Nitrate Is Nitrate: The Status Quo of Using Nitrate through Vegetable Extracts in Meat Products
Abstract
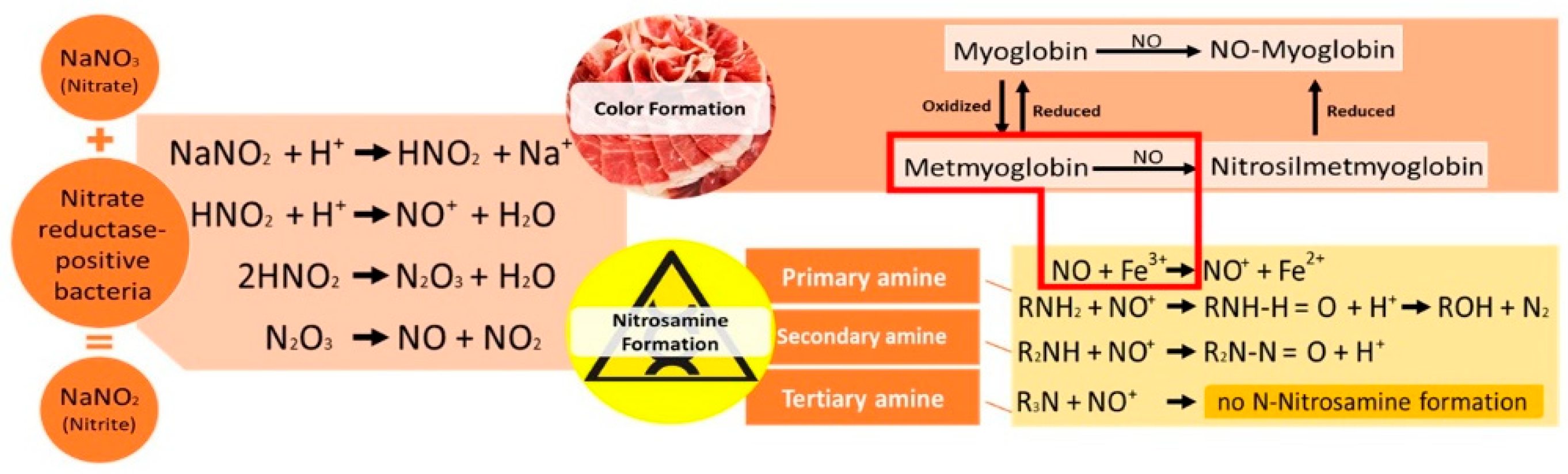
:1. Introduction
2. The Need for Nitrates and Nitrites in Cured Meat Products: Control of the Use
3. The Potential Health Risks Linked to the Consumption of Nitrite-Cured Meat Products
4. Strategic Use of Vegetable Extracts as a Source of Nitrates and Nitrites
5. Status Quo of Green Nitrate in Meat Products and Potential Hazards Associated
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cassens, R.D. Nitrite-cured Meat. In A Food Safety Issue in Perspective; Food & Nutrition Press Inc.: Trumbull, CT, USA, 1990. [Google Scholar]
- Sebranek, J.G. Basic Curing Ingredients. In Ingredients in Meat Products; Tarté, R., Ed.; Springer: New York, NY, USA, 2009; pp. 1–23. [Google Scholar] [CrossRef]
- Bedale, W.; Sindelar, J.J.; Milkowski, A.L. Dietary nitrate and nitrite: Benefits, risks, and evolving perceptions. Meat Sci. 2016, 120, 85–92. [Google Scholar] [CrossRef]
- Food and Agricultural Oganization (FAO). Available online: https://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/en/ (accessed on 28 July 2021).
- European Food Safety Authority (EFSA). Available online: https://www.efsa.europa.eu/en/topics/topic/food-additives (accessed on 28 July 2021).
- U.S. Food and Drug Administration (FDA). Available online: https://www.fda.gov/food/food-ingredients-packaging/overview-food-ingredients-additives-colors#how (accessed on 28 July 2021).
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of potassium nitrite (E 249) and sodium nitrite (E 250) as food additives. EFSA J. 2017, 15, e04786. [Google Scholar] [CrossRef]
- Higuero, N.; Moreno, I.; Lavado, G.; Vidal-Aragón, M.C.; Cava, R. Reduction of nitrate and nitrite in Iberian dry cured loins and its effects during drying process. Meat Sci. 2020, 163, 108062. [Google Scholar] [CrossRef]
- Fraqueza, M.J.; Laranjo, M.; Elias, M.; Patarata, L. Microbiological hazards associated with salt and nitrite reduction in cured meat products: Control strategies based on antimicrobial effect of natural ingredients and protective microbiota. Curr. Opin. Food Sci. 2020, 38, 32–39. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Working Group on the Evaluation of Carcinogenic Risks to Humans Red Meat and Processed Meat; WHO: Geneva, Switzerland, 2018; Volume 114. [Google Scholar]
- Grosse, Y.; Baan, R.; Straif, K.; Secretan, B.; El Ghissassi, F.; Cogliano, V.; WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Original Text. Lancet Oncol. 2006, 7, 628–629. [Google Scholar] [CrossRef] [Green Version]
- Molognoni, L.; Daguer, H.; Motta, G.E.; Merlo, T.C.; Lindner, J.D.D. Interactions of preservatives in meat processing: Formation of carcinogenic compounds, analytical methods, and inhibitory agents. Food Res. Int. 2019, 125, 108608. [Google Scholar] [CrossRef]
- Oostindjer, M.; Alexander, J.; Amdam, G.V.; Andersen, G.; Bryan, N.S.; Chen, D.; Corpet, D.E.; De Smet, S.; Dragsted, L.O.; Haug, A.; et al. The role of red and processed meat in colorectal cancer development: A perspective. Meat Sci. 2014, 97, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Linseisen, J. Processed meat: The real villain? Proc. Nutr. Soc. 2015, 75, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Crowe, W.; Elliott, C.T.; Green, B.D. A Review of the In Vivo Evidence Investigating the Role of Nitrite Exposure from Processed Meat Consumption in the Development of Colorectal Cancer. Nutrients 2019, 11, 2673. [Google Scholar] [CrossRef] [Green Version]
- An, R.; Nickols-Richardson, S.; Alston, R.; Shen, S.; Clarke, C. Total, Fresh, Lean, and Fresh Lean Beef Consumption in Relation to Nutrient Intakes and Diet Quality among U.S. Adults, 2005–2016. Nutrients 2019, 11, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, S.J.; Yoon, Y.; Jo, C.; Jeong, J.Y.; Lee, S.Y. Effect of Dietary Red Meat on Colorectal Cancer Risk—A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1812–1824. [Google Scholar] [CrossRef] [Green Version]
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food—Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilde, P.; Pomeranz, J.L.; Lizewski, L.J.; Ruan, M.; Mozaffarian, D.; Zhang, F.F. Legal Feasibility of US Government Policies to Reduce Cancer Risk by Reducing Intake of Processed Meat. Milbank Q. 2019, 97, 420–448. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Erim, F.B.; Kalaycioglu, Z. Nitrate and Nitrites in Foods: Worldwide Regional Distribution in View of Their Risks and Benefits. J. Agric. Food Chem. 2019, 67, 7205–7222. [Google Scholar] [CrossRef]
- Habermeyer, M.; Roth, A.; Guth, S.; Diel, P.; Engel, K.-H.; Epe, B.; Fürst, P.; Heinz, V.; Humpf, H.-U.; Joost, H.-G.; et al. Nitrate and nitrite in the diet: How to assess their benefit and risk for human health. Mol. Nutr. Food Res. 2014, 59, 106–128. [Google Scholar] [CrossRef]
- Van Breda, S.G.; Mathijs, K.; Sági-Kiss, V.; Kuhnle, G.G.; Van Der Veer, B.; Jones, R.R.; Sinha, R.; Ward, M.H.; De Kok, T.M. Impact of high drinking water nitrate levels on the endogenous formation of apparent N-nitroso compounds in combination with meat intake in healthy volunteers. Environ. Health Glob. Access Sci. Source 2019, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hsu, J.; Arcot, J.; Lee, N.A. Nitrate and nitrite quantification from cured meat and vegetables and their estimated dietary intake in Australians. Food Chem. 2008, 115, 334–339. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Dalgaard, F.; Blekkenhorst, L.C.; Murray, K.; Lewis, J.R.; Croft, K.D.; Kyrø, C.; Torp-Pedersen, C.; Gislason, G.; Tjønneland, A.; et al. Vegetable nitrate intake, blood pressure and incident cardiovascular disease: Danish Diet, Cancer, and Health Study. Eur. J. Epidemiol. 2021, 36, 813–825. [Google Scholar] [CrossRef]
- Archer, D.L. Evidence that Ingested Nitrate and Nitrite Are Beneficial to Health. J. Food Prot. 2002, 65, 872–875. [Google Scholar] [CrossRef]
- Martínez, G.N.; Bastida, P.; Castillo, J.; Ros, G.; Nieto, G. Green Alternatives to Synthetic Antioxidants, Antimicrobials, Nitrates, and Nitrites in Clean Label Spanish Chorizo. Antioxidants 2019, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Franco-Arellano, B.; Vanderlee, L.; Ahmed, M.; Oh, A.; L’Abbé, M. Influence of front-of-pack labelling and regulated nutrition claims on consumers’ perceptions of product healthfulness and purchase intentions: A randomized controlled trial. Appetite 2020, 149, 104629. [Google Scholar] [CrossRef]
- Mansfield, E.; Wahba, R.; De Grandpré, E. Integrating a Health Literacy Lens into Nutrition Labelling Policy in Canada. Int. J. Environ. Res. Public Health 2020, 17, 4130. [Google Scholar] [CrossRef]
- Hung, Y.; de Kok, T.M.; Verbeke, W. Consumer attitude and purchase intention towards processed meat products with natural compounds and a reduced level of nitrite. Meat Sci. 2016, 121, 119–126. [Google Scholar] [CrossRef]
- Hung, Y.; Verbeke, W. Sensory attributes shaping consumers’ willingness-to-pay for newly developed processed meat products with natural compounds and a reduced level of nitrite. Food Qual. Prefer. 2018, 70, 21–31. [Google Scholar] [CrossRef]
- Roncalés, P. Additives. In Handbook of Fermented Meat and Poultry, 2nd ed.; Toldrá, F., Hui, Y.H., Astiasaran, I., Sebranek, J.G., Talon, R., Eds.; Wiley Blackwell: West Sussex, UK, 2015; pp. 55–68. [Google Scholar]
- Sebranek, J.G.; Bacus, J.N. Cured meat products without direct addition of nitrate or nitrite: What are the issues? Meat Sci. 2007, 77, 136–147. [Google Scholar] [CrossRef]
- Garriga, M.; Aymerich, T. The Microbiology of Fermentation and Ripening. In Handbook of Fermented Meat and Poultry, 2nd ed.; Toldrá, F., Hui, Y.H., Astiasaran, I., Sebranek, J.G., Talon, R., Eds.; Wiley Blackwell: West Sussex, UK, 2015; pp. 107–116. [Google Scholar]
- Patarata, L.; Martins, S.; Silva, J.A.; Fraqueza, M.J. Red Wine and Garlic as a Possible Alternative to Minimize the Use of Nitrite for Controlling Clostridium Sporogenes and Salmonella in a Cured Sausage: Safety and Sensory Implications. Foods 2020, 9, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honikel, K.O. Curing. In Handbook of Meat Processing; Toldrá, F., Ed.; Blackwel Publishing: Hoboken, NJ, USA, 2010; pp. 125–143. [Google Scholar]
- Fraqueza, M.J.; Patarata, L.; Lauková, A. Protective cultures and bacteriocins in fermented meats. In Fermented Meat Products: Health Aspects, 1st ed.; Zdolec, N., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 228–269. [Google Scholar]
- Jo, K.; Lee, S.; Yong, H.I.; Choi, Y.-S.; Jung, S. Nitrite sources for cured meat products. LWT 2020, 129, 109583. [Google Scholar] [CrossRef]
- Pegg, R.B.; Honikel, K.O. Principles of Curing. In Handbook of Fermented Meat and Poultry, 2nd ed.; Toldrá, F., Hui, Y.H., Astiasaran, I., Sebranek, J.G., Talon, R., Eds.; Wiley Blackwell: West Sussex, UK, 2015; pp. 19–29. [Google Scholar]
- Ferysiuk, K.; Wójciak, K.M.; Materska, M.; Chilczuk, B.; Pabich, M. Modification of lipid oxidation and antioxidant capacity in canned refrigerated pork with a nitrite content reduced by half and addition of sweet pepper extract. LWT 2020, 118, 108738. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Chemistry, safety, and regulatory considerations in the use of nitrite and nitrate from natural origin in meat products-Invited review. Meat Sci. 2020, 171, 108272. [Google Scholar] [CrossRef]
- Majou, D.; Christieans, S. Mechanisms of the bactericidal effects of nitrate and nitrite in cured meats. Meat Sci. 2018, 145, 273–284. [Google Scholar] [CrossRef]
- Gunvig, A.; Hansen, F.; Borggaard, C. A mathematical model for predicting growth/no-growth of psychrotrophic C. botulinum in meat products with five variables. Food Control 2013, 29, 309–317. [Google Scholar] [CrossRef]
- Morita, H.; Yoshikawa, H.; Suzuki, T.; Hisamatsu, S.; Kato, Y.; Sakata, R.; Nagata, Y.; Yoshimura, T. Anti-microbial Action against VerotoxigenicEscherichia coliO157:H7 of Nitric Oxide Derived from Sodium Nitrite. Biosci. Biotechnol. Biochem. 2004, 68, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Biological Hazards on the request from the Commission related to the effects of Nitrites/Nitrates on the Microbiological Safety of Meat Products. EFSA J. 2003, 14, 1–31. [Google Scholar]
- Feiner, G. Meat Products Handbook: Practical Science and Technology, 1st ed.; Wood-Head Publishing: Cambridge, UK, 2006. [Google Scholar]
- Commission Regulation (EU) No 1129/2011 d of 11 November 2011 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by Establishing a Union List of Food Additives. Official Journal of the European Union, L 295/1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R1129&from=PT (accessed on 26 October 2021).
- United States Department of Agriculture (USDA). Food Safety and Inspection Service, USDA § 172.175. Sodium Nitrite. 21 CFR Ch. I. US Code of Federal Regulations, 2021 (Last Amended). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.175 (accessed on 20 October 2021).
- United States Department of Agriculture (USDA). Food Safety and Inspection Service, USDA § 172.170. Sodium Nitrate. 21 CFR Ch. I. US Code of Federal Regulations, 2021 (Last Amended). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.170 (accessed on 20 October 2021).
- Fraqueza, M.J.; Patarata, L. Fermented Meat Products: From the Technology to the Quality Control. In Fermented Food Products; Sankaranarayanan, A., Amaresan, N., Dhanasekaran, D., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 197–237. [Google Scholar]
- Halagarda, M.; Kędzior, W.; Pyrzyńska, E. Nutritional value and potential chemical food safety hazards of selected Polish sausages as influenced by their traditionality. Meat Sci. 2018, 139, 25–34. [Google Scholar] [CrossRef]
- Fraqueza, M.; Patarata, L. Produtos Cárneos Tradicionais. O Regresso ao Passado Como Caminho Para o Futuro. ACPA 2018, 1, 36–41. [Google Scholar]
- Verbeke, W.; Guerrero, L.; Almli, V.L.; Vanhonacker, F. Traditional Foods; Springer Science+ Business Media: New York, NY, USA, 2016; pp. 3–16. [Google Scholar] [CrossRef]
- Reig, M.; Toldrá, F. Toxic Compounds of Chemical Origin. In Handbook of Fermented Meat and Poultry, 2nd ed.; Toldrá, F., Hui, Y.H., Astiasaran, I., Sebranek, J.G., Talon, R., Eds.; Wiley Blackwell: West Sussex, UK, 2015; pp. 429–434. [Google Scholar]
- Püssa, T. Toxicological issues associated with production and processing of meat. Meat Sci. 2013, 95, 844–853. [Google Scholar] [CrossRef]
- Fraqueza, M.J.; Borges, A.; Patarata, L. Strategies to Reduce the Formation of Carcinogenic Chemicals in Dry Cured Meat Products. In Handbook of Food Bioengineering; Holban, A.M., Grumezescu, A.M., Eds.; Elsevier: London, UK, 2018; Volume 16, pp. 295–342. [Google Scholar]
- Flores, M.; Mora, L.; Reig, M.; Toldrá, F. Risk assessment of chemical substances of safety concern generated in processed meats. Food Sci. Hum. Wellness 2019, 8, 244–251. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 835/2011 of 19 August 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs. Official Journal of the European Union L 215/4. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R0835&from=PT (accessed on 26 October 2021).
- Laranjo, M.; Gomes, A.; Agulheiro-Santos, A.C.; Potes, M.E.; Cabrita, M.J.; Garcia, R.; Rocha, J.M.; Roseiro, L.C.; Fernandes, M.J.; Fraqueza, M.J.; et al. Impact of salt reduction on biogenic amines, fatty acids, microbiota, texture and sensory profile in traditional blood dry-cured sausages. Food Chem. 2016, 218, 129–136. [Google Scholar] [CrossRef]
- Linares, D.M.; Martin, M.C.; Ladero, V.; Alvarez, M.A.; Fernandez, M. Biogenic Amines in Dairy Products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef]
- Vidal-Carou, M.; Veciana-Nogués, M.; Latorre-Moratalla, M.; Bover-Cid, S. Biogenic Amines: Risks and Control. In Handbook of Fermented Meat and Poultry, 2nd ed.; Toldrá, F., Hui, Y.H., Astiasaran, I., Sebranek, J.G., Talon, R., Eds.; Wiley Blackwell: West Sussex, UK, 2015; pp. 413–428. [Google Scholar]
- Herrmann, S.S.; Duedahl-Olesen, L.; Granby, K. Occurrence of volatile and non-volatile N-nitrosamines in processed meat products and the role of heat treatment. Food Control 2015, 48, 163–169. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer (IARC). Working Group on the Evaluation of Carcinogenic Risks to Humans Some N-Nitroso Compounds; WHO: Geneva, Switzerland, 1978; Volume 17. [Google Scholar]
- Mohammed, G.; Bashammakh, A.; Alsibaai, A.; Alwael, H.; El-Shahawi, M. A critical overview on the chemistry, clean-up and recent advances in analysis of biogenic amines in foodstuffs. TrAC Trends Anal. Chem. 2016, 78, 84–94. [Google Scholar] [CrossRef]
- López-Rodríguez, R.; McManus, J.A.; Murphy, N.S.; Ott, M.; Burns, M.J. Pathways for N-Nitroso Compound Formation: Secondary Amines and Beyond. Org. Process. Res. Dev. 2020, 24, 1558–1585. [Google Scholar] [CrossRef]
- De Mey, E.; De Maere, H.; Paelinck, H.; Fraeye, I. VolatileN-nitrosamines in meat products: Potential precursors, influence of processing, and mitigation strategies. Crit. Rev. Food Sci. Nutr. 2017, 57, 2909–2923. [Google Scholar] [CrossRef]
- Honikel, K.-O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Available online: https://www.efsa.europa.eu/en/consultations/call/extensive-literature-search-nitrosocompounds-food (accessed on 28 July 2021).
- European Food Safety Authority (EFSA). Available online: https://www.efsa.europa.eu/en/call/call-continuous-collection-chemical-contaminants-occurrence-data-0 (accessed on 28 July 2021).
- Mavelle, T.; Bouchikhi, B.; Debry, G. The occurrence of volatile N-nitrosamines in French foodstuffs. Food Chem. 1991, 42, 321–338. [Google Scholar] [CrossRef]
- Biaudet, H.; Mavelle, T.; DeBry, G. Mean daily intake of N-nitrosodimethylamine from foods and beverages in France in 1987–1992. Food Chem. Toxicol. 1994, 32, 417–421. [Google Scholar] [CrossRef]
- Campillo, N.; Viñas, P.; Martínez-Castillo, N.; Hernández-Córdoba, M. Determination of volatile nitrosamines in meat products by microwave-assisted extraction and dispersive liquid–liquid microextraction coupled to gas chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 1815–1821. [Google Scholar] [CrossRef]
- Wang, P.; Yu, W.; Qiu, Y. Levels of nine volatile N-nitrosamines in Chinese-style sausages as determined by Quechers-based gas chromatography-tandem mass spectrometry. Ann. Public Health Res. 2016, 3, 1–7. [Google Scholar]
- Huang, M.-C.; Chen, H.-C.; Fu, S.-C.; Ding, W.-H. Determination of volatile N-nitrosamines in meat products by microwave-assisted extraction coupled with dispersive micro solid-phase extraction and gas chromatography—Chemical ionisation mass spectrometry. Food Chem. 2012, 138, 227–233. [Google Scholar] [CrossRef]
- Ozel, M.Z.; Gogus, F.; Yagci, S.; Hamilton, J.F.; Lewis, A.C. Determination of volatile nitrosamines in various meat products using comprehensive gas chromatography–nitrogen chemiluminescence detection. Food Chem. Toxicol. 2010, 48, 3268–3273. [Google Scholar] [CrossRef]
- Sannino, A.; Bolzoni, L. GC/CI–MS/MS method for the identification and quantification of volatile N-nitrosamines in meat products. Food Chem. 2013, 141, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Tricker, A.; Pfundstein, B.; Theobald, E.; Preussmann, R.; Spiegelhalder, B. Mean daily intake of volatile N-nitrosamines from foods and beverages in West Germany in 1989–1990. Food Chem. Toxicol. 1991, 29, 729–732. [Google Scholar] [CrossRef]
- Mitacek, E.; Brunnemann, K.; Suttajit, M.; Martin, N.; Limsila, T.; Ohshima, H.; Caplan, L. Exposure to N-nitroso compounds in a population of high liver cancer regions in Thailand: Volatile nitrosamine (VNA) levels in Thai food. Food Chem. Toxicol. 1999, 37, 297–305. [Google Scholar] [CrossRef]
- Glória, M.B.A.; Barbour, A.J.F.; Scanlan, R.A. Volatile Nitrosamines in Fried Bacon. J. Agric. Food Chem. 1997, 45, 1816–1818. [Google Scholar] [CrossRef]
- Domańska, K.; Kowalski, B. Occurrence of volatile N-nitrosamines in Polish processed meat products. Bull. Vet. Inst. Pulawy 2003, 47, 507–514. [Google Scholar]
- Reinik, M.; Tamme, T.; Roasto, M.; Juhkam, K.; Jurtšenko, S.; Tenno, T.; Kiis, A. Nitrites, nitrates and N-nitrosoamines in Estonian cured meat products: Intake by Estonian children and adolescents. Food Addit. Contam. 2005, 22, 1098–1105. [Google Scholar] [CrossRef]
- SanPiN. 2.3.2.560-96 Gigienicheskie Trebovaniya k Kachestvu i Bezopasnosti Prodovol’stvennogo Syr’ya i Pishchevykh Produktov. Sanitarnye Pravila i Normy (Hygienic Requirements to the Quality and Safety of Food Resources and Food Substances. Sanitary Rules and Norms), Russia. 1997. Available online: https://docs.cntd.ru/document/9052436 (accessed on 20 October 2021).
- United States Department of Agriculture (USDA). Food Safety and Inspection Service, USDA § 424.22. Certain Other Permitted Uses. 9 CFR Ch. III. US Code of Federal Regulations, 2021 (Last Amended). Available online: https://www.law.cornell.edu/cfr/text/9/424.22 (accessed on 20 October 2021).
- Rath, S.; Reyes, F. Nitrosamines. In Safety Analysis of Foods of Animal Origin; Nollet, L., Toldrá, F., Eds.; CRC Press: New York, NY, USA, 2011; pp. 421–440. [Google Scholar]
- Ministerio de Salud (MINSAL). Reglamento Sanitario de los Alimentos DTO. N° 977/96. Diário Oficial de la Republica de Chile; Ministerio de Salud Republica de Chile: Santiago, Chile, 1997. Available online: https://www.minsal.cl/sites/default/files/files/DECRETO_977_96%20actualizado%20a%20Enero%202015(1).pdf (accessed on 20 October 2021).
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Official Journal of the European Union, L 364/5. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 20 October 2021).
- Di Domenico, G.; Sit, J.; Ishizaka, A.; Nunan, D. Fake news, social media and marketing: A systematic review. J. Bus. Res. 2020, 124, 329–341. [Google Scholar] [CrossRef]
- Verbeke, W.; Frewer, L.J.; Scholderer, J.; De Brabander, H.F. Why consumers behave as they do with respect to food safety and risk information. Anal. Chim. Acta 2007, 586, 2–7. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Jayasena, D.D.; Ramachandra, S.; Jo, C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. Technol. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Lamas, A.; Miranda, J.M.; Vázquez, B.; Cepeda, A.; Franco, C.M. An Evaluation of Alternatives to Nitrites and Sulfites to Inhibit the Growth of Salmonella enterica and Listeria monocytogenes in Meat Products. Foods 2016, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as Potential Source of Natural Additives for Meat Industry. A Review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, M.M.; Munekata, P.E.S.; Jacinto-Valderrama, R.A.; Efraim, P.; Pateiro, M.; Lorenzo, J.M.; Pollonio, M.A.R. Beetroot and radish powders as natural nitrite source for fermented dry sausages. Meat Sci. 2021, 171, 108275. [Google Scholar] [CrossRef]
- Pinton, M.B.; dos Santos, B.A.; Lorenzo, J.M.; Cichoski, A.J.; Boeira, C.P.; Campagnol, P.C.B. Green technologies as a strategy to reduce NaCl and phosphate in meat products: An overview. Curr. Opin. Food Sci. 2020, 40, 1–5. [Google Scholar] [CrossRef]
- Hospital, X.F.; Hierro, E.; Stringer, S.; Fernández, M. A study on the toxigenesis by Clostridium botulinum in nitrate and nitrite-reduced dry fermented sausages. Int. J. Food Microbiol. 2016, 218, 66–70. [Google Scholar] [CrossRef]
- Possas, A.; Valdramidis, V.; García-Gimeno, R.M.; Pérez-Rodríguez, F. High hydrostatic pressure processing of sliced fermented sausages: A quantitative exposure assessment for Listeria monocytogenes. Innov. Food Sci. Emerg. Technol. 2019, 52, 406–419. [Google Scholar] [CrossRef]
- Di Gioia, D.; Mazzola, G.; Nikodinoska, I.; Aloisio, I.; Langerholc, T.; Rossi, M.; Raimondi, S.; Melero, B.; Rovira, J. Lactic acid bacteria as protective cultures in fermented pork meat to prevent Clostridium spp. growth. Int. J. Food Microbiol. 2016, 235, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Kurcubic, V.; Mašković, P.Z.; Vujić, J.M.; Vranić, D.V.; Vesković-Moračanin, S.M.; Okanović, G.; Lilić, S.V. Antioxidant and antimicrobial activity of Kitaibelia vitifolia extract as alternative to the added nitrite in fermented dry sausage. Meat Sci. 2014, 97, 459–467. [Google Scholar] [CrossRef]
- Eisinaite, V.; Vinauskiene, R.; Viskelis, P.; Leskauskaite, D. Effects of Freeze-Dried Vegetable Products on the Technological Process and the Quality of Dry Fermented Sausages. J. Food Sci. 2016, 81, C2175–C2182. [Google Scholar] [CrossRef]
- Aquilani, C.; Sirtori, F.; Flores, M.; Bozzi, R.; Lebret, B.; Pugliese, C. Effect of natural antioxidants from grape seed and chestnut in combination with hydroxytyrosol, as sodium nitrite substitutes in Cinta Senese dry-fermented sausages. Meat Sci. 2018, 145, 389–398. [Google Scholar] [CrossRef]
- Pini, F.; Aquilani, C.; Giovannetti, L.; Viti, C.; Pugliese, C. Characterization of the microbial community composition in Italian Cinta Senese sausages dry-fermented with natural extracts as alternatives to sodium nitrite. Food Microbiol. 2020, 89, 103417. [Google Scholar] [CrossRef]
- Eisinaitė, V.; Tamkutė, L.; Vinauskienė, R.; Leskauskaitė, D. Freeze-dried celery as an indirect source of nitrate in cold-smoked sausages: Effect on safety and color formation. LWT 2020, 129, 109586. [Google Scholar] [CrossRef]
- Beya, M.; Netzel, M.; Sultanbawa, Y.; Smyth, H.; Hoffman, L. Plant-Based Phenolic Molecules as Natural Preservatives in Comminuted Meats: A Review. Antioxidants 2021, 10, 263. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Li, X.; He, Q. The Nitrite-Scavenging Properties of Catechol, Resorcinol, and Hydroquinone: A Comparative Study on Their Nitration and Nitrosation Reactions. J. Food Sci. 2016, 81, C2692–C2696. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyżowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2020, 61, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K.C. Phytochemical Profiles and Health-Promoting Effects of Cool-Season Food Legumes As Influenced by Thermal Processing. J. Agric. Food Chem. 2009, 57, 10718–10731. [Google Scholar] [CrossRef]
- Cristino, R.; Costa, E.; Cosme, F.; Jordão, A.M. General phenolic characterisation, individual anthocyanin and antioxidant capacity of matured red wines from two Portuguese Appellations of Origins. J. Sci. Food Agric. 2013, 93, 2486–2493. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Domínguez, R.; Campagnol, P.C.B.; Franco, D.; Trindade, M.A.; Lorenzo, J.M. Effect of natural antioxidants on physicochemical properties and lipid stability of pork liver pâté manufactured with healthy oils during refrigerated storage. J. Food Sci. Technol. 2017, 54, 4324–4334. [Google Scholar] [CrossRef]
- Gonçalves, B.; Falco, V.; Moutinho-Pereira, J.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of Elevated CO2 on Grapevine (Vitis vinifera L.): Volatile Composition, Phenolic Content, and in Vitro Antioxidant Activity of Red Wine. J. Agric. Food Chem. 2008, 57, 265–273. [Google Scholar] [CrossRef]
- García-Díez, J.; Alheiro, J.; Falco, V.; Fraqueza, M.J.; Patarata, L. Chemical characterization and antimicrobial properties of herbs and spices essential oils against pathogens and spoilage bacteria associated to dry-cured meat products. J. Essent. Oil Res. 2016, 29, 117–125. [Google Scholar] [CrossRef]
- Alirezalu, K.; Yaghoubi, M.; Poorsharif, L.; Aminnia, S.; Kahve, H.I.; Pateiro, M.; Lorenzo, J.M.; Munekata, P.E.S. Antimicrobial Polyamide-Alginate Casing Incorporated with Nisin and ε-Polylysine Nanoparticles Combined with Plant Extract for Inactivation of Selected Bacteria in Nitrite-Free Frankfurter-Type Sausage. Foods 2021, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Chatkitanan, T.; Harnkarnsujarit, N. Development of nitrite compounded starch-based films to improve color and quality of vacuum-packaged pork. Food Packag. Shelf Life 2020, 25, 100521. [Google Scholar] [CrossRef]
- Chatkitanan, T.; Harnkarnsujarit, N. Effects of nitrite incorporated active films on quality of pork. Meat Sci. 2020, 172, 108367. [Google Scholar] [CrossRef] [PubMed]
- Rivera, N.; Bunning, M.; Martin, J. Uncured-Labeled Meat Products Produced Using Plant-Derived Nitrates and Nitrites: Chemistry, Safety, and Regulatory Considerations. J. Agric. Food Chem. 2019, 67, 8074–8084. [Google Scholar] [CrossRef] [PubMed]
- Sebranek, J.; Jackson-Davis, A.; Myers, K.; Lavieri, N. Beyond celery and starter culture: Advances in natural/organic curing processes in the United States. Meat Sci. 2012, 92, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Ferysiuk, K.; Wójciak, K.M. Reduction of Nitrite in Meat Products through the Application of Various Plant-Based Ingredients. Antioxidants 2020, 9, 711. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.; Pateiro, M.; Domínguez, R.; Santos, E.M.; Lorenzo, J.M. Cruciferous vegetables as sources of nitrate in meat products. Curr. Opin. Food Sci. 2020, 38, 1–7. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Working Group on the Evaluation of Carcinogenic Risks to Humans Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins; WHO: Geneva, Switzerland, 2010; Volume 94. [Google Scholar]
- Commission Regulation (EU) No 1258/2011 of 2 December 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Nitrates in Foodstuffs. Official Journal of the European Union, L 320/15. Available online: https://eur-lex.europa.eu/eli/reg/2011/1258/oj (accessed on 26 October 2021).
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Official Journal of the European Union, L 354/16. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32008R1333 (accessed on 26 October 2021).
- Standing Committee on Plants, Animals, Food and Feed (ScoPAFF). Summary Report of the Standing Committee on Plants, Animals, Food and Feed Held in Brussels on 17 September 2018. Available online: https://ec.europa.eu/food/horizontal-topics/committees/paff-committees_en (accessed on 26 October 2021).
| Product | Country | Average Concentration µg/kg | Analysis Method | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NDMA | NDEA | NDBA | NPIP | NMOR | NPYR | NMEA | NDPA | NTHZ | ||||
| Pork meat with salt | France | 0.09 | 0.03 | NA | NA | NA | NA | NA | NA | NA | GC-TEA | [69] |
| Pork meat | France | 0.28 | NA | NA | NA | NA | NA | NA | NA | NA | GLC | [70] |
| Spain | 1.7 | ND | ND | 1.0 | NA | 1.5 | ND | ND | NA | GC-MS | [71] | |
| Meat sausage | China | 0.8 | ND | 0.1 | ND | 0.7 | 3.5 | ND | ND | NA | GC-MS/MS | [72] |
| China | 2.4 | ND | 0.6 | 1.4 | NA | ND | 0.6 | ND | NA | GC-CI-MS | [73] | |
| Spain * | 2.2 | ND | 3.3 | 1.3 | NA | ND | ND | ND | NA | GC-MS | [71] | |
| Spain * | 2.4 | ND | ND | ND | NA | 1.5 | ND | ND | NA | GC-MS | [71] | |
| Spain * | 4.1 | 2.8 | ND | 1.5 | NA | 2.6 | ND | ND | NA | GC-MS | [71] | |
| Spain * | 3.3 | 2.2 | 1.9 | 1.1 | NA | 1.8 | ND | ND | NA | GC-MS | [71] | |
| Spain * | 4.0 | 1.9 | ND | ND | NA | ND | ND | ND | NA | GC-MS | [71] | |
| Spain * | 3.1 | 3.6 | 1.2 | 2.2 | NA | 1.5 | ND | 1.0 | NA | GC-MS | [71] | |
| Turkey * | 0.19 | 0.95 | ND | 1.05 | NA | 0.54 | NA | 0.5 | NA | GCXGC-NCD | [74] | |
| Turkey * | 0.11 | 0.10 | 0.15 | 0.16 | NA | 0.11 | NA | ND | NA | GCXGC-NCD | [74] | |
| Turkey * | 0.30 | 0.49 | 0.35 | 1.02 | NA | 0.57 | NA | 0.59 | NA | GCXGC-NCD | [74] | |
| Turkey * | 0.11 | ND | 0.19 | 1.49 | NA | 0.82 | NA | 0.47 | NA | GCXGC-NCD | [74] | |
| Turkey * | ND | ND | ND | 2.71 | NA | 1.36 | NA | 0.27 | NA | GCXGC-NCD | [74] | |
| Turkey * | 0.78 | 0.47 | 1.68 | 0.23 | NA | 0.18 | NA | 1.35 | NA | GCXGC-NCD | [74] | |
| Italy | 0,6 | ND | ND | ND | ND | ND | ND | ND | NA | GC-CI/MS/MS | [75] | |
| bacon | Germany | 1.01 | NA | NA | ND | NA | 0.02 | NA | NA | NA | GC-TEA | [76] |
| Thailand | 0.95 | NA | NA | ND | NA | ND | NA | NA | NA | GC-TEA | [77] | |
| Belgium | 1.6 | NA | NA | 0.2 | NA | 2.2 | NA | NA | NA | HPLC-MS/MS | [61] | |
| Denmark | 1.2 | NA | NA | 0.07 | NA | 1.4 | 0.5 | NA | NA | HPLC-MS/MS | [61] | |
| China | 1.4 | 0.4 | 1.7 | 0.4 | NA | ND | 0.2 | 0.3 | NA | GC-CI-MS | [73] | |
| Smoked bacon | France | 0.25 | 0.97 | 0.17 | 0.11 | NA | NA | NA | NA | NA | GC-TEA | [69] |
| Fried bacon | USA | 1 | 0.2 | 0.2 | ND | NA | 7.1 | NA | NA | 1.1 | GC-TEA | [78] |
| Ham | France | 0.14 | 0.03 | 0.09 | 0.25 | NA | 0.12 | NA | NA | NA | GC-TEA | [69] |
| France | 0.31 | NA | NA | NA | NA | NA | NA | NA | NA | GLC | [70] | |
| Thailand | 0.79 | NA | NA | ND | NA | ND | NA | NA | NA | GC-TEA | [77] | |
| Belgium | 1.5 | NA | NA | 0.07 | NA | 1.5 | 0.4 | NA | NA | HPLC-MS/MS | [61] | |
| Denmark | 2 | NA | NA | 0.04 | 0.08 | 1.2 | 0.2 | NA | NA | HPLC-MS/MS | [61] | |
| Italy | 0.3 | ND | ND | ND | ND | ND | ND | ND | NA | GC-CI/MS/MS | [75] | |
| Poland | 0.62 | 0.18 | NA | NA | NA | NA | NA | NA | NA | GC-TEA | [79] | |
| Spain | 2.6 | 2.4 | ND | 1.9 | NA | 3.4 | ND | ND | NA | GC-MS | [71] | |
| China | 0.6 | ND | 0.2 | 0.2 | NA | ND | 0.6 | ND | NA | GC-CI-MS | [73] | |
| Bologna | Italy | 0.4 | ND | ND | ND | ND | ND | ND | ND | NA | GC-CI/MS/MS | [75] |
| Spain | 1.5 | ND | ND | 0.8 | NA | 1.6 | ND | 1.2 | NA | GC-MS | [71] | |
| Black pudding | Spain | 3.5 | ND | 3.4 | 2.0 | NA | 2.1 | ND | ND | NA | GC-MS | [71] |
| Smoked pork brisket | France | 0.53 | 1.6 | 0.45 | 2.9 | 0.25 | 1.2 | NA | NA | NA | GC-TEA | [69] |
| Prosciutto | France | 0.54 | 1.1 | 0.29 | 0.24 | 0.48 | NA | NA | NA | NA | GC-TEA | [69] |
| Italy | 0.3 | ND | ND | ND | ND | ND | ND | ND | NA | GC-CI/MS/MS | [75] | |
| Spain | 2.0 | ND | ND | 1.8 | NA | 2.9 | 2.5 | ND | NA | GC-MS | [71] | |
| Other sausages | France | 0.91 | 2.4 | 0.43 | 0.18 | 0.74 | 0.28 | NA | NA | NA | GC-TEA | [69] |
| Germany | 0.84 | NA | NA | 0.03 | NA | ND | NA | NA | NA | GC-TEA | [76] | |
| France | 0.45 | NA | NA | NA | NA | NA | NA | NA | NA | GLC | [70] | |
| Belgium | 2.6 | NA | NA | 0.3 | 0.5 | 2.7 | NA | NA | NA | HPLC-MS/MS | [61] | |
| Denmark | 1.6 | 0.3 | NA | 0.1 | NA | 2.1 | 0.5 | NA | NA | HPLC-MS/MS | [61] | |
| China | 3.2 | 1.1 | 1.4 | 1.1 | NA | 0.7 | 0.4 | 0.7 | NA | GC-CI-MS | [73] | |
| Salami | France | 0.45 | 4.6 | 0.56 | 0.17 | NA | NA | NA | NA | NA | GC-TEA | [69] |
| Italy | 0.7 | ND | ND | ND | ND | ND | ND | ND | NA | GC-CI/MS/MS | [75] | |
| Turkey * | ND | 0.22 | ND | 1.44 | NA | ND | NA | ND | NA | GCXGC-NCD | [74] | |
| Turkey * | 0.3 | 0.28 | 0.10 | 0.73 | NA | 0.47 | NA | 0.35 | NA | GCXGC-NCD | [74] | |
| Turkey * | 0.10 | 0.11 | ND | 0.19 | NA | 0.14 | NA | 0.26 | NA | GCXGC-NCD | [74] | |
| Turkey * | ND | ND | 0.21 | 0.53 | NA | 0.37 | NA | 0.51 | NA | GCXGC-NCD | [74] | |
| Turkey * | ND | 0.15 | 0.56 | 0.82 | NA | 0.53 | NA | ND | NA | GCXGC-NCD | [74] | |
| Country | Maximum Permitted Level (µg/kg) | Nitrosamines | Products | References |
|---|---|---|---|---|
| Estonia | 2 | Σ NDMA and NDEA | Meat sausages submitted to heat treatment | [80] |
| 4 | Smoked meat sausages | [80] | ||
| Russia | 2 | Σ NDMA and NDEA | Meat sausages | [81] |
| 4 | Smoked meat sausages | [81] | ||
| USA | 10 | Total volatile nitrosamines | Cured meat product (bacon) | [82] |
| Canada | 10 | NDMA, NDEA, NDPA, NDBA, NPIP, and NMOR | Cured meat | [83] |
| 15 | NPYR | Cured meat | [83] | |
| Chile | 30 | NDMA | Cured meat product (cecinas) | [84] |
| Product | Country | Alternative | Objectives | Methods | Main Conclusions | Some Disadvantages | References |
|---|---|---|---|---|---|---|---|
| Fermented dried sausage | Serbia | Kitaibelia vitifolia extract | Evaluate the impact of nitrite’s replacement on quality characteristics. | 3 formulas: control with 27 g/kg of nitrite salt; with 30 g/kg of extract; and with 12.5 g/kg of extract. Drying process: 1 day, 22 °C, 92%RH; 1 day, 20 °C, 88%RH; 1 day, 19 °C, 86% RH; 1 day, 18 °C, 82% RH; 1 day, 15 °C, 72% RH; 21 days, 15 °C, 72% RH. Smoking process: at 3rd, 4th and 5th days, 5 h; 18 °C. | K. vitifolia extract revealed strong antioxidant capacity and moderate antimicrobial capacity against E. coli. K. vitifolia extract’s addition did not interfere with expected physicochemical characteristics, nor with the product’s overall acceptance. The great potential of K. vitifolia extracts in product’s preservation during processing and refrigerated storage | Products with addition of K. vitifolia extract showed lower consistency. | [96] |
| Fermented dried sausage | Lithuania | Lyophilized vegetable powder: celery, celery juice, parsnip, and leek | Evaluate the effect on ripening processes and final product’s properties. | 5 formulas: with 3% celery powder; with 3% celery juice powder; with 3% parsnip powder; with 3% leek powder; and control without addition. Drying process: 14 days. Initial temperature 24 °C, 92% RH and gradual decrease until 15 °C, 76% RH. Smoking process: cold smoking after 4th day of ripening. | The analysis of quality parameters such as pH, aw, LAB, coagulase-positive Staphylococci, and coliform revealed that the incorporation of these vegetable powders does not have a negative effect on fermentation and ripening processes. Formulas with celery powder and celery juice powder presented relatively stable color parameters during processing. | The incorporation of these vegetable powders resulted in softer products; formulas with celery powder, celery juice powder and leek were less red. | [97] |
| Fermented dried sausage | Italy | Grape seed with olive pomace hydroxytyrosol and chestnut extract with olive pomace hydroxytyrosol | Evaluate the effects in physicochemical, aromatic, and sensory characteristics and microbiological safety. | 3 formulas: control with 30 mg/kg of sodium nitrite; with 10 g/kg of grape seed extract; with 10 g/kg of chestnut extract. Drying process: 4 days, 28 °C, 85% RH. Ripening process: 21 days, 13 °C, 70% RH. | The replacement did not affect the overall acceptability of the products. All formulas were in agreement with European regulations for Listeria monocytogenes, Salmonella, and Clostridium botulinum. All formulas presented a similar aromatic profile. | Nitrite’s replacement resulted in some physical characteristics differences when compared with control. Some color characteristics of products with extracts (namely a* and b* parameters) showed significant differences when compared with control. | [98] |
| Chorizo | Spain | Natural extract of citric, acerola, rosemary, paprika, garlic, oregano, beet, lettuce, arugula, spinach, chard, celery, and watercress | Evaluate the antioxidant and antimicrobial capacity of the extracts. | 7 formulas: 6 with different combinations of extracts; control without extracts. All formulas were inoculated with C. perfringens. Drying process: 2 days, 22 ± 1 °C, 90 ± 5 °% RH; 20 days, 14 ± 1 °C, 70 ± 5 °% RH. Storage: 125 days, packed in vacuum 5 ± 1 °C, 65 ± 5 °% RH. | Rosemary extract revealed the best antimicrobial capacity. Paprika, garlic, and oregano extracts also revealed good antimicrobial capacity. Citric extract presented high antioxidant capacity. When combined, citric extract and rosemary extract have the potential to be a good alternative to the use of synthetic additives. | Citric extract presented low antimicrobial capacity. Celery extract presented lower phenolic content and lower antioxidant capacity. | [26] |
| Fermented dried sausage | Italy | Grape seed with olive pomace hydroxytyrosol and chestnut extract with olive pomace hydroxytyrosol | Evaluate the effect on the prokaryotic community. | 3 formulas: control with 30 mg/kg of sodium nitrite; with 10 g/kg of grape seed extract; with 10 g/kg of chestnut extract. Drying process: 4 days, 28 °C, 85% RH. Ripening process: 21 days, 13 °C, 70% RH. | Formulas without nitrite revealed lower pH values. Lactobacillaceae were significantly more present in chestnut extract formula. Although all three formulas showed significant differences, natural extracts did not present drastic changes in the prokaryotic community or other physicochemical parameters. | Grape seed extract presented less antioxidant capacity than sodium nitrite. Products without nitrite were less red and dark when compared with control. | [99] |
| Fermented smoked sausage | Lithuania | Lyophilized celery, with the addition of S. xylosus or S. xylosus and P. pentosaceus mixture | Evaluate lyophilized celery as possible substitute for both nitrite and nitrate regarding quality and safety. | 6 formulas: with 150 mg/kg of sodium nitrate and S. xylosus; with 150 mg/kg of sodium nitrate and S. xylosus and P. pentosaceus mixture; with 150 mg/kg d of sodium nitrite and S. xylosus; with 150 mg/kg of sodium nitrite and S. xylosus and P. pentosaceus mixture; with lyophilized celery and S. xylosus; with lyophilized celery and S. xylosus and P. pentosaceus mixture. Fermentation and drying processes: 14 days. Initial temperature 24 °C, 92% RH and gradual decrease until 15 °C, 76% RH (9th–10th day) and then remained constant. Smoking process: cold smoking at 96, 120 e 168 h. | Sausages with S. xylosus revealed less residual nitrate content than those with the addition of S. xylosus and P. pentosaceus mixture. Starter culture with S. xylosus and P. pentosaceus mixture presented positive effect in reddish color, higher than the effect of the S. xylosus culture; Lyophilized celery might have potential as an alternative for nitrites and nitrates if conjugated with starter cultures that help control fermentation and ripening processes. | The reddish color was less intense in sausages with lyophilized celery’s addition. | [100] |
| Fermented cured sausage | Brazil | Radish and beetroot powder, with the addition of Staphylococcus carnosus | Evaluate the effect on the development of cured characteristics during ripening and storage in physicochemical and microbiological parameters. | 6 formulas: control with 150 mg/kg of sodium nitrate and 150 mg/kg of sodium nitrite; control without nitrate nor nitrite; with 0.5% of beetroot powder; with 1% of beetroot powder; with 0.5% of radish powder; with 1% of radish powder. Formulas with beetroot and radish powder were complemented with a starter culture S. carnosus. Fermentation and drying process: 1st day, 25 °C/95%; 2nd day, 24 °C/93%; 3rd day, 23 °C/91%; 4th day, 22 °C/89%; 5th day, 21 °C/87%; 6th day, 20 °C/87%; 7th day, 18 °C/85%; 8th to 35th day, 15 °C/75%. The ripening process ended when aw < 0.91. Storage: packed in vacuum, 60 days, 5 °C. | Vegetable powder addition lowered humidity and aw, increasing the weight loss of the sausages. Of all formulas studied, the addition of 1% radish powder was the best alternative to nitrite, considering the following parameters: pH, colour, residual nitrate and nitrite content, and LAB development. | The main negative impact of beetroot’s powder addition was its effect in sausage’s color. | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardo, P.; Patarata, L.; Lorenzo, J.M.; Fraqueza, M.J. Nitrate Is Nitrate: The Status Quo of Using Nitrate through Vegetable Extracts in Meat Products. Foods 2021, 10, 3019. https://doi.org/10.3390/foods10123019
Bernardo P, Patarata L, Lorenzo JM, Fraqueza MJ. Nitrate Is Nitrate: The Status Quo of Using Nitrate through Vegetable Extracts in Meat Products. Foods. 2021; 10(12):3019. https://doi.org/10.3390/foods10123019
Chicago/Turabian StyleBernardo, Patrícia, Luís Patarata, Jose M. Lorenzo, and Maria João Fraqueza. 2021. "Nitrate Is Nitrate: The Status Quo of Using Nitrate through Vegetable Extracts in Meat Products" Foods 10, no. 12: 3019. https://doi.org/10.3390/foods10123019
APA StyleBernardo, P., Patarata, L., Lorenzo, J. M., & Fraqueza, M. J. (2021). Nitrate Is Nitrate: The Status Quo of Using Nitrate through Vegetable Extracts in Meat Products. Foods, 10(12), 3019. https://doi.org/10.3390/foods10123019








