Heat-Stress Responses Differ among Species from Different ‘Bangia’ Clades of Bangiales (Rhodophyta)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of a Species Belonging to ‘Bangia’ 3
2.2. Morphological and Developmental Properties of ‘Bangia’ sp. ESS2
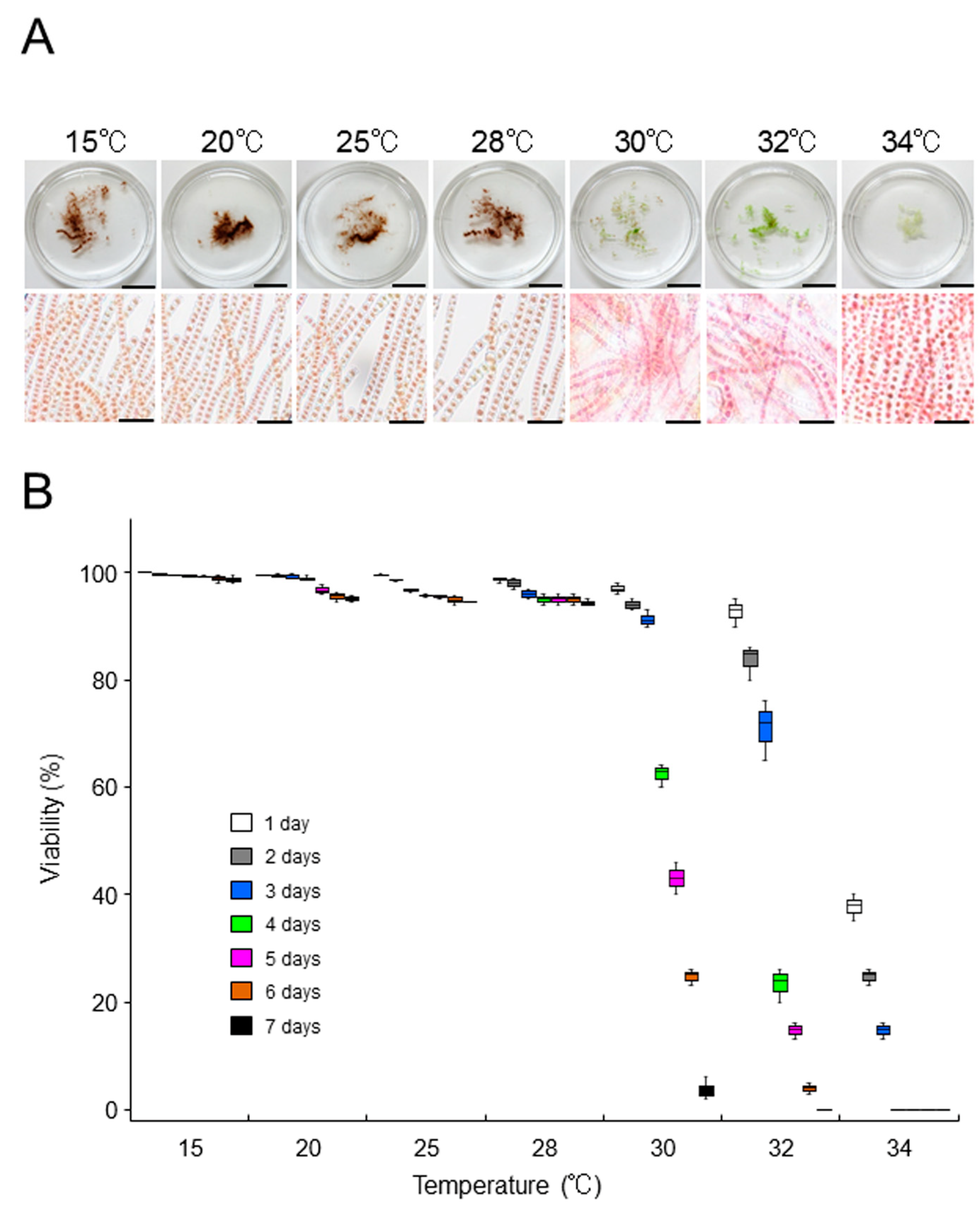
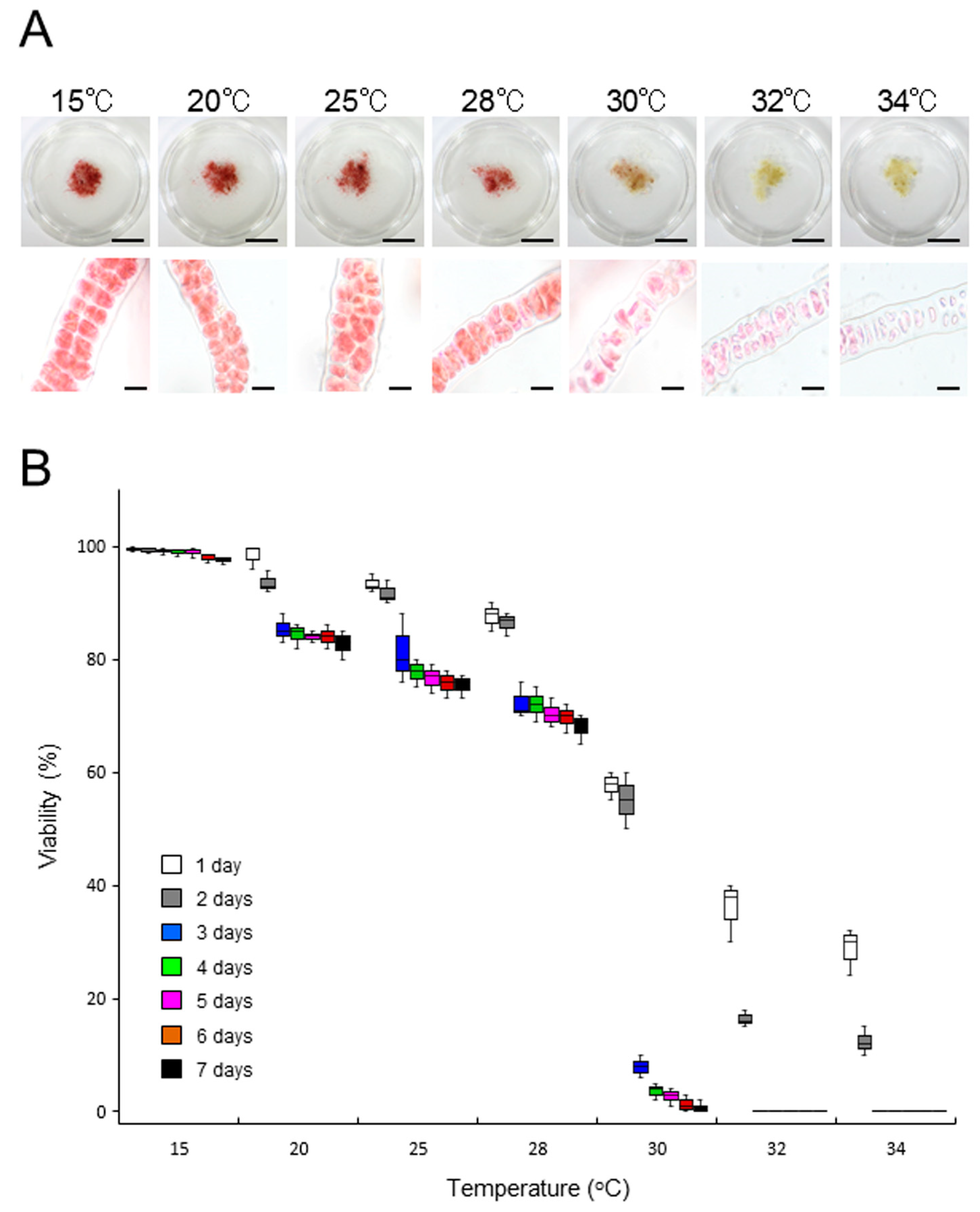
2.3. Growth-Limiting Temperatures of Gametophytic Thalli in ‘Bangia’ sp. ESS2 and Bangia Atropurpurea
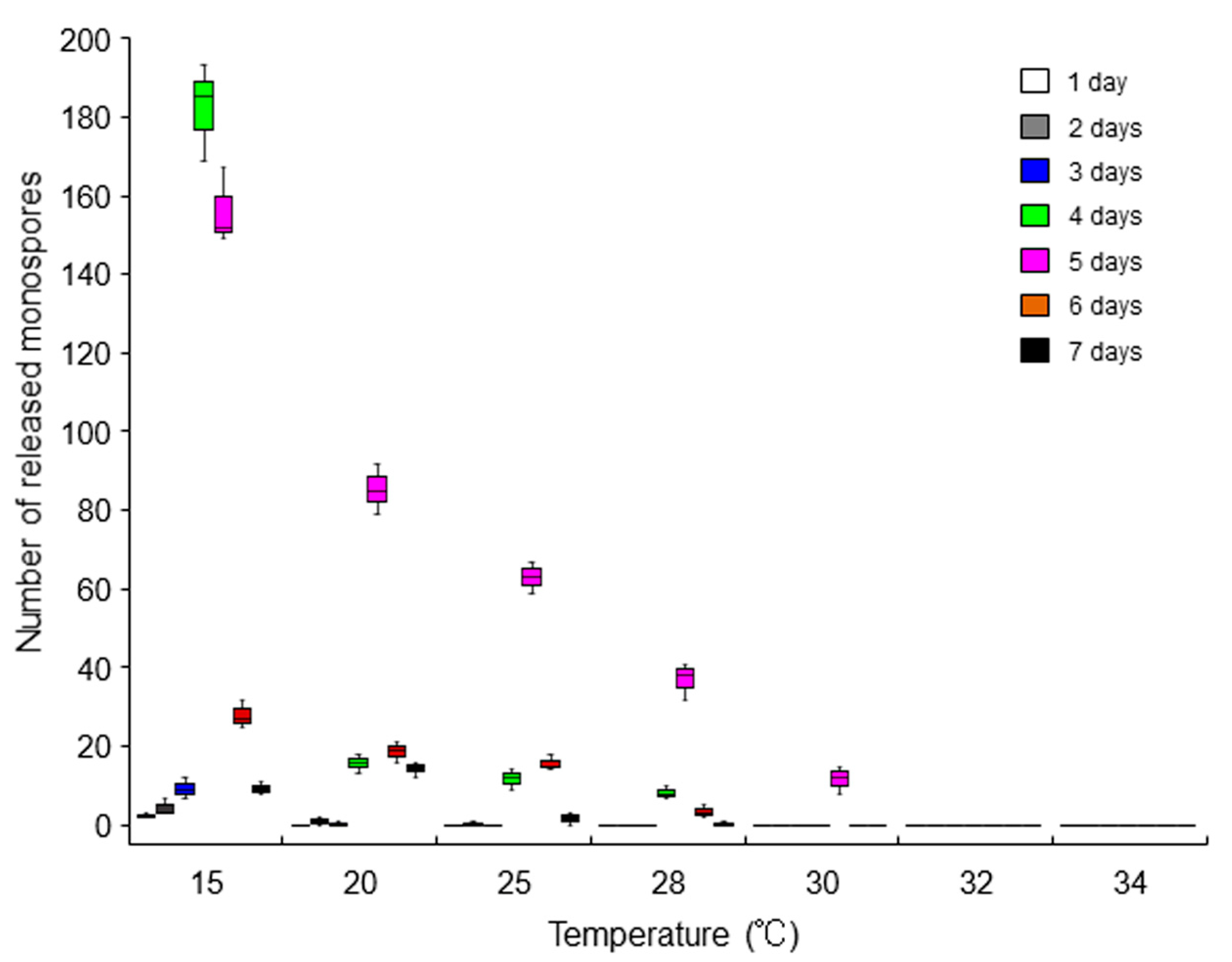
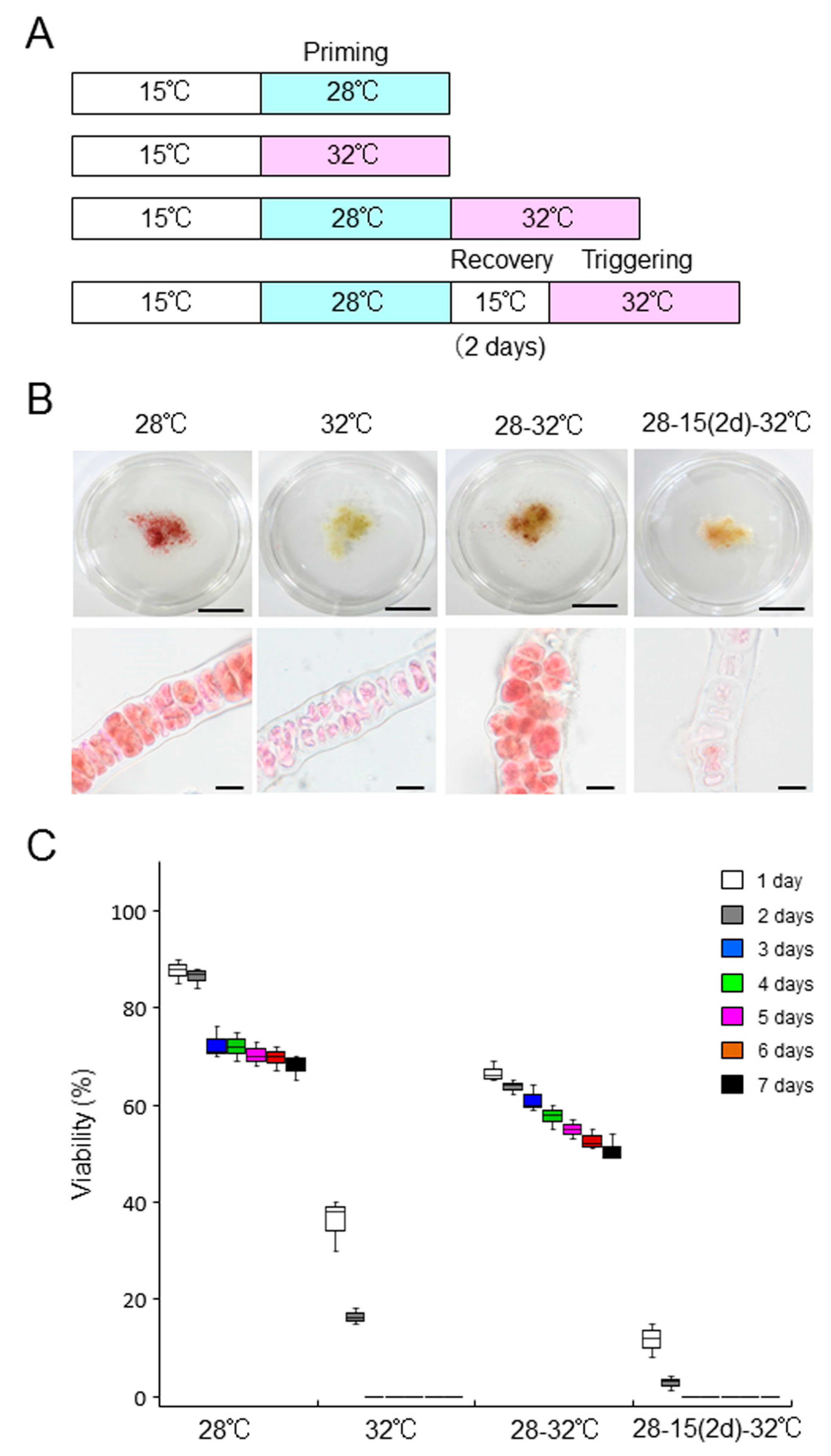
2.4. Repression of the Asexual Life Cycle by Heat Stress
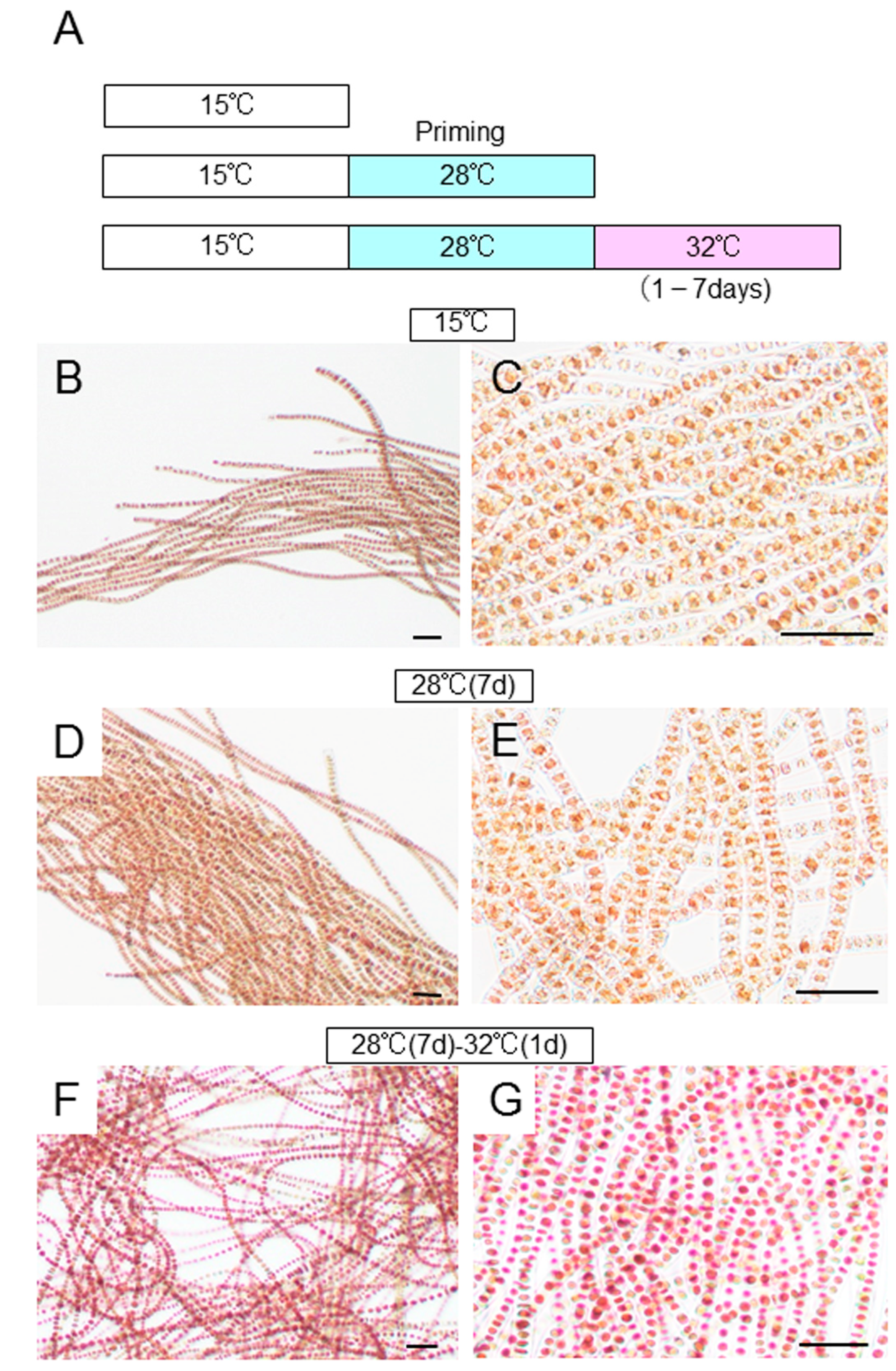
2.5. Defect in the Acquisition of Heat-Stress Tolerance
3. Materials and Methods
3.1. Algal Materials, Culture Conditions, and Morphological Observation
3.2. Phylogenetic Analysis
3.3. Determining the Growth-Limiting Temperature
3.4. Quantification of Released Asexual Spores
3.5. Confirmation of Acquisition of Thermotolerance
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milstein, D.; de Oliveira, M.C. Molecular phylogeny of Bangiales (Rhodophyta) based on small subunit rDNA sequencing: Emphasis on Brazilian Porphyra species. Phycologia 2005, 44, 212–221. [Google Scholar] [CrossRef]
- Brodie, J.; Mortensen, A.M.M.; Ramirez, M.E.; Russell, S.; Rinkel, B. Making the links: Towards a global taxonomy for the red algal genus Porphyra (Bangiales, Rhodophyta). J. Appl. Phycol. 2008, 20, 939–949. [Google Scholar] [CrossRef]
- Müller, K.M.; Cannone, J.J.; Sheath, R.G. A molecular phylogenetic analysis of the Bangiales (Rhodophyta) and description of a new genus and species, Pseudobangia kaycoleia. Phycologia 2005, 44, 146–155. [Google Scholar] [CrossRef]
- Neefus, C.D.; Mathieson, A.C.; Klein, A.S.; Teasdale, B.; Gray, T.; Yarish, C. Porphyra birdiae sp. nov. (Bangiales, Rhodophyta): A new species from the northwest Atlantic. Algae 2002, 17, 203–216. [Google Scholar] [CrossRef]
- Lindstrom, S.C.; Fredericq, S. rbcL gene sequences reveal relationships among north-east Pacific species of Porphyra (Bangiales, Rhodophyta) and a new species, P. aestivalis. Phycol. Res. 2003, 51, 211–224. [Google Scholar] [CrossRef]
- Brodie, J.; Bartsch, I.; Neefus, C.; Orfanidis, S.; Bray, T.; Mathieson, A.C. New insights into the cryptic diversity of the North Atlantic-Mediterranean ‘Porphyra leucosticta’ complex: P. olivii sp. nov. and P. rosengurttii (Bangiales, Rhodophyta). Eur. J. Phycol. 2007, 42, 3–28. [Google Scholar] [CrossRef]
- Lindstrom, S.C. Cryptic diversity, biogeography and genetic variation in northeast Pacific species of Porphyra sensu lato (Bangiales, Rhodophyta). J. Appl. Phycol. 2008, 20, 951–962. [Google Scholar] [CrossRef]
- Niwa, K.; Iida, S.; Kato, A.; Kawai, H.; Kikuchi, N.; Kobiyama, A.; Aruga, Y. Genetic diversity and introgression in two cultivated species (Porphyra yezoensis and Porphyra tenera) and closely related wild species of Porphyra (Bangiales, Rhodophyta). J. Phycol. 2009, 45, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Niwa, K.; Kikuchi, N.; Hwang, M.-S.; Choi, H.-G.; Aruga, Y. Cryptic species in the Pyropia yezoensis complex (Bangiales, Rhodophyta): Sympatric occurrence of two cryptic species even on same rocks. Phycol. Res. 2014, 62, 36–43. [Google Scholar] [CrossRef]
- Sutherland, J.; Lindstrom, S.; Nelson, W.; Brodie, J.; Lynch, M.; Hwang, M.S.; Choi, H.G.; Miyata, M.; Kikuchi, N.; Oliveira, M.; et al. A new look at an ancient order: Generic revision of the Bangiales. J. Phycol. 2011, 47, 1131–1151. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, N.; Vergés, A.; Peteiro, C.; Sutherland, J.E.; Brodie, J. Diversity of bladed Bangiales (Rhodophyta) in western Mediterranean: Recognition of the genus Themis and descriptions of T. ballesterosii sp. nov., T. iberica sp. nov., and Pyropia parva sp. nov. J. Phycol. 2014, 50, 908–929, Corrigendum in 2015, 46, 206. [Google Scholar] [CrossRef]
- Li, C.; Irie, R.; Shimada, S.; Mikami, K. Requirement of different normalization genes for quantitative gene expression analysis under abiotic stress conditions in ‘Bangia’ sp. ESS1. J. Aquat. Res. Mar. Sci. 2019, 2019, 194–205. [Google Scholar]
- Notoya, M.; Iijima, N. Life history and sexuality of archeospore and apogamy of Bangia atropurpurea (Roth) Lyngbye (Bangiales, Rhodophyta) from Fukaura and Enoshima, Jpn. Fish. Sci. 2003, 69, 799–805. [Google Scholar] [CrossRef]
- Wang, W.-J.; Zhu, J.-Y..; Xu, P.; Xu, J.R.; Lin, X.Z.; Huang, C.K.; Song, W.L.; Peng, G.; Wang, G.C. Characterization of the life history of Bangia fuscopurpurea (Bangiaceae, Rhodophyta) in connection with its cultivation in China. Aquaculture 2008, 278, 101–109. [Google Scholar] [CrossRef]
- Mikami, K.; Kishimoto, I. Temperature promoting the asexual life cycle program in Bangia fuscopurpurea (Bangiales, Rhodophyta) from Esashi in the Hokkaido Island, Japan. Algal Resour. 2018, 11, 25–32. [Google Scholar]
- Kishimoto, I.; Ariga, I.; Itabashi, Y.; Mikami, K. Heat-stress memory is responsible for acquired thermotolerance in Bangia fuscopurpurea. J. Phycol. 2019, 55, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Yokono, M.; Uchida, H.; Suzawa, Y.; Akiomoto, S.; Murakami, A. Stabilization and modulation of the phycobilisome by calcium in the calciphilic freshwater red alga Bangia atropurpurea. Biochim. Biophys. Acta 2012, 1817, 306–3011. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Bi, G.; Mao, Y.; Li, G.; Kong, F. The first plastid genome of a filamentous taxon ‘Bangia’ sp. OUCPT-01 in the Bangiales. Sci. Rep. 2018, 8, 10688. [Google Scholar] [CrossRef]
- Müller, K.M.; Sheath, R.G.; Vis, M.L.; Crease, T.J.; Cole, K.M. Biogeography and systematics of Bangia (Bangiales, Rhodophyta) based on the Rubisco spacer, rbcL gene and 18S rRNA gene sequences and morphometric analyses. 1. North America. Phycologia 1998, 37, 195–207. [Google Scholar] [CrossRef]
- Knight, G.A.; Nelson, W.A. An evaluation of characters obtained from life history studies for distinguishing New Zealand Porphyra species. J. Appl. Phycol. 1999, 11, 411–419. [Google Scholar] [CrossRef]
- Kim, N.-G. Culture studies of Porphyra dentate and P. pseudolinearis (Bangia, Rhodophyta), two dioecious species from Korea. Hydrobiologia 1999, 398/399, 127–135. [Google Scholar] [CrossRef]
- Takahashi, M.; Mikami, K. Phototropism in the marine red macroalga Pyropia yezoensis. Am. J. Plant Sci. 2016, 7, 2412–2428. [Google Scholar] [CrossRef]
- Hirata, R.; Takahashi, M.; Saga, N.; Mikami, K. Transient gene expression system established in Porphyra yezoensis is widely applicable in Bangiophycean algae. Mar. Biotechnol. 2011, 13, 1038–1047. [Google Scholar] [CrossRef]
- Omuro, Y.; Khoa, H.V.; Mikami, K. The absence of hydrodynamic stress promotes acquisition of freezing tolerance and freeze-dependent asexual reproduction in the red alga ‘Bangia’ sp. ESS1. Plants 2021, 10, 465. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Bäurle, I. Plant heat adaptation: Priming in response to heat stress. F1000Res. 2016, 5, 694. [Google Scholar] [CrossRef]
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef]
- Sanyal, R.P.; Misra, H.S.; Saini, A. Heat-stress priming and alternative splicing-linked memory. J. Exp. Bot. 2018, 69, 2431–2434. [Google Scholar] [CrossRef]
- Li, C.; Ariga, I.; Mikami, K. Difference in nitrogen starvation-inducible expression patterns among phylogenetically diverse ammonium transporter genes in the red seaweed Pyropia yezoensis. Am. J. Plant Sci. 2019, 10, 1325–1349. [Google Scholar] [CrossRef]
- Ohnishi, M.; Kikuchi, N.; Iwasaki, T.; Kawaguchi, R.; Shimada, S. Population genomic structures of endangered species (CR+EN), Pyropia tenera (Bangiales, Rhodophyta). Jpn. J. Phycol. (Sorui) 2013, 61, 87–96. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoa, H.V.; Kumari, P.; Uchida, H.; Murakami, A.; Shimada, S.; Mikami, K. Heat-Stress Responses Differ among Species from Different ‘Bangia’ Clades of Bangiales (Rhodophyta). Plants 2021, 10, 1733. https://doi.org/10.3390/plants10081733
Khoa HV, Kumari P, Uchida H, Murakami A, Shimada S, Mikami K. Heat-Stress Responses Differ among Species from Different ‘Bangia’ Clades of Bangiales (Rhodophyta). Plants. 2021; 10(8):1733. https://doi.org/10.3390/plants10081733
Chicago/Turabian StyleKhoa, Ho Viet, Puja Kumari, Hiroko Uchida, Akio Murakami, Satoshi Shimada, and Koji Mikami. 2021. "Heat-Stress Responses Differ among Species from Different ‘Bangia’ Clades of Bangiales (Rhodophyta)" Plants 10, no. 8: 1733. https://doi.org/10.3390/plants10081733







