 751–754. [Google Scholar] [CrossRef]
- Wolfe, M.S.; Xia, W.; Ostaszewski, B.L.; Diehl, T.S.; Kimberly, W.T.; Selkoe, D.J. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 1999, 398, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Beel, A.J.; Sanders, C.R. Substrate specificity of γ-secretase and other intramembrane proteases. Cell. Mol. Life Sci. 2008, 65, 1311–1334. [Google Scholar] [CrossRef]
- Shah, S.; Lee, S.-F.; Tabuchi, K.; Hao, Y.-H.; Yu, C.; LaPlant, Q.; Ball, H.; Dann, C.E.; Südhof, T.; Yu, G. Nicastrin Functions as a γ-Secretase-Substrate Receptor. Cell 2005, 122, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hoey, R.J.; Lin, G.; Koide, A.; Leung, B.; Ahn, K.; Dolios, G.; Paduch, M.; Ikeuchi, T.; Wang, R.; et al. Identification of a tetratricopeptide repeat-like domain in the nicastrin subunit of -secretase using synthetic antibodies. Proc. Natl. Acad. Sci. USA 2012, 109, 8534–8539. [Google Scholar] [CrossRef] [PubMed]
- Esler, W.P.; Kimberly, W.T.; Ostaszewski, B.L.; Ye, W.; Diehl, T.S.; Selkoe, D.J. Activity-dependent isolation of the presenilin-gamma-secretase complex reveals NICTastrin and a gamma substrate. Proc. Natl. Acad. Sci. USA 2002, 99, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Ishihara, S.; Nobuhara, M.; Higashide, H.; Funamoto, S. Glycosylation status of nicastrin influences catalytic activity and substrate preference of γ-secretase. Biochem. Biophys. Res. Commun. 2018, 502, 98–103. [Google Scholar] [CrossRef]
- Petit, D.; Hitzenberger, M.; Lismont, S.; Zoltowska, K.M.; Ryan, N.S.; Mercken, M.; Bischoff, F.; Zacharias, M.; Chávez-Gutiérrez, L. Extracellular interface between APP and Nicastrin regulates Aβ length and response to γ-secretase modulators. EMBO J. 2019, 38, e101494. [Google Scholar] [CrossRef]
- Zonooz, M.F.; Sabbagh-Kermani, F.; Fattahi, Z.; Fadaee, M.; Akbari, M.R.; Amiri, R.; Vahidnezhad, H.; Uitto, J.; Najmabadi, H.; Kariminejad, A. Whole Genome Linkage Analysis Followed by Whole Exome Sequencing Identifies Nicastrin (NCSTN) as a Causative Gene in a Multiplex Family with γ-Secretase Spectrum of Autoinflammatory Skin Phenotypes. J. Investig. Dermatol. 2016, 136, 1283–1286. [Google Scholar] [CrossRef]
- He, Y.; Li, C.; Xu, H.; Duan, Z.; Liu, Y.; Zeng, R.; Li, M.; Wang, B. AKT-dependent hyperproliferation of keratinocytes in familial hidradenitis suppurativa with a NCSTN mutation: A potential role of defective miR-100-5p. Br. J. Dermatol. 2020, 182, 500–502. [Google Scholar] [CrossRef]
- Takeichi, T.; Matsumoto, T.; Nomura, T.; Takeda, M.; Niwa, H.; Kono, M.; Shimizu, H.; Ogi, T.; Akiyama, M. A novel NCSTN missense mutation in the signal peptide domain causes hidradenitis suppurativa, which has features characteristic of an autoinflammatory keratinization disease. Br. J. Dermatol. 2019, 182, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Liou, G.-G.; Jiang, Y.-J. Nicastrin Deficiency Induces Tyrosinase-Dependent Depigmentation and Skin Inflammation. J. Investig. Dermatol. 2020, 140, 404–414.e13. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Ha, M.; Kim, S.W.; Kim, M.J.; Lee, C.; Oh, C.; Han, M.; Oh, S.; Kim, Y.H. Evaluation of the prognostic significances of γ-secretase genes in pancreatic cancer. Oncol. Lett. 2019, 17, 4614–4620. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, G.C.; Napoleão-Pêgo, P.; Bottino, C.C.G.; Pinho, R.T.; Provance, D.W., Jr.; De-Simone, S.G. Trypanosoma cruzi Presenilin-Like Transmembrane Aspartyl Protease: Characterization and Cellular Localization. Biomolecules 2020, 10, 1564. [Google Scholar] [CrossRef]
- Coura, J.R. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions—A comprehensive review. Mem. Inst. Oswaldo Cruz 2015, 110, 277–282. [Google Scholar] [CrossRef]
- De-Simone, S.; De Carvalho, L.C.; Oliva, O.F.; Andrade, S.G.; Galvao-Castro, B. Trypanosoma cruzi strain-specific monoclonal antibodies: Identification of Colombian strain flagellates in the insect vector. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 750–754. [Google Scholar] [CrossRef]
- Piras, M.M.; Piras, R.; Henriquez, D.; Negri, S. Changes in morphology and infectivity of cell culture-derived trypomastigotes of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1982, 6, 67–81. [Google Scholar] [CrossRef]
- De-Simone, S.G.; Napoleão-Pêgo, P.; De-Simone, T.S. Spot Synthesis: An Optimized Microarray to Detect IgE Epitopes. Adv. Struct. Saf. Stud. 2016, 1352, 263–277. [Google Scholar] [CrossRef]
- De-Simone, S.; Napoleão-Pego, P.; Teixeira-Pinto, L.A.; Santos, J.D.; De-Simone, T.S.; Melgarejo, A.R.; Aguiar, A.S.; Marchi-Salvador, D.P. Linear B-cell epitopes in BthTX-1, BthTX-II and BthA-1, phospholipase A2’s from Bothrops jararacussu snake venom, recognized by therapeutically neutralizing commercial horse antivenom. Toxicon 2013, 72, 90–101. [Google Scholar] [CrossRef]
- Bottino, C.G.; Gomes, L.P.; Pereira, J.B.; Coura, J.R.; Provance, D.W.; De-Simone, S.G. Chagas disease-specific antigens: Characterization of epitopes in CRA/FRA by synthetic peptide mapping and evaluation by ELISA-peptide assay. BMC Infect. Dis. 2013, 13, 568. [Google Scholar] [CrossRef]
- Pinho, R.T.; Beltramini, L.M.; Alves, C.R.; De-Simone, S.G. Trypanosoma cruzi: Isolation and characterization of aspartyl proteases. Exp. Parasitol. 2009, 122, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Boratyn, G.M.; Schäffer, A.A.; Agarwala, R.; Altschul, S.F.; Lipman, D.J.; Madden, T.L. Domain enhanced lookup time accelerated BLAST. Biol. Direct 2012, 7, 1–14. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Lomize, M.A.; Pogozheva, I.D.; Joo, H.; Mosberg, H.I.; Lomize, A.L. OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucleic Acids Res. 2011, 40, D370–D376. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar] [PubMed]
- Dries, D.R.; Yu, D.R.D.A.G. Assembly, Maturation, and Trafficking of the γ-Secretase Complex in Alzheimers Disease. Curr. Alzheimer Res. 2008, 5, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Nishimura, M.; Arawaka, S.; Levitan, D.; Zhang, L.; Tandon, A. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and beta APP processing. Nature 2000, 407, 48–54. [Google Scholar] [CrossRef]
- Hayashi, I.; Takatori, S.; Urano, Y.; Miyake, Y.; Takagi, J.; Sakata-Yanagimoto, M.; Iwanari, H.; Osawa, S.; Morohashi, Y.; Li, T.; et al. Neutralization of the γ-secretase activity by monoclonal antibody against extracellular domain of nicastrin. Oncogene 2011, 31, 787–798. [Google Scholar] [CrossRef]
- Quevillon, E.; Silventoinen, V.; Pillai, S.; Harte, N.; Mulder, N.; Apweiler, R.; Lopez, R.; Abernathy, K.J. InterProScan: Protein domains identifier. Nucleic Acids Res. 2005, 33, W116–W120. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Arndt, W.; Miller, B.L.; Wheeler, T.J.; Schreiber, F.; Bateman, A.; Eddy, S. HMMER web server: 2015 update. Nucleic Acids Res. 2015, 43, W30–W38. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Arawaka, S.; Hasegawa, H.; Tandon, A.; Janus, C.; Chen, F.; Yu, G.; Kikuchi, K.; Koyama, S.; Kato, T.; Fraser, P.E.; et al. The levels of mature glycosylated nicastrin are regulated and correlate with γ-secretase processing of amyloid β-precursor protein. J. Neurochem. 2002, 83, 1065–1071. [Google Scholar] [CrossRef]
- Morais, V.; Brito, C.; Pijak, D.S.; Crystal, A.S.; Fortna, R.R.; Li, T.; Wong, P.C.; Doms, R.W.; Costa, J.; Montes, A.C.M.B.A. N-glycosylation of human nicastrin is required for interaction with the lectins from the secretory pathway calnexin and ERGIC-53. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2006, 1762, 802–810. [Google Scholar] [CrossRef]
- Field, M.C.; Carrington, M. The trypanosome flagellar pocket. Nat. Rev. Genet. 2009, 7, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; De Strooper, B. Presenilins: Members of the γ-Secretase Quartets, But Part-Time Soloists Too. Physiology 2008, 23, 194–204. [Google Scholar] [CrossRef]
- McConville, M.J.; Mullin, K.A.; Ilgoutz, S.C.; Teasdale, R.D. Secretory Pathway of Trypanosomatid Parasites. Microbiol. Mol. Biol. Rev. 2002, 66, 122–154. [Google Scholar] [CrossRef]
- Haffner, C.; Haass, C. The biochemical and genetic odyssey to the function of a nicastrin-like protein. Neurodegener Dis. 2004, 1, 192–195. [Google Scholar] [CrossRef]
- Zhang, M.; Haapasalo, A.; Kim, D.Y.; Ingano, L.A.M.; Pettingell, W.H.; Kovacs, D.M. Presenilin/γ-secretase activity regulates protein clearance from the endocytic recycling compartment. FASEB J. 2006, 20, 1176–1178. [Google Scholar] [CrossRef]
- Batista, C.M.; Kessler, R.; Eger, I.; Soares, M. Trypanosoma cruzi Intracellular Amastigotes Isolated by Nitrogen Decompression Are Capable of Endocytosis and Cargo Storage in Reservosomes. PLoS ONE 2015, 10, e0130165. [Google Scholar] [CrossRef]
- Urban, S. Nicastrin guards Alzheimer’s gate. Proc. Natl. Acad. Sci. USA 2016, 113, 1112–1114. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, Z.; Ilagan, M.X.; Kopan, R. Gamma-secretase composed of PS1/Pen2/Aph1a can cleave notch and amyloid precursor protein in the absence of nicastrin. J. Neurosci. 2010, 30, 1648–1656. [Google Scholar] [CrossRef]
- Li, T.; Ma, G.; Cai, H.; Price, D.L.; Wong, P.C. Nicastrin Is Required for Assembly of Presenilin/γ-Secretase Complexes to Mediate Notch Signaling and for Processing and Trafficking of β-Amyloid Precursor Protein in Mammals. J. Neurosci. 2003, 23, 3272–3277. [Google Scholar] [CrossRef] [PubMed]
- McMains, V.C.; Myre, M.; Kreppel, L.; Kimmel, A.R. Dictyostelium possesses highly diverged presenilin/γ-secretase that regulates growth and cell-fate specification and can accurately process human APP: A system for functional studies of the presenilin/γ-secretase complex. Dis. Model. Mech. 2010, 3, 581–594. [Google Scholar] [CrossRef]
- Dries, D.R.; Shah, S.; Han, Y.-H.; Yu, C.; Yu, S.; Shearman, M.S.; Yu, G. Glu-333 of Nicastrin Directly Participates in γ-Secretase Activity. J. Biol. Chem. 2009, 284, 29714–29724. [Google Scholar] [CrossRef] [PubMed]
- De Souza, W.; de Carvalho, T.M.; Barrias, E.S. Review on Trypanosoma cruzi: Host cell interaction. Int. J. Cell Biol. 2010, 2010, 295394. [Google Scholar] [CrossRef]
- Piras, M.M.; Henriquez, D.; Piras, R. The effect of proteolytic enzymes and protease inhibitors on the interaction Trypanosoma cruzi—Fibroblasts. Mol. Biochem. Parasitol. 1985, 14, 151–163. [Google Scholar] [CrossRef]
- Sharma, S.; Schiller, M.R. The carboxy-terminus, a key regulator of protein function. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 85–102. [Google Scholar] [CrossRef]
- Capell, A.; Kaether, C.; Edbauer, D.; Shirotani, K.; Merkl, S.; Steiner, H.; Haass, C. Nicastrin Interacts with γ-Secretase Complex Components via the N-terminal Part of Its Transmembrane Domain. J. Biol. Chem. 2003, 278, 52519–52523. [Google Scholar] [CrossRef]
- Li, Y.; Liew, L.S.Y.; Li, Q.; Kang, C. Structure of the transmembrane domain of human nicastrin-a component of γ-secretase. Sci. Rep. 2016, 6, 19522. [Google Scholar] [CrossRef] [PubMed]
- Fluhrer, R.; Kamp, F.; Grammer, G.; Nuscher, B.; Steiner, H.; Beyer, K.; Haass, C. The Nicastrin ectodomain adopts a highly thermostable structure. Biol. Chem. 2011, 392, 995–1001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hart, G.W.; Copeland, R.J. Glycomics Hits the Big Time. Cell 2010, 143, 672–676. [Google Scholar] [CrossRef] [PubMed]
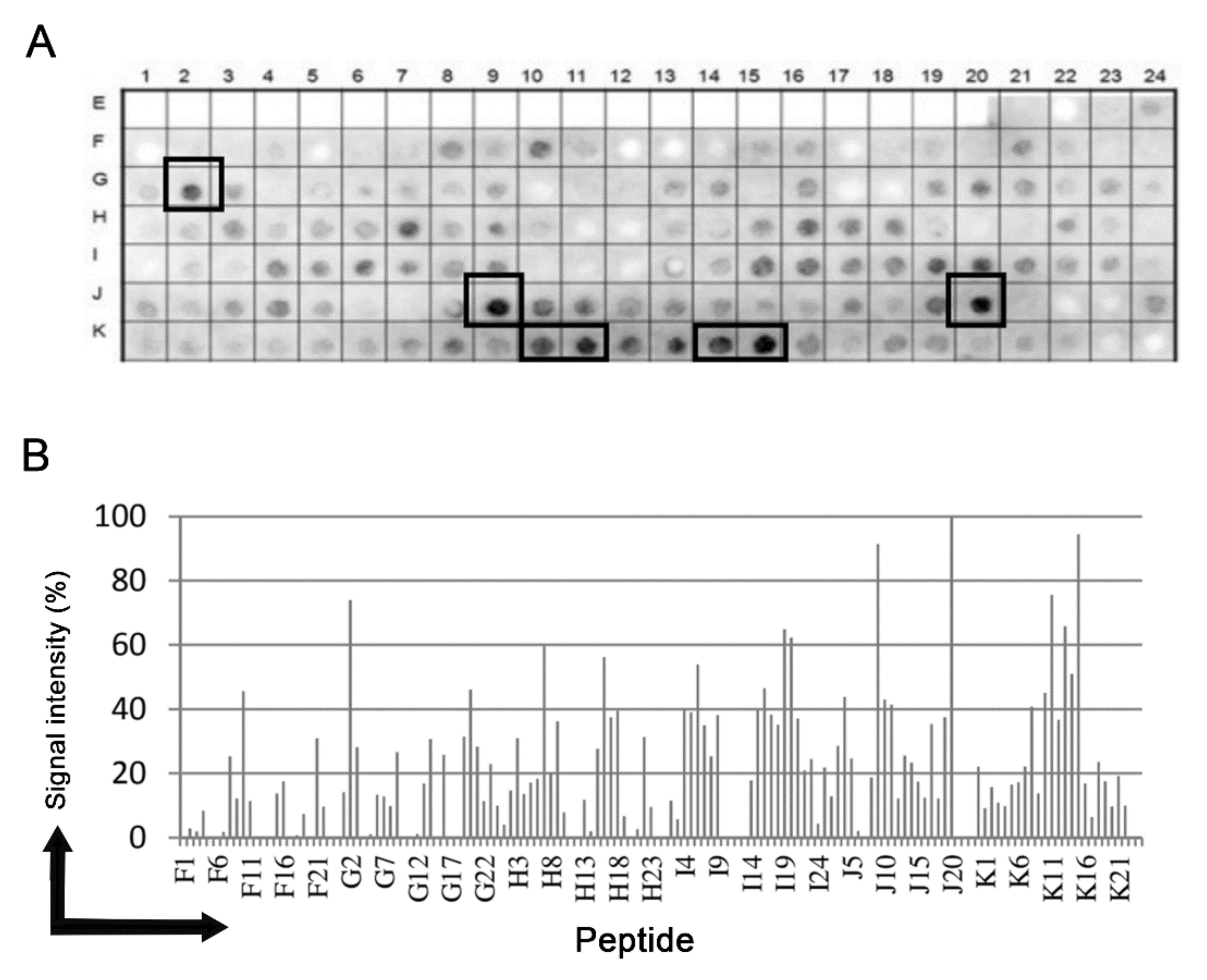



| Epitope | Sequence | aa |
|---|---|---|
| Tc/NICT-1 | SLQDIIRGLSIPDT | 126–139 |
| Tc/NICT-2 | TLTRYNTTFANPDV | 521–534 |
| Tc/NICT-3 | VTSVNR | 576–581 |
| Tx/NICT-4 | KSLRIPHGDWGATW | 651–664 |
| Tc/NICT-5 | MRLHNDSRYELHVM | 671–684 |
| Domain/Properties | H. sapiens (Q92542) | T. cruzi (Q4DEM3) |
|---|---|---|
| Signal peptide | 1–38 | 1–38 |
| Chain | 38–728 | 38–703 |
| Embedded residues | - | 48–54, 58 |
| TM domain | 671–690 | 704–726 |
| Glu, as important residue for gamma-secretase activity | 333 | 330 |
| Motif GGXXP | 300–304 | ND |
| Motif DYIGS | DYIGS (336–340) | GSVGS (402–406) |
| Try previous the conserved domains aa 352–486 | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechuga, G.C.; Napoleão-Pêgo, P.; Gomes, L.R.; da Matta Durans, A.; Provance, D.W., Jr.; De-Simone, S.G. Nicastrin-Like, a Novel Transmembrane Protein from Trypanosoma cruzi Associated to the Flagellar Pocket. Microorganisms 2021, 9, 1750. https://doi.org/10.3390/microorganisms9081750
Lechuga GC, Napoleão-Pêgo P, Gomes LR, da Matta Durans A, Provance DW Jr., De-Simone SG. Nicastrin-Like, a Novel Transmembrane Protein from Trypanosoma cruzi Associated to the Flagellar Pocket. Microorganisms. 2021; 9(8):1750. https://doi.org/10.3390/microorganisms9081750
Chicago/Turabian StyleLechuga, Guilherme Curty, Paloma Napoleão-Pêgo, Larissa Rodrigues Gomes, Andressa da Matta Durans, David William Provance, Jr., and Salvatore Giovanni De-Simone. 2021. "Nicastrin-Like, a Novel Transmembrane Protein from Trypanosoma cruzi Associated to the Flagellar Pocket" Microorganisms 9, no. 8: 1750. https://doi.org/10.3390/microorganisms9081750
APA StyleLechuga, G. C., Napoleão-Pêgo, P., Gomes, L. R., da Matta Durans, A., Provance, D. W., Jr., & De-Simone, S. G. (2021). Nicastrin-Like, a Novel Transmembrane Protein from Trypanosoma cruzi Associated to the Flagellar Pocket. Microorganisms, 9(8), 1750. https://doi.org/10.3390/microorganisms9081750