Effectiveness and Safety of Transthoracic Ultrasound in Guiding Percutaneous Needle Biopsy in the Lung and Comparison vs. CT Scan in Assessing Morphology of Subpleural Consolidations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Contrast-Enhanced Chest CT (CECT)
2.2. Transthoracic Ultrasound (TUS)
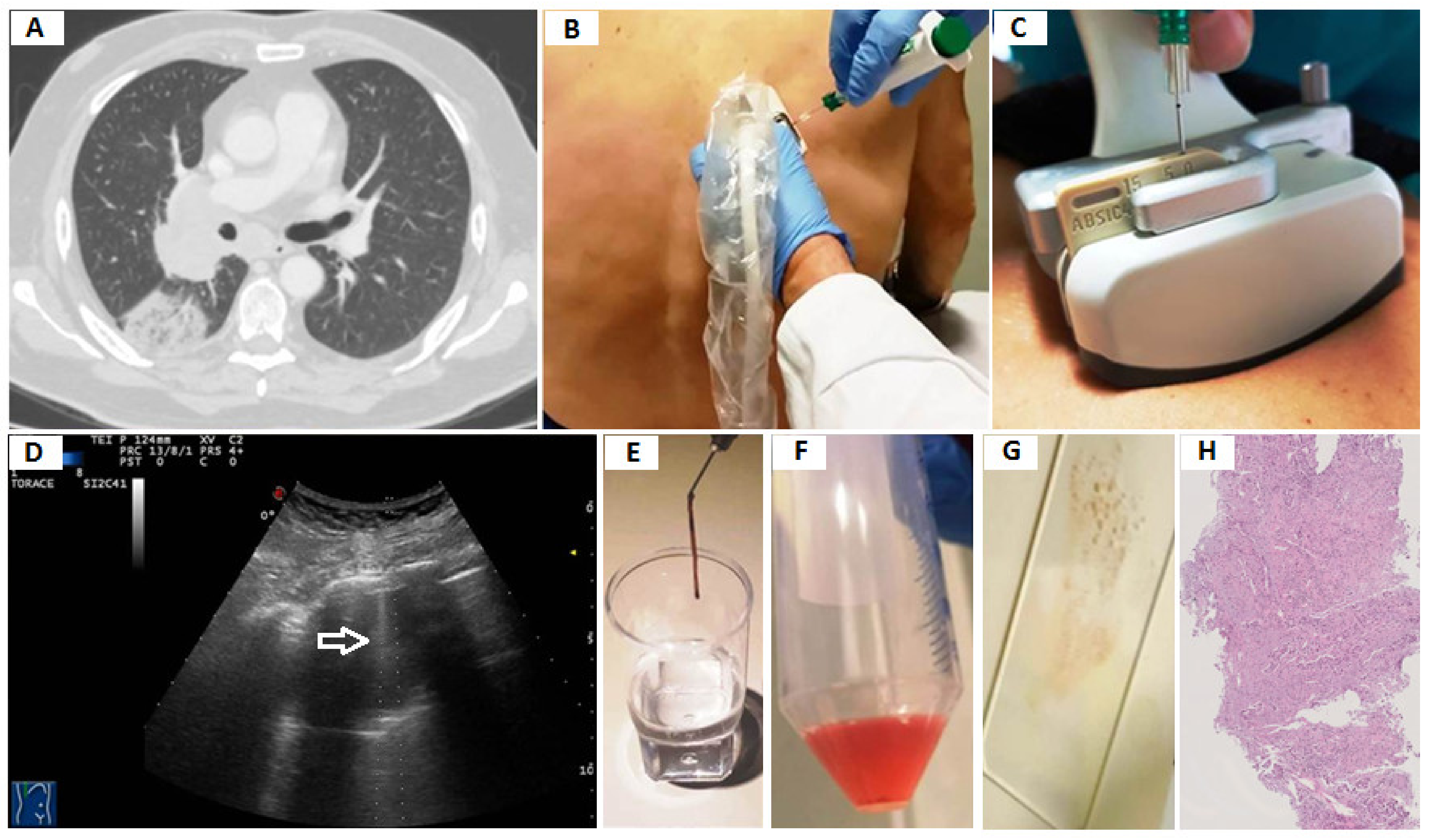
2.3. Transthoracic Ultrasound-Guided Percutaneous Needle Biopsy (TUS-PNB)
2.4. Final Diagnosis
2.5. Statistical Analysis
3. Results
3.1. Diagnostic Yield of TUS-Guided PNB in Diagnosing Peripheral Pulmonary Malignant Lesions
3.2. Diagnostic Accuracy of TUS Examination in Characterizing Peripheral Pulmonary Lesions vs. Chest CT Scan
4. Discussion
4.1. TUS-Guided PNB for the Diagnosis of Peripheral Pulmonary Malignant Lesions
4.2. TUS Examination in the Morphological Characterization of Peripheral Pulmonary Lesions vs. Chest CT Scan
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv1–iv21. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Bepler, G.; Bueno, R.; Chang, A.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; Demmy, T.L.; Feigenberg, S.J.; Grannis, F.W., Jr.; et al. National Comprehensive Cancer Network (NCCN). Non-small cell lung cancer clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2006, 4, 548–582. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef] [Green Version]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. ESMO Guidelines Committee. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv192–iv237. [Google Scholar] [CrossRef]
- Dingemans, A.C.; Früh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. ESMO Guidelines Committee. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Callister, M.E.; Baldwin, D.R.; Akram, A.R.; Barnard, S.; Cane, P.; Draffan, J.; Franks, K.; Gleeson, F.; Graham, R.; Malhotra, P.; et al. British Thoracic Society Pulmonary Nodule Guideline Development Group; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015, 70 (Suppl. 2), ii1–ii54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperandeo, M.; Carnevale, V.; Muscarella, S.; Sperandeo, G.; Varriale, A.; Filabozzi, P.; Piattelli, M.L.; D’Alessandro, V.; Copetti, M.; Pellegrini, F.; et al. Clinical application of transthoracic ultrasonography in inpatients with pneumonia. Eur. J. Clin. Investig. 2011, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, M.; Rea, G.; Carnevale, V.; Grimaldi, M.A.; Saponara, A.R.; Rosenthal, E.; Maggi, M.M.; Dimitri, L.; Sperandeo, M. Assessment of thoracic ultrasound in complementary diagnosis and in follow up of community-acquired pneumonia (CAP). BMC Med. Imaging 2017, 17, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolliger, C.T.; Mathur, P.N. ERS/ATS statement on interventional pulmonology. Eur. Respir. J. 2002, 19, 356–373. [Google Scholar] [CrossRef] [Green Version]
- Lung Cancer: Diagnosis and Management—NICE Guideline. Available online: www.nice.org.uk/guidance/ngAccessed (accessed on 11 October 2019).
- Sperandeo, M.; Rotondo, A.; Guglielmi, G.; Catalano, D.; Feragalli, B.; Trovato, G.M. Transthoracic ultrasound in the assessment of pleural and pulmonary diseases: Use and limitations. Radiol. Med. 2014, 119, 729–740. [Google Scholar] [CrossRef]
- Sperandeo, M.; Filabozzi, P.; Varriale, A.; Carnevale, V.; Piattelli, M.L.; Sperandeo, G.; Brunetti, E.; Decuzzi, M. Role of thoracic ultrasound in the assessment of pleural and pulmonary diseases. J. Ultrasound 2008, 11, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [Green Version]
- Sheth, S.; Hamper, U.M.; Stanley, D.B.; Wheeler, J.H.; Smith, P.A. US Guidance for Thoracic Biopsy: A Valuable Alternative to CT. Radiology 1999, 210, 721–726. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Mauri, G.; Grossi, F.; Truini, M.; Serafini, G.; Sardanelli, F.; Murolo, C. Pleural and peripheral lung lesions: Comparison of US- and CT-guided biopsy. Radiology 2013, 266, 930–935. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.C.; Luh, K.T.; Sheu, J.C.; Kuo, S.H.; Yang, S.P. Peripheral pulmonary lesions: Ultrasonography and ultrasonically guided aspiration biopsy. Radiology 1985, 155, 451–456. [Google Scholar] [CrossRef]
- Liao, W.Y.; Chen, M.Z.; Chang, Y.L.; Wu, H.D.; Yu, C.J.; Kuo, P.H.; Yang, P.C. US-guided transthoracic cutting biopsy for peripheral thoracic lesions less than 3 cm in diameter. Radiology 2000, 217, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Sagar, P.; Gulati, M.; Gupta, S.K.; Gupta, S.; Shankar, S.; Joshi, K.; Jindal, S.K.; Suri, S. Ultrasound-guided transthoracic co-axial biopsy of thoracic mass lesions. Acta Radiol. 2000, 41, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Jarmakani, M.; Duguay, S.; Rust, K.; Conner, K.; Wagner, J.M. Ultrasound Versus Computed Tomographic Guidance for Percutaneous Biopsy of Chest Lesions. J. Ultrasound Med. 2016, 35, 1865–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diacon, A.H.; Schuurmans, M.M.; Theron, J.; Schubert, P.T.; Wright, C.A.; Bolliger, C.T. Safety and Yield of Ultrasound-Assisted Transthoracic Biopsy Performed by Pulmonologists. Respiration 2004, 71, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Watanabe, T.; Yamada, K.; Nakai, T.; Suzumura, T.; Sakagami, K.; Yoshimoto, N.; Sato, K.; Tanaka, H.; Mitsuoka, S.; et al. Efficacy and safety of ultrasound (US) guided percutaneous needle biopsy for peripheral lung or pleural lesion: Comparison with computed tomography (CT) guided needle biopsy. J. Thorac. Dis. 2019, 11, 936–943. [Google Scholar] [CrossRef]
- Jeon, K.N.; Bae, K.; Park, M.J.; Choi, H.C.; Shin, H.S.; Shin, S.; Kim, H.C.; Ha, C.Y. US-guided transthoracic biopsy of peripheral lung lesions: Pleural contact length influences diagnostic yield. Acta Radiol. 2014, 55, 295–301. [Google Scholar] [CrossRef]
- Liang, J.; Wang, D.; Li, H.; Zhao, S.; Chen, M.; Li, H.; Ding, Z.; Liu, J.; Liu, L. Contrast-enhanced ultrasound for needle biopsy of thoracic lesions. Oncol. Lett. 2020, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, W.; Zhang, H.; Xu, Q.; Yan, K. The role of contrast-enhanced ultrasound in selection indication and improveing diagnosis for transthoracic biopsy in peripheral pulmonary and mediastinal lesions. Biomed. Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Han, J.; Feng, X.L.; Xu, T.Y.; Feng, W.Q.; Liu, M.J.; Wang, B.; Qiu, T.L.; Wang, Y. Clinical value of contrast-enhanced ultrasound in transthoracic biopsy of malignant anterior mediastinal masses. J. Thorac. Dis. 2019, 11, 5290–5299. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Boiselle, P.M.; Shepard, J.O.; Trotman-Dickenson, B.; McLoud, T.C. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: Comparison of small and large pulmonary nodules. AJR Am. J. Roentgenol. 1996, 167, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeow, K.M.; Tsay, P.K.; Cheung, Y.C.; Lui, K.W.; Pan, K.T.; Shau-Bin Chou, A. Factors Affecting Diagnostic Accuracy of CTguided Coaxial Cutting Needle Lung Biopsy: Retrospective Analysis of 631 Procedures. J. Vasc. Interv. Radiol. 2003, 14, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, M.; Del Colle, A.; Frongillo, E.; Rea, G.; Dimitri, L.; Cipriani, C.; Lacedonia, D. Safety maximization of percutaneous transthoracic needle biopsy with ultrasound guide in subpleural lesions in the evaluation of pulmonary consolidation. Respir. Res. 2019, 20, 68. [Google Scholar] [CrossRef]
- Trovato, G.M.; Sperandeo, M.; Catalano, D. Optimization of Thoracic US Guidance for Lung Nodule Biopsy. Radiology 2014, 270, 308. [Google Scholar] [CrossRef]
- Reissig, A.; Kroegel, C. Transthoracic Ultrasound of Lung and Pleura in the Diagnosis of Pulmonary Embolism: A Novel Non-Invasive Bedside Approach. Respiration 2003, 70, 441–452. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mezière, G.; Seitz, J. The Dynamic Air Bronchogram: A Lung Ultrasound Sign of Alveolar Consolidation Ruling Out Atelectasis. Chest 2009, 135, 1421–1425. [Google Scholar] [CrossRef]
- Görg, C.; Seifart, U.; Görg, K.; Zugmaier, G. Color Doppler Sonographic Mapping of Pulmonary Lesions. J. Ultrasound Med. 2003, 22, 1033–1039. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehmacher, O.; Mathis, G.; Kopf, A.; Scheier, M. Ultrasound imaging of pneumonia. Ultrasound Med. Biol. 1995, 21, 1119–1122. [Google Scholar] [CrossRef]
- Yang, P.C.; Luh, K.T.; Lee, Y.C.; Chang, D.B.; Yu, C.J.; Wu, H.D.; Lee, L.N.; Kuo, S.H. Lung abscesses: US examination and US-guided transthoracic aspiration. Radiology 1991, 180, 171–175. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, M.A.; D’Agostino, H. La ultrasonografía en neumología: Situación actual [Ultrasound in pneumology: The current situation]. Arch. Bronconeumol. 2003, 39, 535–536. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiang, J.W.; Shen, Y.; Ye, J.D.; Zhang, J.; Zhu, L. Using air bronchograms on multi-detector CT to predict the invasiveness of small lung adenocarcinoma. Eur. J. Radiol. 2016, 85, 571–577. [Google Scholar] [CrossRef]
- Hattori, A.; Suzuki, K.; Maeyashiki, T.; Fukui, M.; Kitamura, Y.; Matsunaga, T.; Miyasaka, Y.; Takamochi, K.; Oh, S. The presence of air bronchogram is a novel predictor of negative nodal involvement in radiologically pure-solid lung cancer. Eur. J. Cardiothorac. Surg. 2014, 45, 699–702. [Google Scholar] [CrossRef] [Green Version]
- Sperandeo, M.; Filabozzi, P.; Carnevale, V. Ultrasound diagnosis of ventilator-associated pneumonia: A not-so-easy issue. Chest 2016, 149, 1350–1351. [Google Scholar] [CrossRef] [Green Version]
- Lacedonia, D.; Quarato, C.M.I.; Borelli, C.; Dimitri, L.; Graziano, P.; Foschino Barbaro, M.P.; Scioscia, G.; Mirijello, A.; Maggi, M.M.; Rea, G.; et al. Transthoracic Ultrasound in Infectious Organizing Pneumonia: A Useful Guide for Percutaneous Needle Biopsy. Front. Med. 2021, 8, 1074. [Google Scholar] [CrossRef]
- Campbell, S.C. Doppler ultrasound artifacts. In Ultrasound Secrets, 1st ed.; Dogra, V., Rubens, D., Eds.; Hanley & Belfus: Philadelphia, PA, USA, 2004; pp. 14–22. [Google Scholar]






| Characteristics | N = 762 | % |
|---|---|---|
| Age | 47.80 ± 14.42 | |
| Sex | ||
| Male | 622 | 81.63 |
| Female | 140 | 18.37 |
| Smokers | 518 | 67.98 |
| Patients with multiple lesions on CT scan | 590 | 77.43 |
| Lesion size on CT scan | 3.28 ± 1.01 | |
| <2 cm | 104 | 13.65 |
| 2 cm–5 cm | 570 | 74.80 |
| >5 cm | 88 | 11.55 |
| Lesion location | ||
| Right upper lobe | 80 | 10.50 |
| Right middle lobe | 148 | 19.42 |
| Right lower lobe | 213 | 27.95 |
| Left upper lobe | 103 | 13.52 |
| Left lower lobe | 218 | 28.61 |
| Repeated biopsy in the single session | 24 | 3.15 |
| Needle passes per biopsy | 2.75 ± 0.56 | |
| Diagnostic biopsy | 709 | 93.04 |
| Inconclusive biopsy | 53 | 6.96 |
| <2 cm | 11 | |
| 2 cm–5 cm | 33 | |
| >5 cm | 9 |
| Final Diagnosis | N = 762 | % |
|---|---|---|
| Malignant | ||
| Metastasis | 18 | 2.36 |
| Small cell lung carcinoma | 49 | 6.43 |
| Squamous carcinoma | 89 | 11.68 |
| Adenocarcinoma | 305 | 40.03 |
| Undifferentiated carcinoma | 166 | 21.78 |
| Total | 627 | 82.28 |
| Benign | ||
| Organizing pneumonia | 53 | 6.95 |
| Lung abscess | 29 | 3.81 |
| Total | 82 | 10.76 |
| Inconclusive | ||
| Necrotic tissue | 8 | 1.05 |
| Normal lung | 10 | 1.31 |
| Fibrous tissue | 14 | 1.84 |
| Chronic inflammation | 21 | 3.15 |
| Total | 53 | 1.97 |
| Variable | Malignant (n = 627) | Benign (n = 281) | p-Value |
|---|---|---|---|
| Age, years, (mean ± SD) | 47.96 ± 14.63 | 50.28 ± 14.36 | 0.03 |
| Gender: | |||
| Male n (%) | 511 (81.50) | 212 (75.44) | 0.04 |
| Female n (%) | 116 (18.50) | 69 (24.56) | |
| Smokers, n (%) | 438 (69.86) | 187 (66.55) | 0.49 |
| Patients with multiple lesions at CT, n (%) | 496 (79.11) | 198 (70.46) | 0.005 |
| CT findings: | |||
| Diameter, cm (mean ± SD) | 3.21 ± 0.97 | 3.20 ± 0.86 | 0.88 |
| Irregular margins, n (%) | 576 (91.87) | 85 (30.25) | <0.0001 |
| Smooth margins, n (%) | 51 (8.13) | 196 (69.75) | |
| Air bronchogram, n (%) | 218 (34.77) | 82 (29.18) | 0.11 |
| Necrosis, n (%) | 158 (25.20) | 104 (37.01) | 0.0004 |
| Pleural effusion, n (%) | 286 (45.61) | 155 (55.16) | 0.19 |
| US findings: | |||
| Diameter, cm (mean ± SD) | 3.17 ± 0.99 | 3.15 ± 0.89 | 0.77 |
| Irregular margins, n (%) | 429 (84.10) | 205 (72.95) | 0.18 |
| Smooth margins, n (%) | 198 (31.58) | 76 (27.05) | |
| Hypoechoic pattern, n (%) | 378 (60.29) | 167 (59.43) | 0.10 |
| Anechoic pattern, n (%) | 93 (14.83) | 29 (10.32) | 0.07 |
| Mixed (hyper/hypoechoic) pattern, n (%) | 156 (24.88) | 85 (30.25) | 0.72 |
| Sonographic “air bronchogram”, n (%) | 164 (26.16) | 89 (31.67) | 0.09 |
| Sonographic “necrosis”, n (%) | 132 (21.05) | 57 (20.28) | 0.86 |
| Pleural effusion, n (%) | 417 (66.51) | 202 (71.89) | <0.0001 |
| Malignant | Metastasis | Small Cell Lung Carcinoma | Squamous Carcinoma | Adeno-Carcinoma | Undifferentiated Carcinoma |
|---|---|---|---|---|---|
| CT findings | n = 18 | n = 49 | n = 89 | n = 305 | n = 166 |
| Diameter, cm (mean ± SD) | 3.33 ± 0.90 | 3.21 ± 0.89 | 3.25 ± 0.99 | 3.24 ± 1.01 | 3.12 ± 0.91 |
| Irregular margins, n (%) | 6 (33.33) | 45 (91.84) | 86 (96.63) | 281 (92.13) | 158 (95.18) |
| Air bronchogram, n (%) | 5 (27.78) | 8 (16.33) | 12 (13.48) | 156 (51.15) | 37 (22.29) |
| Necrosis, n (%) | 4 (22.22) | 27 (55.10) | 14 (15.73) | 50 (16.39) | 56 (33.73) |
| Pleural effusion, n (%) | 4 (22.22) | 11 (22.45) | 26 (29.21) | 146 (47.87) | 83 (50.00) |
| US findings | |||||
| Diameter, cm (mean ± SD) | 3.26 ± 0.93 | 3.15 ± 0.92 | 3.20 ± 1.01 | 3.22 ± 1.03 | 3.07 ± 0.94 |
| Irregular margins, n (%) | 8 (44.44) | 43 (87.76) | 40 (44.94) | 243 (79.67) | 95 (57.23) |
| Hypoechoic pattern, n (%) | 9 (50.00) | 34 (69.39) | 51 (57.30) | 181 (59.34) | 103 (62.05) |
| Anechoic pattern, n (%) | 3 (16.67) | 11 (22.45) | 12 (13.48) | 12 (3.93) | 55 (33.13) |
| Mixed pattern, n (%) | 6 (33.33) | 4 (8.16) | 26 (29.21) | 112 (36.72) | 8 (4.82) |
| Sonographic “air bronchogram”, n (%) | 7 (38.89) | 9 (18.37) | 14 (15.73) | 98 (32.13) | 36 (21.69) |
| Sonographic “necrosis”, n (%) | 2 (11.11) | 32 (65.31) | 21 (23.60) | 40 (13.11) | 37 (22.29) |
| Pleural effusion, n (%) | 6 (33.33) | 17 (34.69) | 39 (43.82) | 217 (71.15) | 109 (65.66) |
| Benign | Pneumonia | Organizing Pneumonia | Lung Abscess | Obstructive Atelectasis | Compressive Atelectasis |
|---|---|---|---|---|---|
| CT findings | n = 164 | n = 53 | n = 29 | n = 21 | n = 14 |
| Diameter, cm (mean ± SD) | 3.01 ± 0.75 | 3.36 ± 0.78 | 4.07 ± 1.05 | 3.23 ± 1.02 | 2.98 ± 0.73 |
| Irregular margins, n (%) | 32 (19.51) | 47 (88.68) | 6 (20.69) | 0 (0.00) | 0 (0.00) |
| Air bronchogram, n (%) | 67 (40.85) | 6 (11.32) | 3 (10.34) | 4 (19.05) | 2 (14.29) |
| Necrosis, n (%) | 52 (31.71) | 17 (32.08) | 29 (100.00) | 4 (19.05) | 2 (14.29) |
| Pleural effusion, n (%) | 92 (56.10) | 25 (47.17) | 16 (55.17) | 8 (38.10) | 14 (100.00) |
| US findings | |||||
| Diameter, cm (mean ± SD) | 2.99 ± 0.78 | 3.26 ± 0.80 | 4.03 ± 1.10 | 3.15 ± 1.10 | 2.91 ± 0.73 |
| Irregular margins, n (%) | 128 (78.05) | 42 (79.25) | 14 (48.28) | 21 (100.00) | 0 (0.00) |
| Hypoechoic pattern, n (%) | 126 (76.83) | 21 (39.62) | 0 (0.00) | 12 (57.14) | 8 (57.14) |
| Anechoic pattern, n (%) | 0 (0.00) | 0 (0.00) | 29 (100.00) | 0 (0.00) | 0 (0.00) |
| Mixed pattern, n (%) | 38 (23.17) | 32 (60.38) | 0 (0.00) | 9 (42.86) | 6 (42.86) |
| Sonographic “air bronchogram”, n (%) | 36 (21.95) | 38 (71.70) | 12 (41.38) | 1 (4.76) | 2 (14.29) |
| Sonographic “necrosis”, n (%) | 34 (20.73) | 14 (26.42) | 9 (31.03) | 0 (0.00) | 0 (0.00) |
| Pleural effusion, n (%) | 120 (73.17) | 37 (69.81) | 20 (68.97) | 11 (52.38) | 14 (100.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperandeo, M.; Maiello, E.; Graziano, P.; Simeone, A.; De Cosmo, S.; Dimitri, L.; Di Micco, C.; Perrone, E.; Taurchini, M.; Ferretti, G.; et al. Effectiveness and Safety of Transthoracic Ultrasound in Guiding Percutaneous Needle Biopsy in the Lung and Comparison vs. CT Scan in Assessing Morphology of Subpleural Consolidations. Diagnostics 2021, 11, 1641. https://doi.org/10.3390/diagnostics11091641
Sperandeo M, Maiello E, Graziano P, Simeone A, De Cosmo S, Dimitri L, Di Micco C, Perrone E, Taurchini M, Ferretti G, et al. Effectiveness and Safety of Transthoracic Ultrasound in Guiding Percutaneous Needle Biopsy in the Lung and Comparison vs. CT Scan in Assessing Morphology of Subpleural Consolidations. Diagnostics. 2021; 11(9):1641. https://doi.org/10.3390/diagnostics11091641
Chicago/Turabian StyleSperandeo, Marco, Evaristo Maiello, Paolo Graziano, Annalisa Simeone, Salvatore De Cosmo, Lucia Dimitri, Concetta Di Micco, Elio Perrone, Marco Taurchini, Gianmaria Ferretti, and et al. 2021. "Effectiveness and Safety of Transthoracic Ultrasound in Guiding Percutaneous Needle Biopsy in the Lung and Comparison vs. CT Scan in Assessing Morphology of Subpleural Consolidations" Diagnostics 11, no. 9: 1641. https://doi.org/10.3390/diagnostics11091641
APA StyleSperandeo, M., Maiello, E., Graziano, P., Simeone, A., De Cosmo, S., Dimitri, L., Di Micco, C., Perrone, E., Taurchini, M., Ferretti, G., Mirijello, A., Varriale, A., Grimaldi, M. A., Lacedonia, D., & Quarato, C. M. I. (2021). Effectiveness and Safety of Transthoracic Ultrasound in Guiding Percutaneous Needle Biopsy in the Lung and Comparison vs. CT Scan in Assessing Morphology of Subpleural Consolidations. Diagnostics, 11(9), 1641. https://doi.org/10.3390/diagnostics11091641









