RETRACTED: Combined Toxicity of Xenobiotics Bisphenol A and Heavy Metals on Zebrafish Embryos (Danio rerio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance and Embryo Collection
2.2. Dose Preparation and Exposure Protocols
2.3. Fish Embryo Toxicity (FET) Test
2.4. Viability, Morphology and Hatching Rate
2.5. Total RNA Extraction and RT-PCR
2.6. Detection of ROS and Antioxidative Enzyme Assay
2.7. Materials
2.8. Statistical Evaluation
3. Results
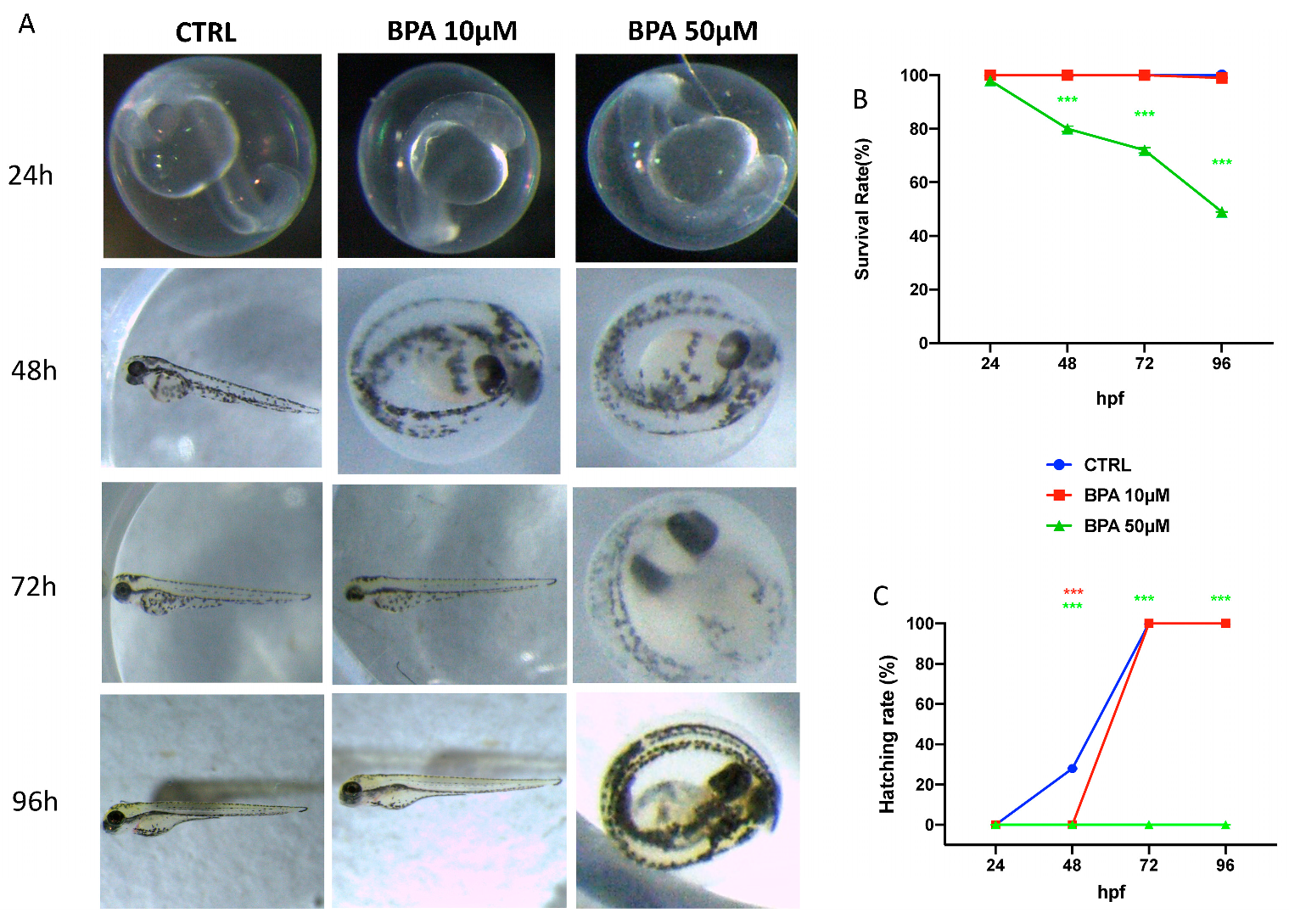
3.1. Effect of Bisphenol A on Morphology, Viability, and Hatching Rate
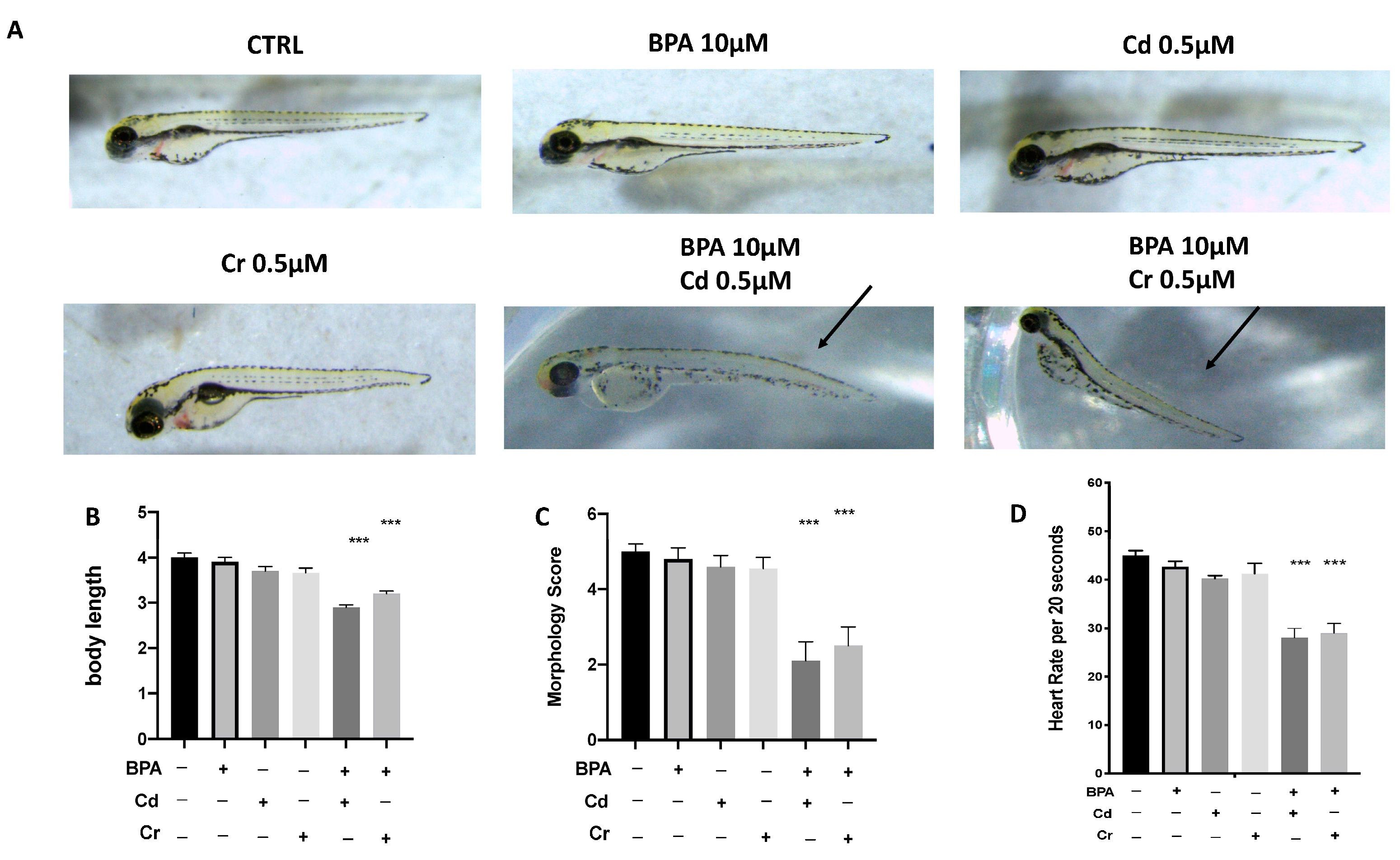
3.2. Malformation Scores, Body Length, and Heart Rate
3.3. Effect of BPA, Cd, and Cr on Gene Expression of Antioxidant Pathway
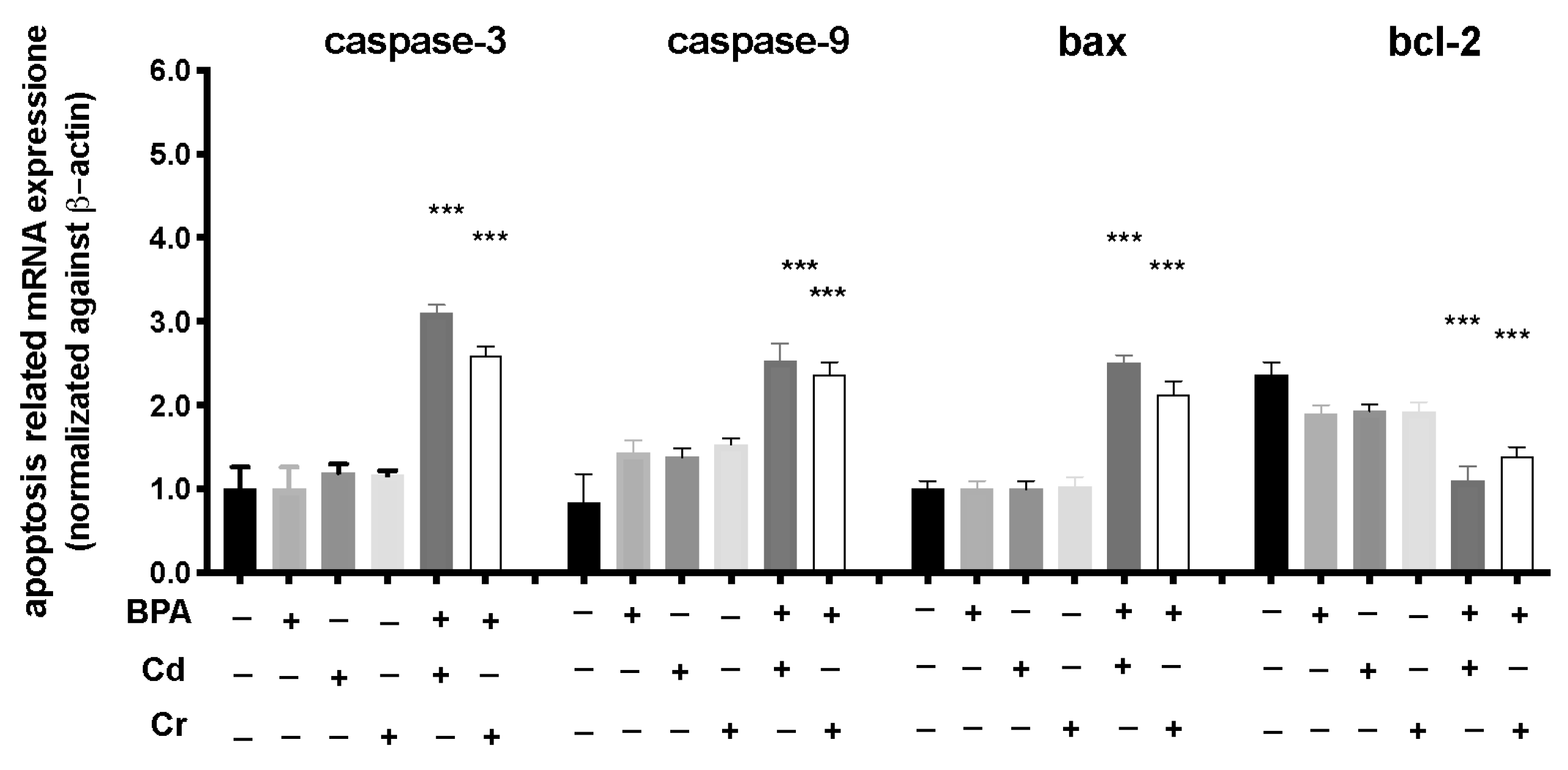
3.4. Effect of BPA, Cd, and Cr on Gene Expression of Apoptotic Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, C.; Kannan, K. High levels of bisphenol A in paper currencies from several countries, and implications for dermal exposure. Environ. Sci. Technol. 2011, 45, 6761–6768. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.-P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.-M.; Pussemier, L.; Scippo, M.-L. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- Li, X.; Yin, P.; Zhao, L. Effects of individual and combined toxicity of bisphenol A, dibutyl phthalate and cadmium on oxidative stress and genotoxicity in HepG 2 cells. Food Chem. Toxicol. 2017, 105, 73–81. [Google Scholar] [CrossRef]
- Experts, I. Bisphenol-A-A Global Market Overview; Hexion Inc.: Columbus, OH, USA, 2016. [Google Scholar]
- Huang, Y.; Wong, C.; Zheng, J.; Bouwman, H.; Barra, R.; Wahlström, B.; Neretin, L.; Wong, M.H. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012, 42, 91–99. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef]
- Lam, S.H.; Hlaing, M.M.; Zhang, X.; Yan, C.; Duan, Z.; Zhu, L.; Ung, C.Y.; Mathavan, S.; Ong, C.N.; Gong, Z. Toxicogenomic and phenotypic analyses of bisphenol-A early-life exposure toxicity in zebrafish. PLoS ONE 2011, 6, e28273. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.S.; Chan, W.K.L.; Chan, K.M. Toxicity assessment and vitellogenin expression in zebrafish (Danio rerio) embryos and larvae acutely exposed to bisphenol A, endosulfan, heptachlor, methoxychlor and tetrabromobisphenol A. J. Appl. Toxicol. 2013, 33, 670–678. [Google Scholar] [CrossRef]
- Nriagu, J.O. A global assessment of natural sources of atmospheric trace metals. Nature 1989, 338, 47–49. [Google Scholar] [CrossRef]
- Juárez-Franco, M.F.; Sarma, S.; Nandini, S. Effect of cadmium and zinc on the population growth of Brachionus havanaensis (Rotifera: Brachionidae). J. Environ. Sci. Health Part A 2007, 42, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Khangarot, B. Bioaccumulation of copper and toxic effects on feeding, growth, fecundity and development of pond snail Lymnaea luteola L. J. Hazard. Mater. 2011, 185, 295–305. [Google Scholar] [CrossRef]
- Bagchi, D.; Joshi, S.S.; Bagchi, M.; Balmoori, J.; Benner, E.; Kuszynski, C.; Stohs, S. Cadmium-and chromium-induced oxidative stress, DNA damage, and apoptotic cell death in cultured human chronic myelogenous leukemic K562 cells, promyelocytic leukemic HL-60 cells, and normal human peripheral blood mononuclear cells. J. Biochem. Mol. Toxicol. 2000, 14, 33–41. [Google Scholar] [CrossRef]
- Alghasham, A.; Salem, T.A.; Meki, A.-R.M. Effect of cadmium-polluted water on plasma levels of tumor necrosis factor-α, interleukin-6 and oxidative status biomarkers in rats: Protective effect of curcumin. Food Chem. Toxicol. 2013, 59, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.; Kille, P.; Sweeney, G. Cadmium delays growth hormone expression during rainbow trout development. J. Fish Biol. 2001, 59, 1015–1022. [Google Scholar] [CrossRef]
- Ma, W.; Wang, L.; He, Y.; Yan, Y. Tissue-specific cadmium and metallothionein levels in freshwater crab Sinopotamon henanense during acute exposure to waterborne cadmium. Environ. Toxicol. Int. J. 2008, 23, 393–400. [Google Scholar] [CrossRef]
- Woo, S.; Yum, S.; Park, H.-S.; Lee, T.-K.; Ryu, J.-C. Effects of heavy metals on antioxidants and stress-responsive gene expression in Javanese medaka (Oryzias javanicus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Chen, L.; Wu, Y.-H.; Li, P.; Li, Y.-F.; Ni, Z.-H. Effects of waterborne cadmium on thyroid hormone levels and related gene expression in Chinese rare minnow larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 161, 53–57. [Google Scholar] [CrossRef]
- Bindhumol, V.; Chitra, K.; Mathur, P. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology 2003, 188, 117–124. [Google Scholar] [CrossRef]
- Wu, M.; Xu, H.; Shen, Y.; Qiu, W.; Yang, M. Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol A, nonylphenol, and their mixture. Environ. Toxicol. Chem. 2011, 30, 2335–2341. [Google Scholar] [CrossRef]
- Isani, G.; Monari, M.; Andreani, G.; Fabbri, M.; Carpenè, E. Effect of copper exposure on the antioxidant enzymes in bivalve mollusc Scapharca inaequivalvis. Vet. Res. Commun. 2003, 27, 691–693. [Google Scholar] [CrossRef]
- Vale, G.; Franco, C.; Diniz, M.S.; dos Santos, M.M.; Domingos, R.F. Bioavailability of cadmium and biochemical responses on the freshwater bivalve Corbicula fluminea–the role of TiO2 nanoparticles. Ecotoxicol. Environ. Saf. 2014, 109, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ken, C.-F.; Lin, C.-T.; Shaw, J.-F.; Wu, J.-L. Characterization of fish Cu/Zn–superoxide dismutase and its protection from oxidative stress. Mar. Biotechnol. 2003, 5, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Huang, W.; Liu, J.; Yin, X.; Dou, S. Accumulation and oxidative stress biomarkers in Japanese flounder larvae and juveniles under chronic cadmium exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Won, E.-J.; Lee, J.-S.; Lee, Y.-M. Combined effects of cadmium and copper on the expression of antioxidant enzyme—Coding genes in the polychaete, Perinereis nuntia. Toxicol. Environ. Health Sci. 2013, 5, 26–33. [Google Scholar] [CrossRef]
- Won, E.-J.; Ra, K.; Kim, K.-T.; Lee, J.-S.; Lee, Y.-M. Three novel superoxide dismutase genes identified in the marine polychaete Perinereis nuntia and their differential responses to single and combined metal exposures. Ecotoxicol. Environ. Saf. 2014, 107, 36–45. [Google Scholar] [CrossRef]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic. Biol. Med. 2010, 49, 1328–1341. [Google Scholar] [CrossRef] [PubMed]
- Richetti, S.K.; Rosemberg, D.B.; Ventura-Lima, J.; Monserrat, J.M.; Bogo, M.R.; Bonan, C.D. Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. Neurotoxicology 2011, 32, 116–122. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Lesley, R.; Ramakrishnan, L. Insights into early mycobacterial pathogenesis from the zebrafish. Curr. Opin. Microbiol. 2008, 11, 277–283. [Google Scholar] [CrossRef]
- Chemicals, D. OECD Guideline for Testing of Chemicals; The Organisation for Economic Co-operation and Development: Paris, France, 2015; pp. 1–13. [Google Scholar]
- Brundo, M.V.; Pecoraro, R.; Marino, F.; Salvaggio, A.; Tibullo, D.; Saccone, S.; Bramanti, V.; Buccheri, M.A.; Impellizzeri, G.; Scuderi, V. Toxicity evaluation of new engineered nanomaterials in zebrafish. Front. Physiol. 2016, 7, 130. [Google Scholar] [CrossRef]
- Di Paola, D.; Iaria, C.; Capparucci, F.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S. Aflatoxin B1 Toxicity in Zebrafish Larva (Danio rerio): Protective Role of Hericium erinaceus. Toxins 2021, 13, 710. [Google Scholar] [CrossRef]
- Kuder, R.S.; Gundala, H.P. Developmental toxicity of deltamethrin and 3-phenoxybenzoic acid in embryo-larval stages of zebrafish (Danio rerio). Toxicol. Mech. Methods 2018, 28, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Liu, K.; He, Q.; Sun, C.; Han, J.; Han, L.; Tian, Q. Xiaoaiping induces developmental toxicity in zebrafish embryos through activation of ER stress, apoptosis and the Wnt pathway. Front. Pharmacol. 2018, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yu, L.; Liu, C.; Yu, K.; Shi, X.; Yeung, L.W.; Lam, P.K.; Wu, R.S.; Zhou, B. Hexabromocyclododecane-induced developmental toxicity and apoptosis in zebrafish embryos. Aquat. Toxicol. 2009, 93, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Wei, Y.; Zhang, H.; Xu, M.; Dai, J. Induction of time-dependent oxidative stress and related transcriptional effects of perfluorododecanoic acid in zebrafish liver. Aquat. Toxicol. 2008, 89, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, X.; Shu, L.; Chen, L.; Sun, L.; Qian, H.; Liu, W.; Fu, Z. Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 2010, 78, 846–852. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, L.; Ruan, M.; Liu, J.; Yang, Y.; Zhou, C.; Xu, B.; Fu, Z. Cypermethrin exposure during puberty induces oxidative stress and endocrine disruption in male mice. Chemosphere 2011, 84, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Stucki, G.; Alexander, M. Role of dissolution rate and solubility in biodegradation of aromatic compounds. Appl. Environ. Microbiol. 1987, 53, 2603. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Liu, F.; Ye, Y.; Peng, T.; Fu, Z. Embryonic exposure to cadmium (II) and chromium (VI) induce behavioral alterations, oxidative stress and immunotoxicity in zebrafish (Danio rerio). Neurotoxicology Teratol. 2015, 48, 9–17. [Google Scholar] [CrossRef]
- Kortenkamp, A. Ten years of mixing cocktails: A review of combination effects of endocrine-disrupting chemicals. Environ. Health Perspect. 2007, 115, 98–105. [Google Scholar] [CrossRef]
- Kocaoba, S.; Akcin, G. Removal of chromium (III) and cadmium (II) from aqueous solutions. Desalination 2005, 180, 151–156. [Google Scholar] [CrossRef]
- Samaee, S.M.; Rabbani, S.; Jovanovic, B.; Mohajeri-Tehrani, M.R.; Haghpanah, V. Efficacy of the hatching event in assessing the embryo toxicity of the nano-sized TiO(2) particles in zebrafish: A comparison between two different classes of hatching-derived variables. Ecotoxicol. Environ. Saf. 2015, 116, 121–128. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Coelhan, M.; Chan, H.M.; Ma, W.; Liu, L. Relative developmental toxicity of short-chain chlorinated paraffins in Zebrafish (Danio rerio) embryos. Environ. Pollut. 2016, 219, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Yusof, S. Effect of mercury and cadmium on early life stages of Java medaka (Oryzias javanicus): A potential tropical test fish. Mar. Pollut. Bull. 2011, 63, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Papiya, S.; Kanamadi, R. Effect of mercurial fungicide Emisan®-6 on the embryonic developmental stages of zebrafish, Brachydanio (Danio) rerio. J. Adv. Zool. 2000, 21, 12–18. [Google Scholar]
- Morin, S.; Duong, T.; Dabrin, A.; Coynel, A.; Herlory, O.; Baudrimont, M.; Delmas, F.; Durrieu, G.; Schäfer, J.; Winterton, P. Long-term survey of heavy-metal pollution, biofilm contamination and diatom community structure in the Riou Mort watershed, South-West France. Environ. Pollut. 2008, 151, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Lakind, J.S.; Naiman, D.Q. Bisphenol A (BPA) daily intakes in the United States: Estimates from the 2003–2004 NHANES urinary BPA data. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 608–615. [Google Scholar] [CrossRef]
- Kusch, R.C.; Krone, P.H.; Chivers, D.P. Chronic exposure to low concentrations of waterborne cadmium during embryonic and larval development results in the long-term hindrance of antipredator behavior in zebrafish. Environ. Toxicol. Chem. Int. J. 2008, 27, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Jiang, L.; Liu, X.; Geng, C.; Wang, W.; Zhong, L.; Yang, G.; Chen, M. Bisphenol A induces oxidative stress-associated DNA damage in INS-1 cells. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2014, 769, 29–33. [Google Scholar] [CrossRef]
- Youn, C.-K.; Kim, S.-H.; Song, S.H.; Chang, I.-Y.; Hyun, J.-W.; Chung, M.-H.; You, H.J. Cadmium down-regulates human OGG1 through suppression of Sp1 activity. J. Biol. Chem. 2005, 280, 25185–25195. [Google Scholar] [CrossRef]
- Nazıroğlu, M. Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem. Res. 2009, 34, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- Baillie, T.A.; Rettie, A.E. Role of biotransformation in drug-induced toxicity: Influence of intra- and inter-species differences in drug metabolism. Drug Metab. Pharm. 2011, 26, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.; Kalgutkar, A.S.; Obach, R.S. Metabolic activation in drug-induced liver injury. Drug Metab. Rev. 2012, 44, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.; Kim, E.A.; Kang, M.C.; Lee, W.W.; Lee, H.S.; Vairappan, C.S.; Jeon, Y.J. Assessment of anti-inflammatory effect of 5beta-hydroxypalisadin B isolated from red seaweed Laurencia snackeyi in zebrafish embryo in vivo model. Environ. Toxicol. Pharmacol. 2014, 37, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhu, L.; Shao, B.; Zhu, S.; Wang, J.; Xie, H.; Wang, J.; Wang, F. The effects of endosulfan on cytochrome P450 enzymes and glutathione S-transferases in zebrafish (Danio rerio) livers. Ecotoxicol. Environ. Saf. 2013, 92, 1–9. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-L.; Yuan, S.-S.; Wu, C.-W.; Lv, Z.-M. Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish (Danio rerio). Aquat. Toxicol. 2016, 180, 36–44. [Google Scholar] [CrossRef]
- Shaw, P.; Mondal, P.; Bandyopadhyay, A.; Chattopadhyay, A. Environmentally relevant concentration of chromium activates Nrf2 and alters transcription of related XME genes in liver of zebrafish. Chemosphere 2019, 214, 35–46. [Google Scholar] [CrossRef]
- Cai, G.; Zhu, J.; Shen, C.; Cui, Y.; Du, J.; Chen, X. The effects of cobalt on the development, oxidative stress, and apoptosis in zebrafish embryos. Biol. Trace Elem. Res. 2012, 150, 200–207. [Google Scholar] [CrossRef]
- Cole, L.; Ross, L. Apoptosis in the developing zebrafish embryo. Dev. Biol. 2001, 240, 123–142. [Google Scholar] [CrossRef]
- Corda, S.; Laplace, C.; Vicaut, E.; Duranteau, J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-α is mediated by ceramide. Am. J. Respir. Cell Mol. Biol. 2001, 24, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.; Radeke, H.; Selle, S.; Younes, M.; Sies, H.; Resch, K.; Habermehl, G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-α. Biochem. J. 1989, 263, 539–545. [Google Scholar] [CrossRef]
- Shoji, Y.; Uedono, Y.; Ishikura, H.; Takeyama, N.; Tanaka, T. DNA damage induced by tumour necrosis factor-alpha in L929 cells is mediated by mitochondrial oxygen radical formation. Immunology 1995, 84, 543. [Google Scholar]
- Pulido, M.D.; Parrish, A.R. Metal-induced apoptosis: Mechanisms. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2003, 533, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.B.; Green, D.R. Suicidal tendencies: Apoptotic cell death by caspase family proteinases. J. Biol. Chem. 1999, 274, 20049–20052. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Orientation | Nucleotide Sequence |
|---|---|---|
| b-actin | forward | 5′-AGAGCTATGAGCTGCCTGACG-3′ |
| reverse | 5′-CCGCAAGATTCCATACCCA-3′ | |
| Oxidative stress pathway genes | ||
| sod1 | forward | 5′-GGCCAACCGATAGTGTTAGA-3′ |
| reverse | 5′-CCAGCGTTGCCAGTTTTTAG-3′ | |
| cat | forward | 5′-AGGGCAACTGGGATCTTACA-3′ |
| reverse | 5′-TTTATGGGACCAGACCTTGG-3′ | |
| gstp2 | forward | 5′-CACAGACCTCGCTTTTCACAC-3′ |
| reverse | 5′-GAGAGAAGCCTCACAGTCGT-3′ | |
| Nrf2 | forward | 5′-TCGGGTTTGTCCCTAGATG-3′ |
| reverse | 5′-AGGTTTGGAGTGTCCGCTA-3′ | |
| Apoptosis pathway genes | ||
| casp-3 | forward | 5′-CCGCTGCCCATCACTA-3′ |
| reverse | 5′-ATCCTTTCACGACCATCT-3′ | |
| Bax | forward | 5′-GGCTATTTCAACCAGGGTTCC-3′ |
| reverse | 5′-TGCGAATCACCAATGCTGT-3′ | |
| bcl-2 | forward | 5′-TCACTCGTTCAGACCCTCAT-3′ |
| reverse | 5′-ACGCTTTCCACGCACAT-3′ | |
| casp-9 | forward | 5′-AAATACATAGCAAGGCAACC-3′ |
| reverse | 5′-CACAGGGAATCAAGAAAGG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Paola, D.; Capparucci, F.; Lanteri, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. RETRACTED: Combined Toxicity of Xenobiotics Bisphenol A and Heavy Metals on Zebrafish Embryos (Danio rerio). Toxics 2021, 9, 344. https://doi.org/10.3390/toxics9120344
Di Paola D, Capparucci F, Lanteri G, Cordaro M, Crupi R, Siracusa R, D’Amico R, Fusco R, Impellizzeri D, Cuzzocrea S, et al. RETRACTED: Combined Toxicity of Xenobiotics Bisphenol A and Heavy Metals on Zebrafish Embryos (Danio rerio). Toxics. 2021; 9(12):344. https://doi.org/10.3390/toxics9120344
Chicago/Turabian StyleDi Paola, Davide, Fabiano Capparucci, Giovanni Lanteri, Marika Cordaro, Rosalia Crupi, Rosalba Siracusa, Ramona D’Amico, Roberta Fusco, Daniela Impellizzeri, Salvatore Cuzzocrea, and et al. 2021. "RETRACTED: Combined Toxicity of Xenobiotics Bisphenol A and Heavy Metals on Zebrafish Embryos (Danio rerio)" Toxics 9, no. 12: 344. https://doi.org/10.3390/toxics9120344
APA StyleDi Paola, D., Capparucci, F., Lanteri, G., Cordaro, M., Crupi, R., Siracusa, R., D’Amico, R., Fusco, R., Impellizzeri, D., Cuzzocrea, S., Spanò, N., Gugliandolo, E., & Peritore, A. F. (2021). RETRACTED: Combined Toxicity of Xenobiotics Bisphenol A and Heavy Metals on Zebrafish Embryos (Danio rerio). Toxics, 9(12), 344. https://doi.org/10.3390/toxics9120344















