‘Pinore’: The New Red Wine Variety Cross-Bred between ‘Pinot Noir’ and ‘Regent’ Vines
Abstract
:1. Introduction
2. Results and Discussion
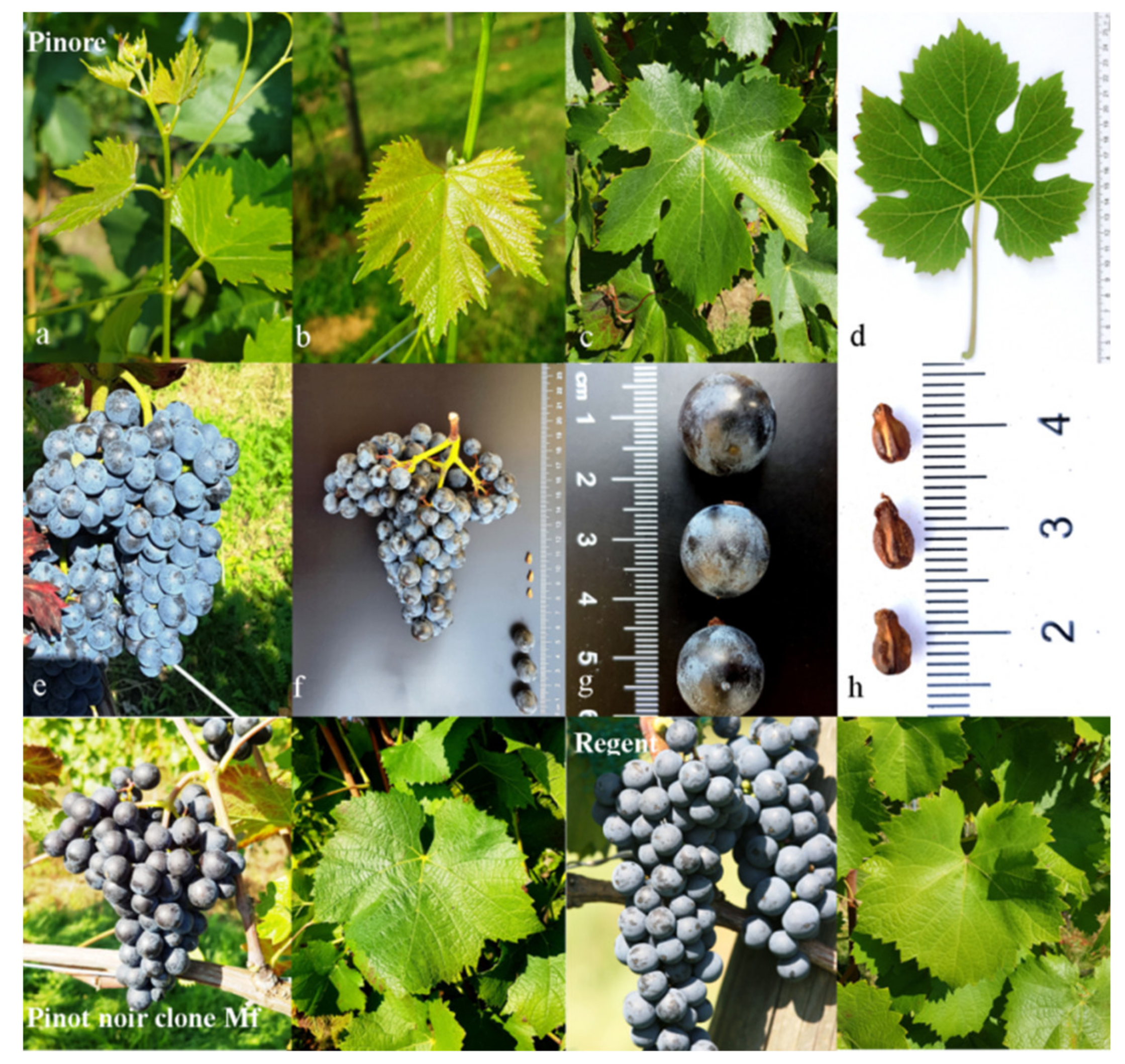
2.1. Genetic and Ampelographic Characteristics
2.2. Phenological, Agronomic, and Qualitative Performances
2.3. Wine Characteristics
3. Materials and Methods
3.1. Origin of the Genotype and Study Site
3.2. Experiment Set Up
3.3. Sampling and Measurements
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broome, J.; Warner, K. Agro-environmental Partnerships Facilitate Sustainable Wine-Grape Production and Assessment. Calif. Agric. 2008, 62, 133. [Google Scholar] [CrossRef] [Green Version]
- Lisek, J. Yielding and Healthiness of Selected Grape Cultivars for Processing in Central Poland. J. Fruit. Ornam. Plant Res. 2010, 18, 265–272. [Google Scholar]
- Gessler, C.; Pertot, I.; Perazzolli, M. Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar]
- Röckel, F.; Trapp, O.; Zyprian, E.; Hausmann, L.; Migliaro, D.; Vezzulli, S.; Töpfer, R.; Maul, E. A ’Regent’ pedigree update: Ancestors, offspring and their confirmed resistance loci. Vitis 2021, 60, 189–192. [Google Scholar]
- Eisenmann, B.; Czemmel, S.; Ziegler, T.; Buchholz, G.; Kortekamp, A.; Trapp, O.; Rausch, T.; Dry, I.; Bogs, I. Rpv3–1 mediated resistance to grapevine downy mildew is associated with specific host transcriptional responses and the accumulation of stilbenes. BMC Plant Biol. 2019, 19, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espino, R.R.C.; Nesbitt, W.B. Inheritance of Downy Mildew Resistence in Grape (Vitis sp.). HortScience 1982, 17, 499. [Google Scholar]
- Breider, H. Untersuchungen ¸über den Einfluss des Traubensaftes von Hybridenreben auf den Tierorganismus. Weinb. Keller Band 1964, 11, 513–517. [Google Scholar]
- Becker, N.J.; Zimmermann, H. Breeding of Yield Varieties Resistant to Downy Mildew. Gentltique et Amelioration de la Vigne. In Proceedings of the IIe Symposiu International sur l’Amelioration de la Vigne Bordeau, Bordeaux, France, 14–18 June; L’Institut National de la Recherche Agronomique: Paris, France, 1977; pp. 209–214. [Google Scholar]
- Mayer, G. Results of cross-breeding. In Proceedings of the 5th International Symposium on Grape Breeding, St. Martin, Pfalz, Germany, 12–16 September 1989; p. 148. [Google Scholar]
- Kozma, P. Qualité du raisin et resistance de la vigne dans les populations hybrids interspecifiques. In Proceedings of the 4th Symposium International Genetic Vitis, April 13-18, Verona, Italy, 13–18 April 1985; 1985; pp. 242–246. [Google Scholar]
- Töpfer, R.; Hausmann, L.; Harst, M.; Maul, E.; Zyprian, E.; Eibach, R.; Flachowsky, H. New Horizons for Grapevine Breeding; Flachowsky, H., Hanke, M.V., Eds.; Fruit, vegetable and cereal science and biotechnology, vol 5. Methods in temperate fruit breeding; Global Science Books: Isleworth, UK, 2011; pp. 79–100. [Google Scholar]
- Skalicky, B. Selection of American vine rootstocks. Kmetovalec 1907, 24, 15–19. [Google Scholar]
- Koruza, B.; Vaupotič, T.; Škvarč, A.; Korošec-Koruza, Z.; Rusjan, D. Catalog of Slovenian Grapevine Clones; Agricultural and Forestry Institute Nova Gorica, Grafika Soča d.o.o.: Nova Gorica, Slovenia, 2012; 96p. [Google Scholar]
- Korošec-Koruza, Z. Vine Selection—Why? 60 Years of Continuous Vine Selection in Slovenia; Agricultural institute of Slovenia: Šentjernej, Slovenia, 2018. [Google Scholar]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in wine grape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Wingerter, C.; Eisenmann, B.; Weber, P.; Dry, I.; Bogs, I. Grapevine Rpv3-, Rpv10- and Rpv12-mediated defense responses against Plasmopara viticola and the impact of their deployment on fungicide use in viticulture. BMC Plant Biol. 2021, 21, 470. [Google Scholar] [CrossRef] [PubMed]
- Peressotti, E.; Wiedemann-Merdinoglu, S.; Delmotte, F.; Bellin, D.; Di Gaspero, G.; Testolin, R.; Merdinoglu, D.; Mestre, P. Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety. BMC Plant Biol. 2010, 10, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargolzaei, M.; Maddalena, G.; Bitsadze, N.; Maghradze, D.; Bianco, P.A.; Failla, O.; Toffolatti, S.L.; De Lorenzis, G. Rpv29, Rpv30 and Rpv31: Three Novel Genomic Loci Associated With Resistance to Plasmopara viticola in Vitis vinifera. Front. Plant Sci. 2020, 11, 1537. [Google Scholar] [CrossRef] [PubMed]
- Eibach, R.; Töpfer, R. Traditional grapevine breeding techniques. Grapevine breeding programs for the wine industry. In Grapevine Breeding Programs for the Wine Industry; Reynolds, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–22. [Google Scholar] [CrossRef]
- Baroň, M.; Kumšta, M. Comparison Of North Italian And South Moravian Wines On The Base Of Their Antioxidant Activity, Phenolic Composition And Sensory Quality. Acta Univ. Agric. Silvic. Mendel. Brun. 2012, 60, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Ju, Y.; Yang, L.; Yue, X.; Li, Y.; He, R.; Deng, S.; Yang, X.; Fanga, Y. Anthocyanin profiles and color properties of red wines made from Vitis davidii and Vitis vinifera grapes. Food Sci. Hum. Wellness 2021, 10, 335–344. [Google Scholar] [CrossRef]
- Sochorova, L.; Prusova, B.; Jurikova, T.; Mlcek, J.; Adamkova, A.; Baron, M.; Sochor, J. The Study of Antioxidant Com-ponents in Grape Seeds. Molecules 2020, 25, 3736. [Google Scholar] [CrossRef] [PubMed]
- Zdunić, G.; Lukšić, K.; Nagy, A.M.; Mucalo, A.; Hančević, K.; Radić, T.; Butorac, L.; Jahnke, G.G.; Kiss, E.; Ledesma-Krist, G.; et al. Genetic Structure and Relationships among Wild and Cultivated Grapevines from Central Europe and Part of the Western Balkan Peninsula. Genes 2020, 11, 962. [Google Scholar] [CrossRef] [PubMed]


| Variety | VVMD27:1 | VVMD27:2 | VVS2:1 | VVS2:2 | VVMD7:1 | VVMD7:2 | VVMD5:1 | VVMD5:2 | VrZAG62:1 | VrZAG62:2 | VrZAG79:1 | VrZAG79:2 | VVMD28:1 | VVMD28:2 | VVMD32:1 | VVMD32:2 | VVMD25:1 | VVMD25:2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| * ‘Pinot Noir’ | 186 | 190 | 137 | 151 | 239 | 243 | 230 | 240 | 188 | 194 | 239 | 245 | 218 | 236 | 240 | 272 | 239 | 249 |
| ‘Pinore’ | 190 | 190 | 137 | 153 | 239 | 251 | 240 | 240 | 194 | 194 | 245 | 251 | 218 | 258 | 240 | 272 | 239 | 241 |
| * ‘Regent’ | 186 | 190 | 133 | 153 | 247 | 251 | 228 | 240 | 194 | 204 | 251 | 259 | 234 | 258 | 240 | 272 | 241 | 241 |
| Ampelographic Characteristics | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Young Shoot | Mature Leaf | Bunch | Berry | ||||||||||
| (OIV code) | 001 | 004 | 051 | 053 | 067 | 068 | 070 | 076 | 079 | 084 | 204 | 223 | 225 | 231 |
| Pinot Noir’ | 5 | 5 | 1/3 | 5 | 3 | 2 | 1 | 2 | 3/5 | 3 | 3 | 2 | 6 | 1 |
| ‘Pinore’ | 5 | 5 | 2/3 | 5 | 4 | 3 | 1 | 2 | 5 | 3 | 3 | 2 | 6 | 3 |
| ‘Regent’ | 5 | 5 | 1/3 | 5 | 2 | 3 | 2 | 2 | 3/5 | 3 | 5 | 1/2 | 6 | 1 |
| 2014–2016 | Date of Vintage | Shoots Number | Bunches per Vine | Yield kg/Vine | Bunch (g) | Nr. of Berries per Bunch | Berry (g) | Sugar °Oe | Titr. Acidity (g/L) | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| Pinore-NS * | 22.9 ± 2.65 | 6.0 ± 0.00 a b | 11.7 ± 2.52 b | 1.36 ± 0.22 b | 117.3 ± 8.79 b | 91.7 ± 9.65 a | 1.2 ± 0.03 b | 89.7 ± 7.57 a | 8.73 ± 0.23 a | 3.09 ± 0.07 a |
| ‘Pinore’ | 22.9 ± 2.65 | 7.0 ± 0.82 a | 15.5 ± 3.63 a | 2.51 ± 0.18 a | 168.7 ± 32.98 a | 93.3 ± 11.32 a | 1.7 ± 0.04 a | 88.7 ± 8.22 a | 9.93 ± 0.75 a | 3.04 ± 0.02 a |
| ‘Pinot Noir’ | 22.9 ± 2.65 | 6.9 ± 0.73 a | 14.6 ± 2.76 a | 2.38 ± 0.23 a | 161.4 ± 26.67 a | 95.2 ± 8.95 a | 1.6 ± 0.03 a b | 90.1 ± 6.27 a | 9.80 ± 1.35 a | 3.1 ± 0.08 a |
| Average 2014–2016 | Alcohol vol% | Total Extract g/L | Tartaric Acid g/L | Anthocyans mg/L | Color Intensity | Sensory Evaluation |
|---|---|---|---|---|---|---|
| ‘Pinore’ | 12.00 ± 0.76 a | 25.3 ± 1.79 a | 5.98 ± 1.07 a | 1534.4 ± 722.33 a | 5.53 ± 0.69 a | 17.42 ± 0.183 a |
| ‘Pinot Noir’ | 12.22 ± 0.59 a | 23.6 ± 1.98 b | 4.80 ± 0.93 b | 70.37 ± 33.21 b | 2.45 ± 0.38 b | 17.29 ± 0.155 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vršič, S.; Vršič, K. ‘Pinore’: The New Red Wine Variety Cross-Bred between ‘Pinot Noir’ and ‘Regent’ Vines. Plants 2021, 10, 2666. https://doi.org/10.3390/plants10122666
Vršič S, Vršič K. ‘Pinore’: The New Red Wine Variety Cross-Bred between ‘Pinot Noir’ and ‘Regent’ Vines. Plants. 2021; 10(12):2666. https://doi.org/10.3390/plants10122666
Chicago/Turabian StyleVršič, Stanko, and Klemen Vršič. 2021. "‘Pinore’: The New Red Wine Variety Cross-Bred between ‘Pinot Noir’ and ‘Regent’ Vines" Plants 10, no. 12: 2666. https://doi.org/10.3390/plants10122666
APA StyleVršič, S., & Vršič, K. (2021). ‘Pinore’: The New Red Wine Variety Cross-Bred between ‘Pinot Noir’ and ‘Regent’ Vines. Plants, 10(12), 2666. https://doi.org/10.3390/plants10122666






