Enhancement of Gel Strength of Itaconic Acid-Based Superabsorbent Polymer Composites Using Oxidized Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the CSAP Composites
2.3. Preparation of SSAP
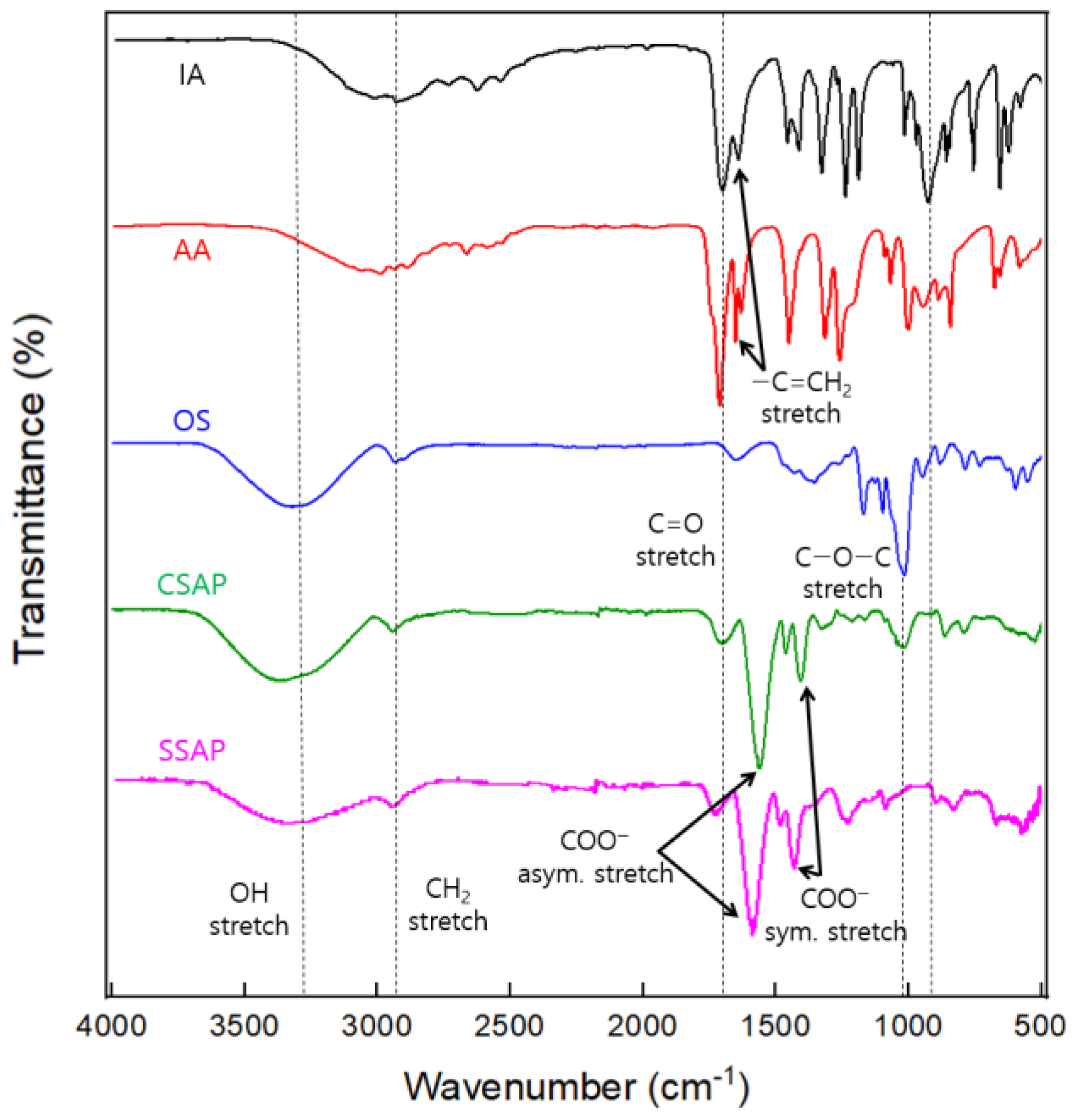
2.4. Fourier Transform Infrared (FT-IR) Characterization
2.5. Centrifuge Retention Capacity (CRC)
2.6. Absorbency under Load (AUL)
2.7. Rheological Analysis of the Gel Strength
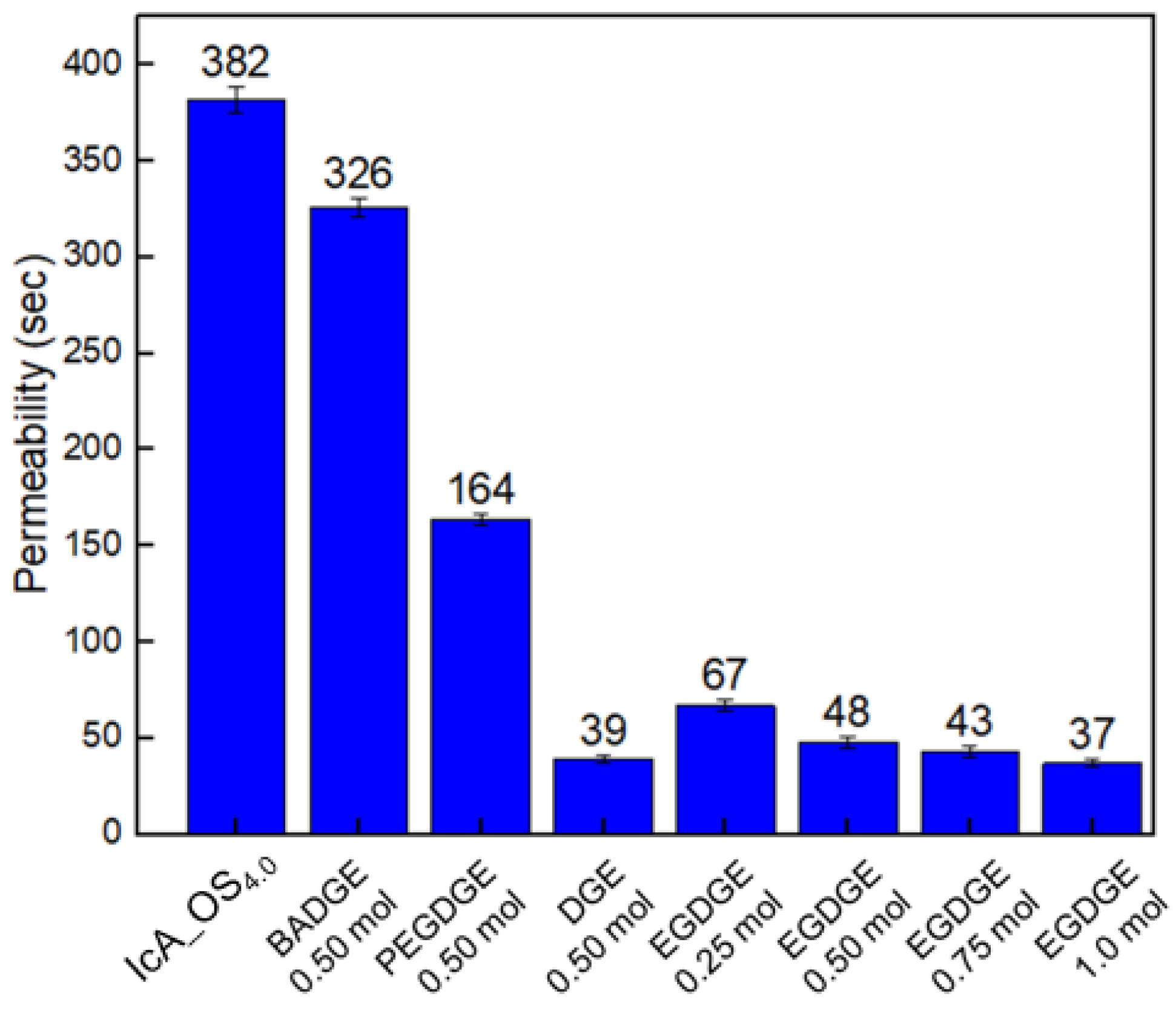
2.8. Permeability Measurement
2.9. Re-Swellability Test
3. Results
3.1. Preparation of the CSAP Composites
3.2. Preparation of the SSAP
3.3. Characterization of the CSAP Composite and the SSAP
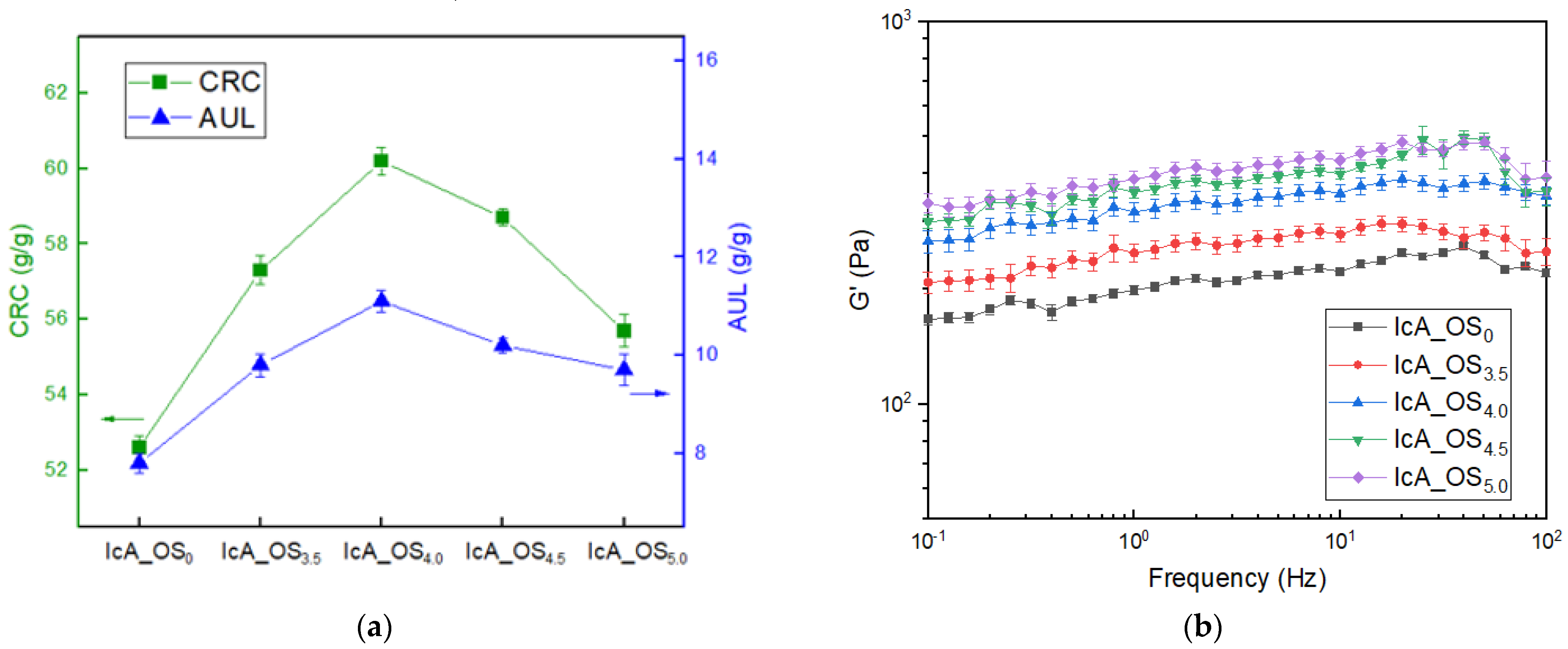
3.4. The Effects of OS Content upon the CRC, AUL, and Gel Strength
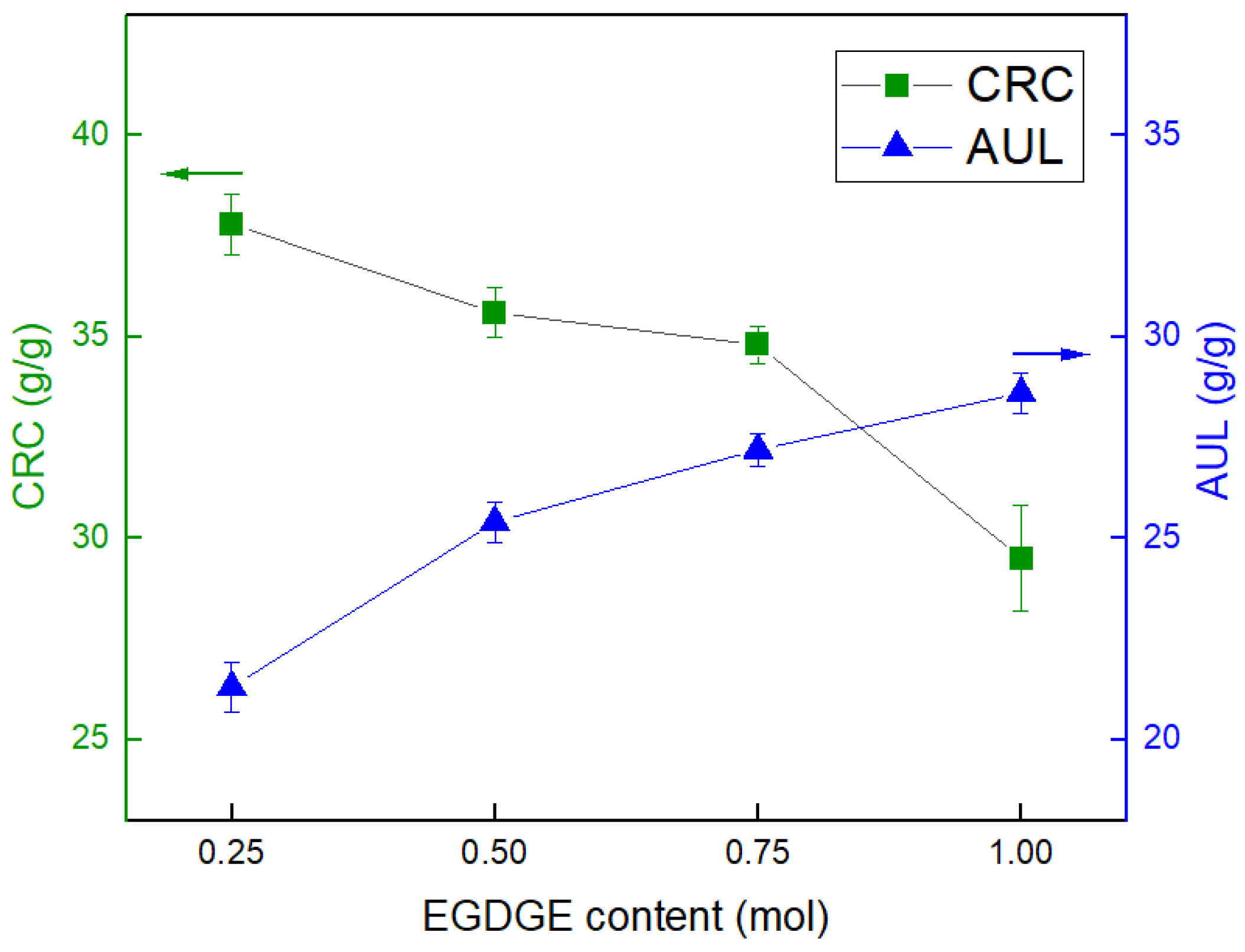
3.5. The Effect of the Type and Content of Surface-Crosslinker
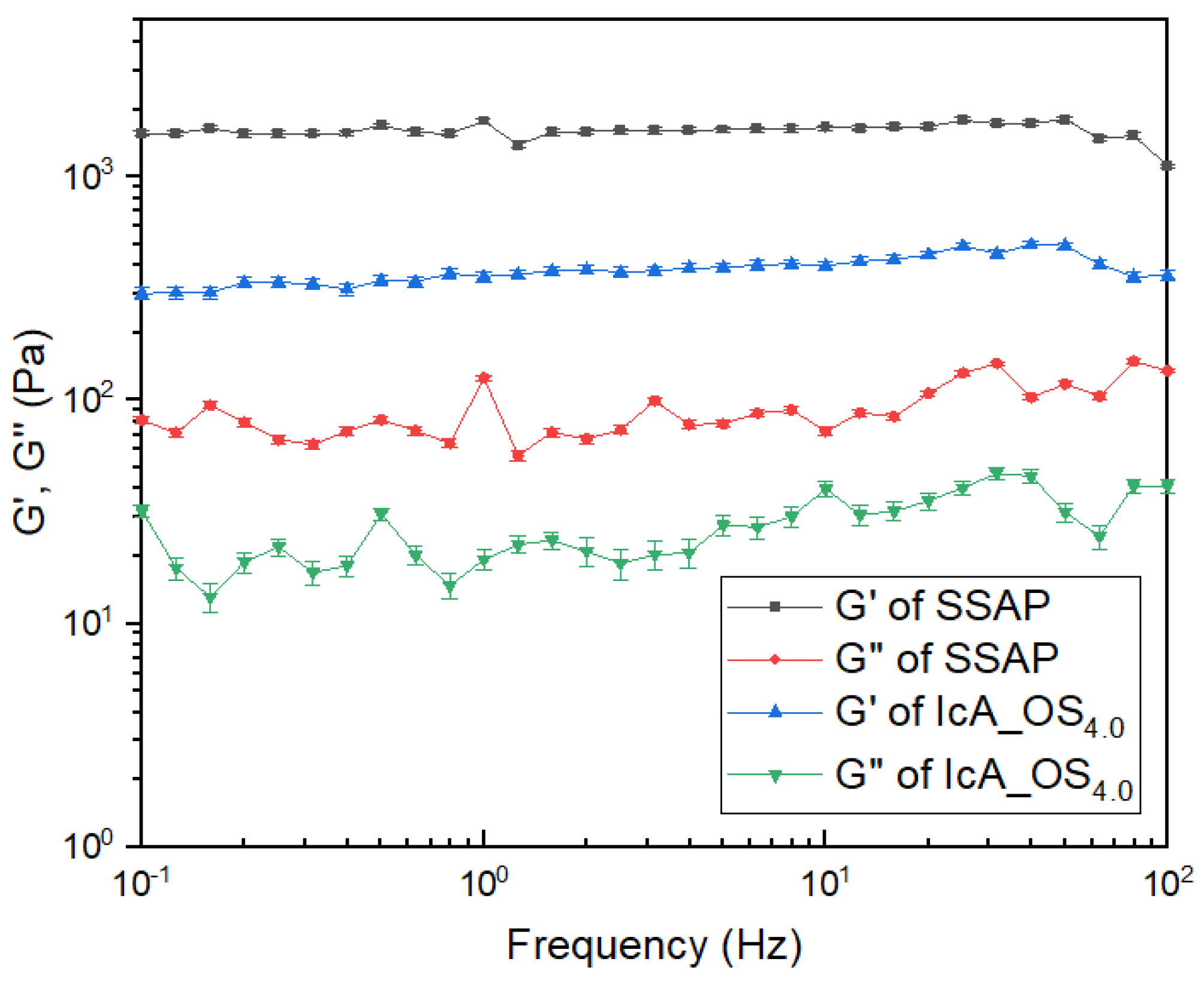
3.6. The Rheological Analysis of Gel Strength
3.7. Re-Swellability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zohuriaan-Mehr, M.A.; Kabiri, K. Superabsorbent Polymer Materials: A Review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Colombo, P. Swelling-controlled release in hydrogel matrices for oral route. Adv. Drug Deliv. Rev. 1993, 11, 37–57. [Google Scholar] [CrossRef]
- Raju, M.P.; Raju, K.M. Design and synthesis of superabsorbent polymers. J. Appl. Polym. Sci. 2001, 80, 2635–2639. [Google Scholar] [CrossRef]
- Kong, W.; Li, Q.; Li, X.; Su, Y.; Yue, Q.; Gao, B. A biodegradable biomass-based polymeric composite for slow release and water retention. J. Environ. Manag. 2019, 230, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Suo, A.; Qian, J.; Yao, Y.; Zhang, W. Synthesis and properties of carboxymethyl cellulose-graft-poly(acrylic acid-co-acrylamide) as a novel cellulose-based superabsorbent. J. Appl. Polym. Sci. 2007, 103, 1382–1388. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Fan, X.; Xu, J.; Zhang, Y.; Yuan, J.; Jin, H.; Cavaco-Paulo, A. Synthesis and characterization of starch-poly(methyl acrylate) graft copolymers using horseradish peroxidase. Carbohydr. Polym. 2016, 136, 1010–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Tan, H.M. Crosslinked carboxymethylchitosan-g-poly(acrylic acid) copolymer as a novel superabsorbent polymer. Carbohydr. Res. 2006, 341, 887–896. [Google Scholar] [CrossRef]
- Sand, A.; Shin, N.J.; Nam, H.G.; Kwark, Y.J. Effects of Reaction Parameters on Water Absorption of Poly(itaconic acid) Superabsorbent Particles Synthesized by Inverse Suspension Polymerization. Fibers Polym. 2021, 22, 898–903. [Google Scholar] [CrossRef]
- Cavus, S.; Gurdag, G. Noncompetitive removal of heavy metal ions from aqueous solutions by poly [2-(acrylamido)-2-methyl-1-propanesulfonic acid-co-itaconic acid] hydrogel. Ind. Eng. Chem. Res. 2009, 48, 2652–2658. [Google Scholar] [CrossRef]
- Sollka, L.; Lienkamp, K. Progress in the Free and Controlled Radical Homo-and Co-Polymerization of Itaconic Acid Derivatives: Toward Functional Polymers with Controlled Molar Mass Distribution and Architecture. Macromol. Rapid Commun. 2021, 42, 2000546. [Google Scholar] [CrossRef]
- Seetapan, N.; Srisithipantakul, N.; Kiatkamjornwong, S. Synthesis of acrylamide-co-(itaconic acid) superabsorbent polymers and associated silica superabsorbent polymer composites. Polym. Eng. Sci. 2001, 51, 764–775. [Google Scholar] [CrossRef]
- Sharika, T.; Mohanan, A. Synthesis and swelling studies of poly(acrylamide-co-itaconic acid)/hydroxyapatite nanocomposite hydrogels. Mater. Today-Proc. 2021, 41, 744–751. [Google Scholar] [CrossRef]
- Sadeghi, M. Synthesis of a biocopolymer carrageenan-g-poly (AAm-co-IA)/montmorilonite superabsorbent hydrogel composite. Braz. J. Chem. Eng. 2012, 29, 295–305. [Google Scholar] [CrossRef]
- Shakib, F.; Dadvand Koohi, A.; Kamran Pirzaman, A. Adsorption of methylene blue by using novel chitosan-g-itaconic acid/bentonite nanocomposite–equilibrium and kinetic study. Water Sci. Technol. 2017, 75, 1932–1943. [Google Scholar] [CrossRef]
- Parovuori, P.; Hamunen, A.; Forssell, P.; Autio, K.; Poutanen, K. Oxidation of Potato Starch by Hydrogen Peroxide. Starch-Stärke 1995, 47, 19–23. [Google Scholar] [CrossRef]
- Gan, D.; Lyon, L.A. Tunable swelling kinetics in core-shell hydrogel nanoparticles. J. Am. Chem. Soc. 2001, 123, 7511–7517. [Google Scholar] [CrossRef] [PubMed]
- Mudiyanselage, T.K.; Neckers, D.C. Highly absorbing superabsorbent polymer. J. Polym. Sci. Pol. Chem. 2008, 46, 1357–1364. [Google Scholar] [CrossRef]
- Moini, N.; Kabiri, K.; Zohuriaan-Mehr, M.J. Practical improvement of SAP hydrogel properties via facile tunable cross-linking of the particles surface. Polym.-Plast. Technol. Eng. 2016, 55, 278–290. [Google Scholar] [CrossRef]
- McKegney, M.; Taggart, I.; Grant, M.H. The influence of crosslinking agents and diamines on the pore size, morphology and the biological stability of collagen sponges and their effect on cell penetration through the sponge matrix. J. Mater. Sci. Mater. Med. 2001, 12, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Bednarz, S.; Błaszczyk, A.; Błażejewska, D.; Bogdał, D. Free-radical polymerization of itaconic acid in the presence of choline salts: Mechanism of persulfate decomposition. Catal. Today 2015, 257, 297–304. [Google Scholar] [CrossRef]
- Dabbaghi, A.; Kabiri, K.; Ramazani, A.; Zohuriaan-Mehr, M.J.; Jahandideh, A. Synthesis of bio-based internal and external cross-linkers based on tannic acid for preparation of antibacterial superabsorbents. Polym. Adv. Technol. 2019, 30, 2894–2905. [Google Scholar] [CrossRef]
- Morgan, J.L.R.; Crist, R.H. The photochemical decomposition of potassium persulfate. I. J. Am. Chem. Soc. 1927, 49, 16–25. [Google Scholar] [CrossRef]
- Costanza, J.; Otano, G.; Callaghan, J.; Pennell, K.D. PCE oxidation by sodium persulfate in the presence of solids. Environ. Sci. Technol. 2010, 44, 9445–9450. [Google Scholar] [CrossRef]
- Tako, M.; Tamaki, Y.; Teruya, T.; Takeda, Y. The principles of starch gelatinization and retrogradation. Food Nutr. Sci. 2014, 5, 280–291. [Google Scholar] [CrossRef] [Green Version]
- Lanthong, P.; Nuisin, R.; Kiatkamjornwong, S. Graft copolymerization, characterization, and degradation of cassava starch-g-acrylamide/itaconic acid superabsorbents. Carbohydr. Polym. 2006, 66, 229–245. [Google Scholar] [CrossRef]
- Matějka, L.; Pokomý, S.; Dušek, K. Network formation involving epoxide and carboxyl groups. Polym. Bull. 1982, 7, 123–128. [Google Scholar]
- Świderski, G.; Wilczewska, A.Z.; Świsłocka, R.; Kalinowska, M.; Lewandowski, W. Spectroscopic (IR, Raman, UV–Vis) study and thermal analysis of 3d-metal complexes with 4-imidazolecarboxylic acid. J. Therm. Anal. Calorim. 2018, 134, 513–525. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.M.; Guo, X.J.; Liang, R.H.; Liu, W.; Chen, J. Alkylated pectin: Molecular characterization, conformational change and gel property. Food Hydrocoll. 2017, 69, 341–349. [Google Scholar] [CrossRef]
- Aranguren, M.I.; Mora, E.; DeGroot, J.V., Jr.; Macosko, C.W. Effect of reinforcing fillers on the rheology of polymer melts. J. Rheol. 1992, 36, 1165–1182. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, D.H.; Lee, Y.S. The Influence of Monomer Composition and Surface-CrossLinking Condition on Biodegradation and Gel Strength of Super Absorbent Polymer. Polymers 2021, 13, 663. [Google Scholar] [CrossRef]
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Pulat, M.; Eksi, H. Determination of swelling behavior and morphological properties of poly(acrylamide-co-itaconic acid) and poly(acrylic acid-co-itaconic acid) copolymeric hydrogels. J. Appl. Polym. Sci. 2006, 102, 5994–5999. [Google Scholar] [CrossRef]




| Sample | IA (mol) | AA (mol) | OS (g) | Neutralization Degree (%) | HDODA (mmol%) | APS (wt.%) |
|---|---|---|---|---|---|---|
| IcA_OS0 | 0.5 | 0.5 | 0 | 75 | 2.0 | 0.5 |
| IcA_OS3.5 | 3.5 | |||||
| IcA_OS4.0 | 4.0 | |||||
| IcA_OS4.5 | 4.5 | |||||
| IcA_OS5.0 | 5.0 |
| Methanol (mL) | DW (mL) | Surface-Crosslinker | ||
|---|---|---|---|---|
| Designation | Chemical Structure | Content (mol%) | ||
| 6 | 3 | BADGE | 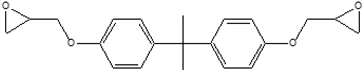 | 0.5 |
| PEGDGE | 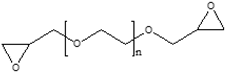 | 0.5 | ||
| EGDGE |  | 0.5 | ||
| DGE |  | 0.5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, J.; Kim, D. Enhancement of Gel Strength of Itaconic Acid-Based Superabsorbent Polymer Composites Using Oxidized Starch. Polymers 2021, 13, 2859. https://doi.org/10.3390/polym13172859
Kim H, Kim J, Kim D. Enhancement of Gel Strength of Itaconic Acid-Based Superabsorbent Polymer Composites Using Oxidized Starch. Polymers. 2021; 13(17):2859. https://doi.org/10.3390/polym13172859
Chicago/Turabian StyleKim, Haechan, Jungsoo Kim, and Donghyun Kim. 2021. "Enhancement of Gel Strength of Itaconic Acid-Based Superabsorbent Polymer Composites Using Oxidized Starch" Polymers 13, no. 17: 2859. https://doi.org/10.3390/polym13172859







