Abstract
The present review covers the recent synthetic strategies and chemical transformations of thiazolo[3,2-a]benzimidazoles and it also presents the highlights of the biological activities of these compounds.
1. Introduction
Thiazolo[3,2-a]benzimidazole systems have been known for more than seven decades [1]. Thiazolo[3,2-a]bezimidazol-3(2H)one (1, Figure 1)) was synthesized in 1926, while thiazolo[3,2-a]-benzimidazole (2, Figure 1) was reported in 1966 [2,3]. Nevertheless, various substituted thiazolo[3,2-a]-benzimidazoles were reported before compound 2 [4,5,6,7,8].

Figure 1.
Structure of compounds 1 and 2.
Figure 1.
Structure of compounds 1 and 2.
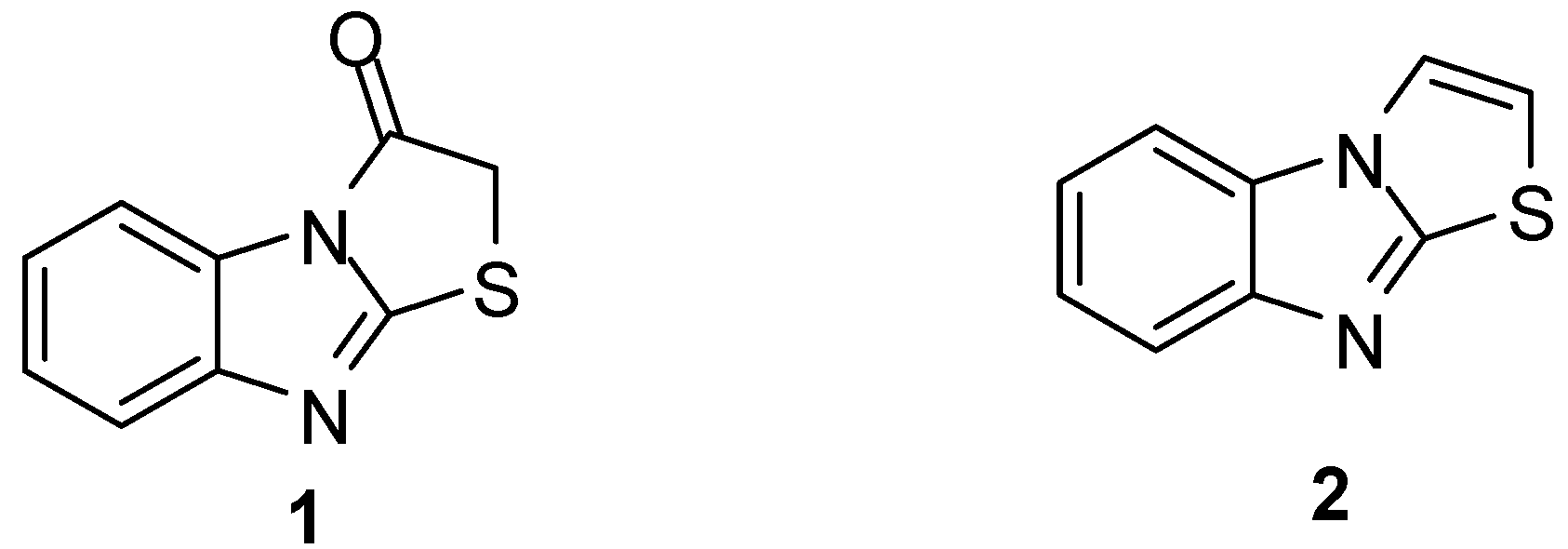
In 1988, a review on fused thiazolobenzimidazoles was published by Chimirri et al. [9]. In recent years, there has been considerable interest of researchers in thiazolo[3,2-a]benzimidazoles, stimulated by their biological activities. Additionally an enormous variety of thiazolo[3,2-a]-benzimidazoles with unique pharmaceutical and medicinal applications have been reported in the patent literature. These considerable biological activities and our contributions to the chemistry and biological activities of these compounds prompted us to compile the present review which deals with the recent synthetic strategies, chemical transformations and biological activities of thiazolo[3,2-a]benzimidazoles. The intention of this review is to focus mainly on publications appeared from 1989 to the end of 2009.
2. Synthetic Strategies
2.1. From 2-mercaptobenzimidazoles
2-Mercaptobenzimidazoles were used in the synthesis of several thiazolo[3,2-a]benzimidazole derivatives by annulations of thiazole ring to a benzimidazole moiety. The reaction of 2-mercapto-benzimidazole (3) with ketones 4 in boiling AcOH/H2SO4 afforded 2-benzimidazolylthioacetophenone derivatives 5 [10,11,12]. The latter sulphides were cyclized to give the corresponding thiazolo[3,2-a]benzimidazoles 6 using PPA or [hydroxy(tosyloxy)iodo]benzene (Scheme 1).
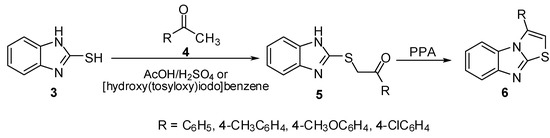
Scheme 1.
Reaction of 2-mercaptobenzimidazole (3) with ketones 4.
Scheme 1.
Reaction of 2-mercaptobenzimidazole (3) with ketones 4.
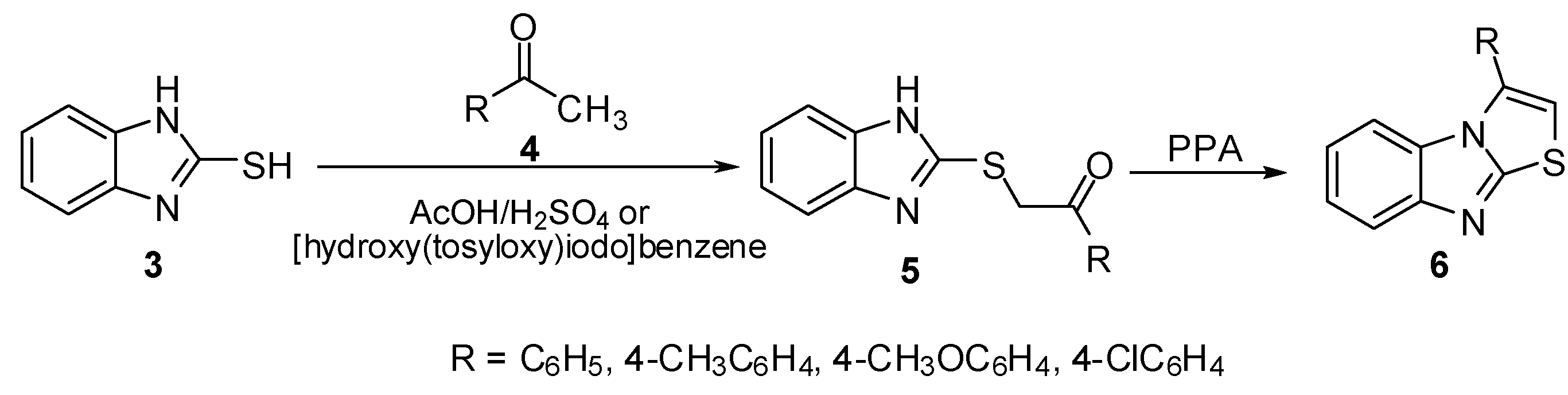
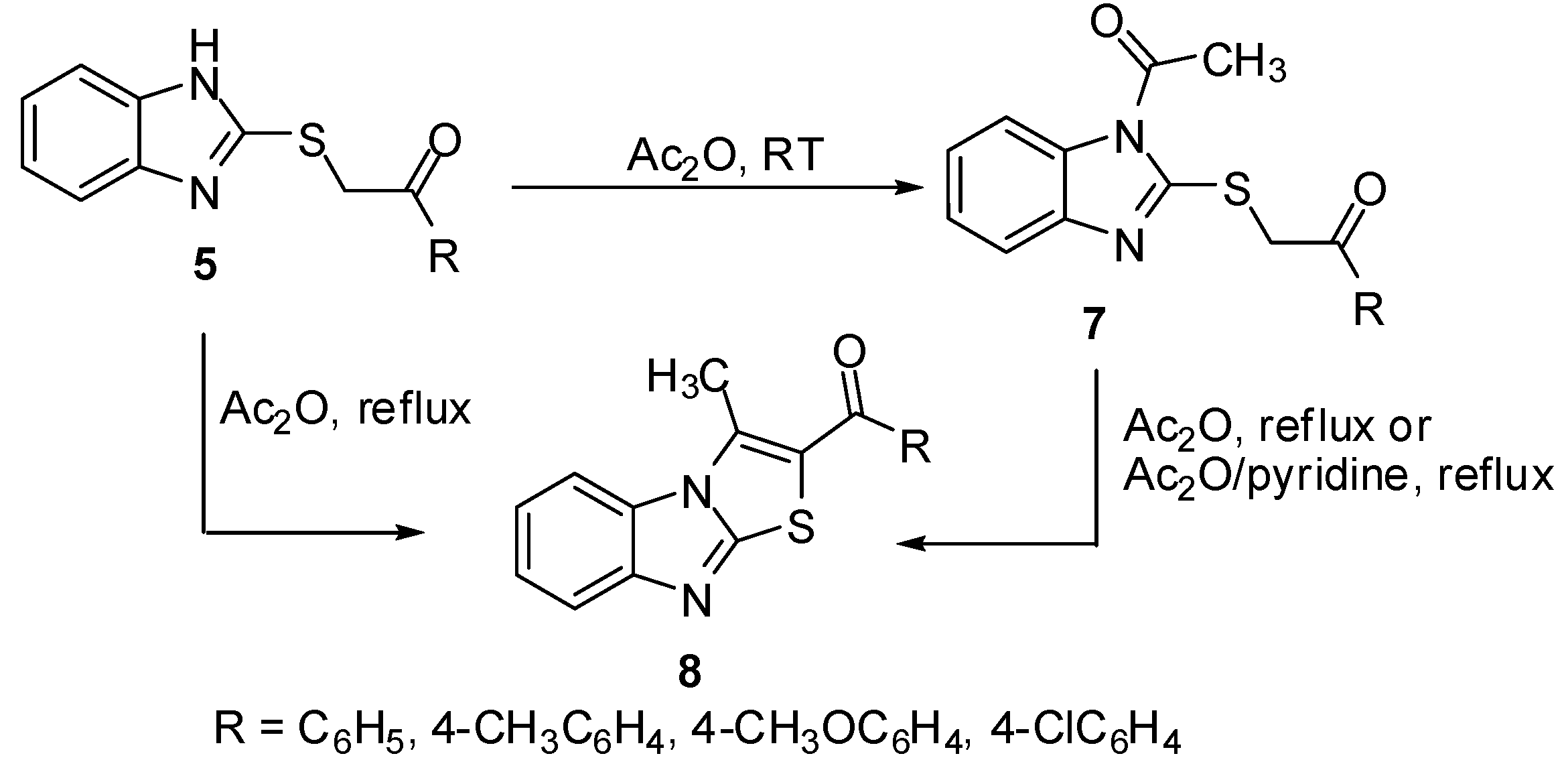
Treatment of sulphides 5 with acetic anhydride at room temperature gave the N-acetyl derivatives 7. On the other hand, heating of thioacetophenones 5 in acetic anhydride or in Ac2O/pyridine mixture afforded the 2-aroyl-3-methylthiazolo[3,2-a]benzimidazoles 8 [11] which were obtained independently by refluxing N-acetyl derivatives 7 in Ac2O (Scheme 2).
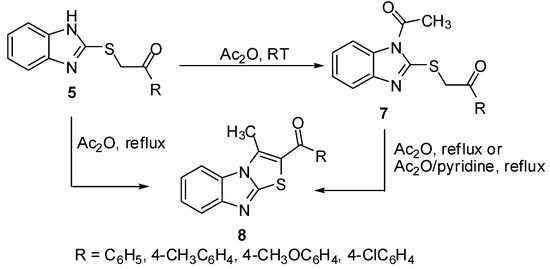
Scheme 2.
Cyclization of sulphides 5.
Scheme 2.
Cyclization of sulphides 5.
The reaction of 2-mercaptobenzimidazole (3) with aliphatic ketones 9a-f [11] such as acetone, acetylacetone, butanone, pentan-2-one and 1-phenylbutan-2-one using acidified acetic acid gave the corresponding thiazolo[3,2-a]benzimidazoles 10a-f in good yield. Alicyclic ketones 11a-d like cyclopentanone, cyclohexanone, 2-methylcyclohexanone and cycloheptanone were allowed to react with 2-mercaptobenzimidazole (3) under the same reaction conditions to obtain the tetracyclic compounds 12a-d [11] (Scheme 3).
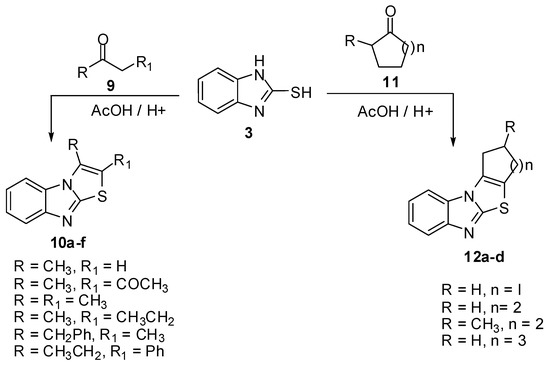
Scheme 3.
Reaction of 2-mercaptobenzimidazole (3) with ketones 9 and 11.
Scheme 3.
Reaction of 2-mercaptobenzimidazole (3) with ketones 9 and 11.
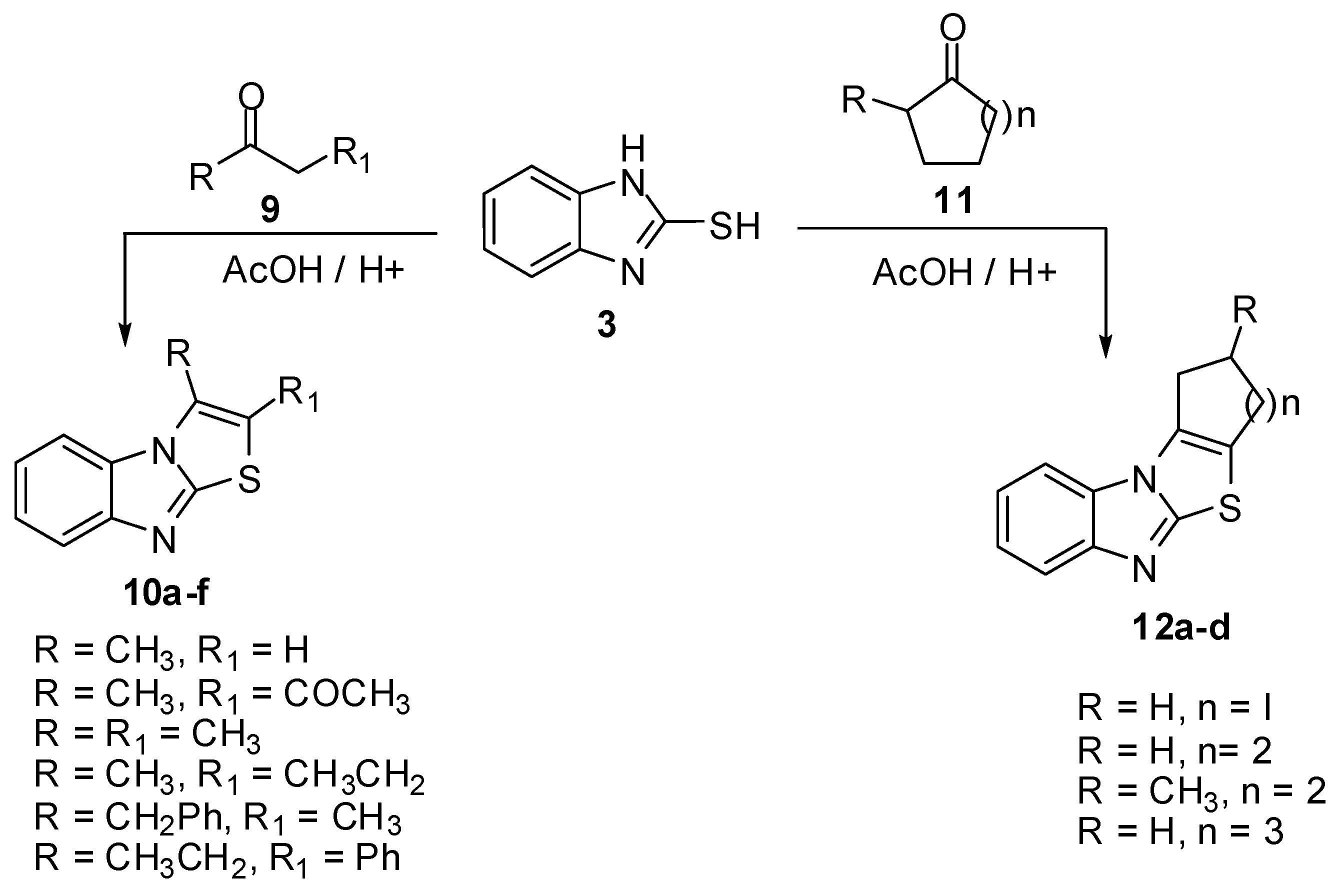
Thiazolo[3,2-a]benzimidazoles 2 and 16 [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] were obtained by the reaction of 2-mercaptobenzimidazoles 3 with various α-halo ketone derivatives 13 (14) which gave the corresponding acyclic intermediates 15. Cyclization of the latter by acetic anhydride/pyridine mixture, polyphosphoric acid or sodium ethoxide gave compounds 16 (Scheme 4). Cyclization of 5-substituted-(2-benzimidazolyl)thioacetic acid 15 (R1 = H, R2 = OH) led to the formation of two isomers with the substituent in 6 or 7 position as established through NMR analysis of the reaction products [25,27] (Scheme 4).
Scheme 4.
Reaction of 2-mercaptobenzimidazoles 3 with α-halo ketones 13.
Scheme 4.
Reaction of 2-mercaptobenzimidazoles 3 with α-halo ketones 13.
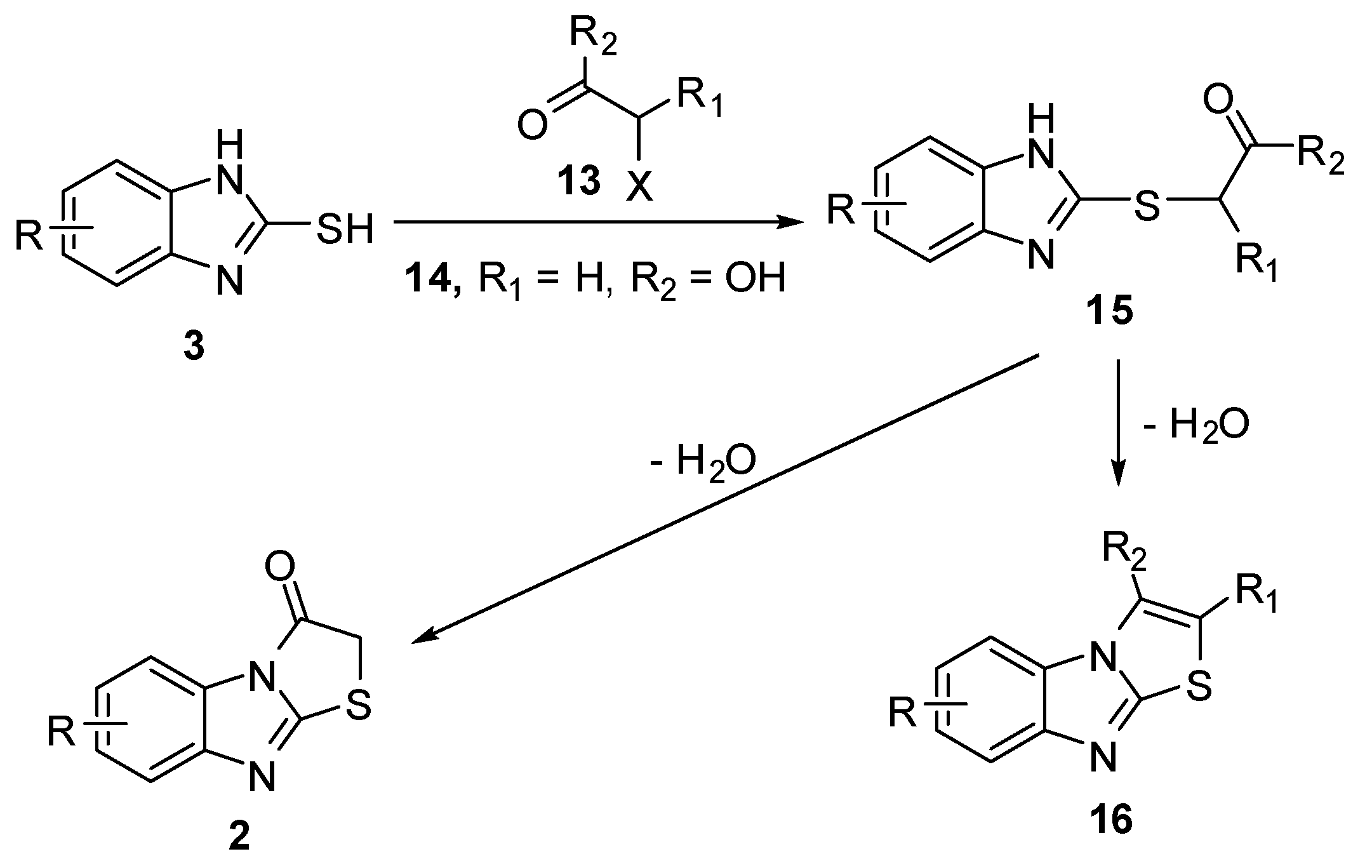
Interaction of 2-mercaptobenzimidazole (3) with 3-chloro-3-acetopropyl acetate (17) [28] with subsequent cyclization of the intermediate 3-acetyl-1-acetoxypropylmercaptobenzimidazole (18) via hydrobromic acid afforded 2-bromoethyl-3-methylthiazolo[3,2-a]benzimidazole 19 (Scheme 5).
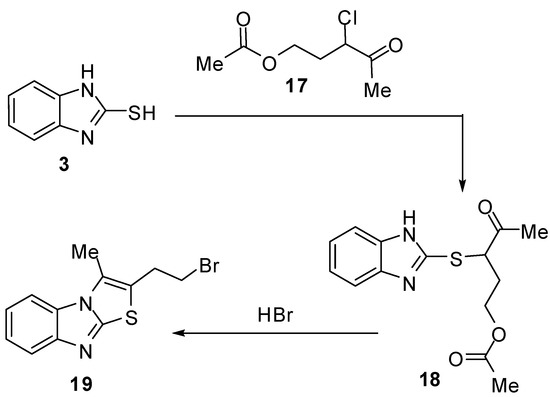
Scheme 5.
Reaction of 2-mercaptobenzimidazoles (3) with α-halo ketone 17.
Scheme 5.
Reaction of 2-mercaptobenzimidazoles (3) with α-halo ketone 17.
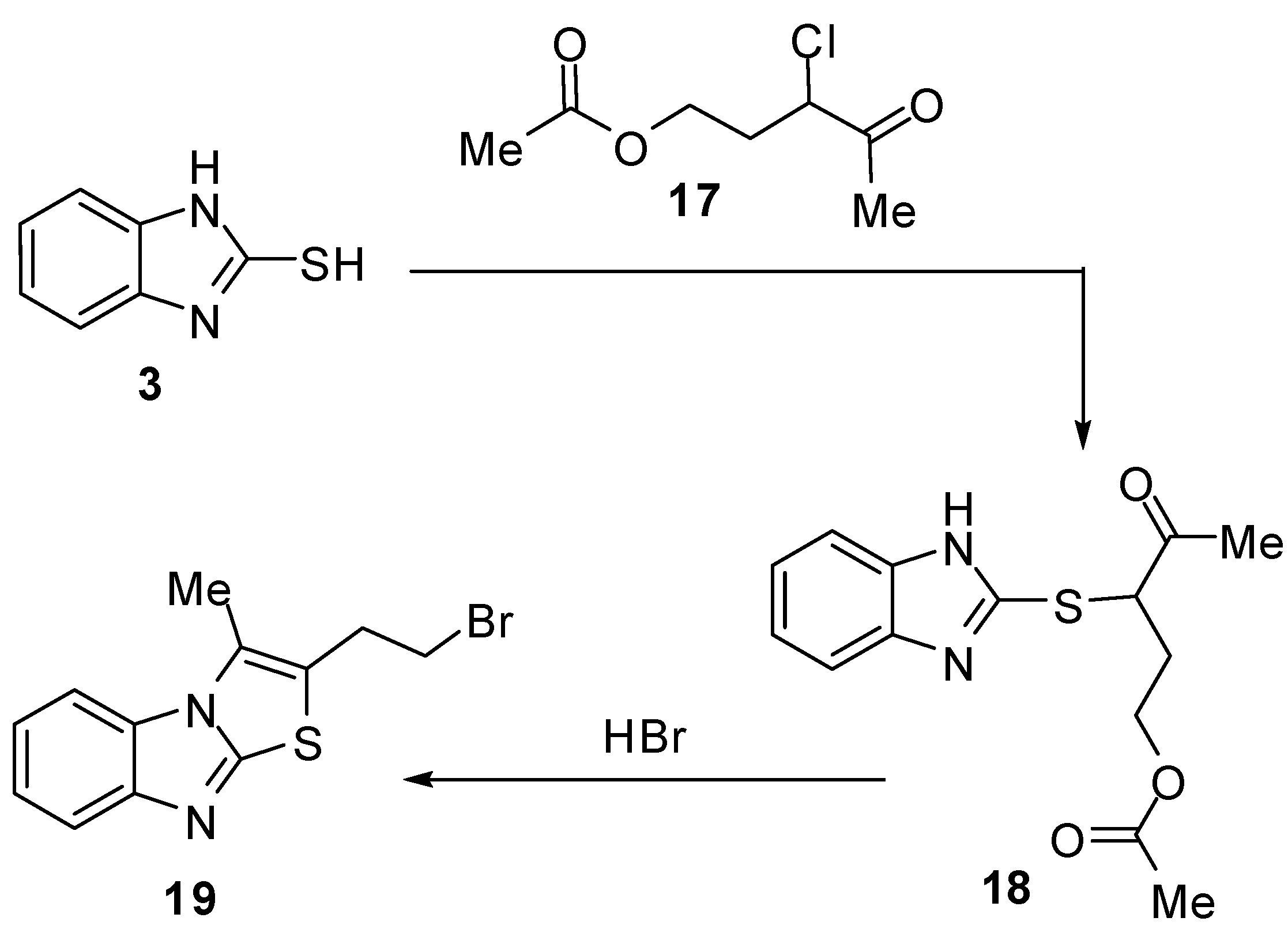
2-Hydrazonolthiazolo[3,2-a]benzimidazoles 22 and 23 [29,30,31,32,33] were prepared by the reaction of 2-mercaptobenzimidazole (3) with hydrazonyl halides 20 followed by cyclization of hydrazones 21(Scheme 6).
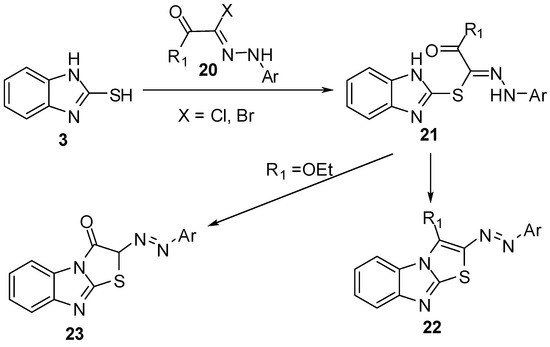
Scheme 6.
Reaction of 2-mercaptobenzimidazole (3) with hydrazonyl halides 20.
Scheme 6.
Reaction of 2-mercaptobenzimidazole (3) with hydrazonyl halides 20.
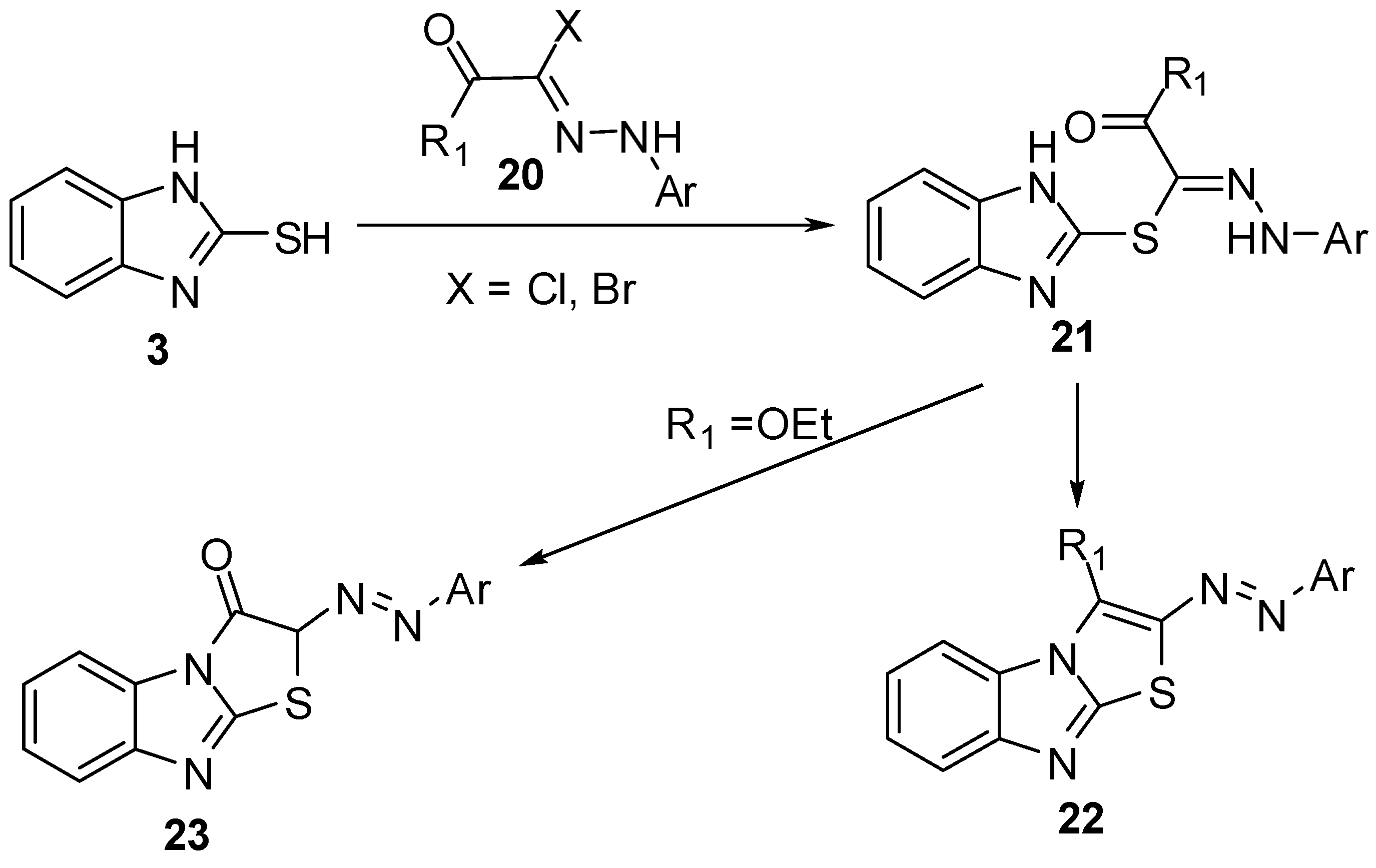
The reaction of 2-mercaptobenzimidazoles 3 with 1,2-dihaloethyl derivatives 24 [34,35,36] in the presence of basic reagents, affords 2-(β-haloethylthio)benzimidazole 25. Cyclization of the latter intermediate gives 2,3-dihydrothiazolo[3,2-a]benzimidazoles 26(Scheme 7).
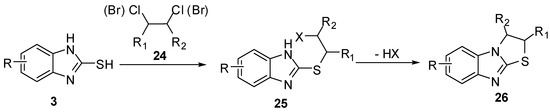
Scheme 7.
Reaction of 2-mercaptobenzimidazoles 3 with 1,2-dihaloethyl derivatives 24.
Scheme 7.
Reaction of 2-mercaptobenzimidazoles 3 with 1,2-dihaloethyl derivatives 24.
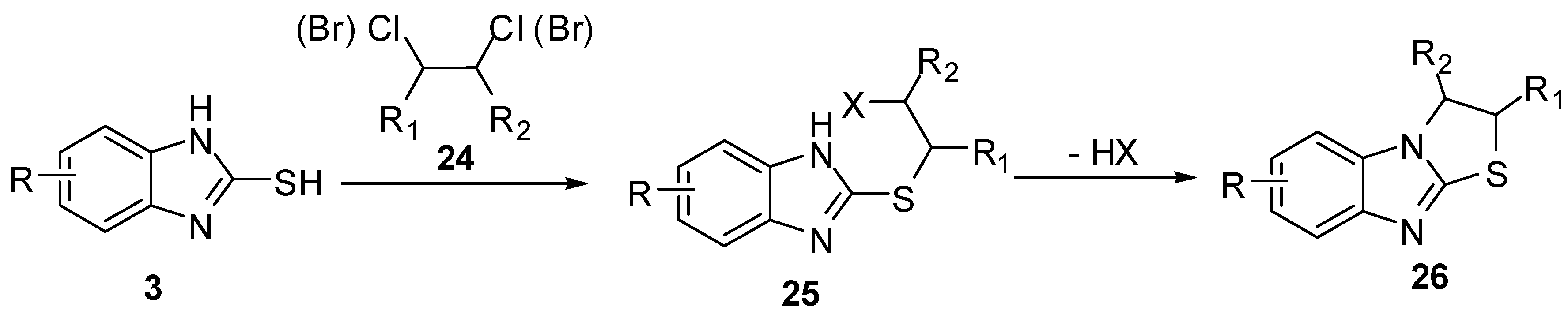
2,3-(Diaryl)hydrazono-2,3-dihydrothiazolo[3,2-a]benzimidazoles 28 [37,38] were synthesized via the reaction of bis-hydrazonoyl chlorides 27 with 2-mercaptobenzimidazole (3). Oxidation of the latter hydrazones resulted in the formation of 2,3-diazothiazolo[3,2-a]benzimidazoles 29 (Scheme 8).
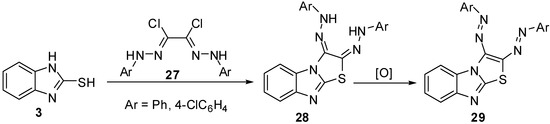
Scheme 8.
Reaction of 2-mercaptobenzimidazole (3) with bis-hydrazonoyl chlorides 27.
Scheme 8.
Reaction of 2-mercaptobenzimidazole (3) with bis-hydrazonoyl chlorides 27.
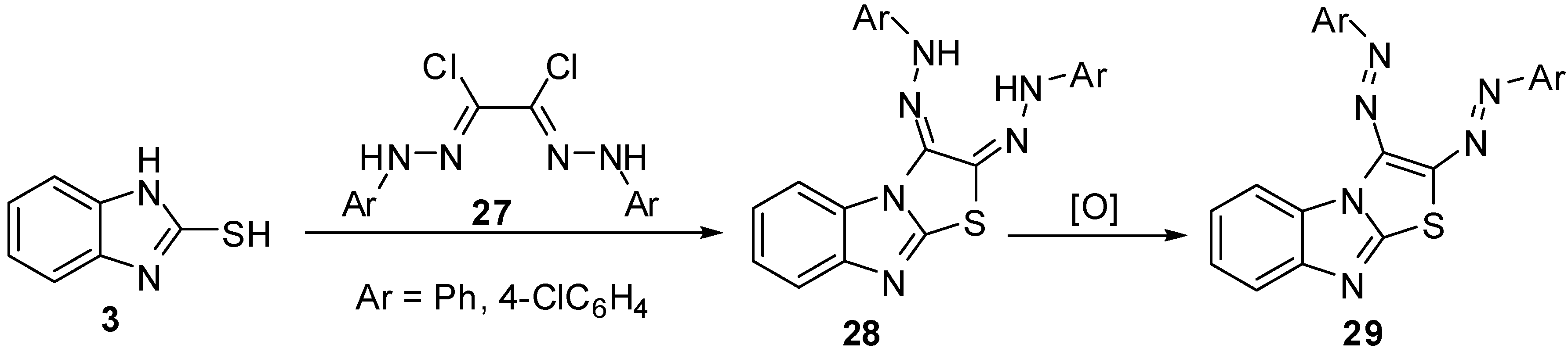
Additionally, arylsulphones 31 [39] were prepared by heterocyclization reaction of 1,2-dibromoethylsulfonyles 30 with 2-mercaptobenzimidazole (3) in presence of potassium hydroxide (Scheme 9).
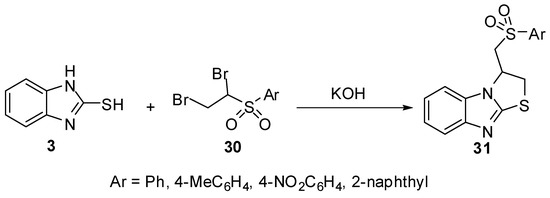
Scheme 9.
Reaction of 2-mercaptobenzimidazole (3) with 1,2-dibromoethylsulfonyles 30.
Scheme 9.
Reaction of 2-mercaptobenzimidazole (3) with 1,2-dibromoethylsulfonyles 30.
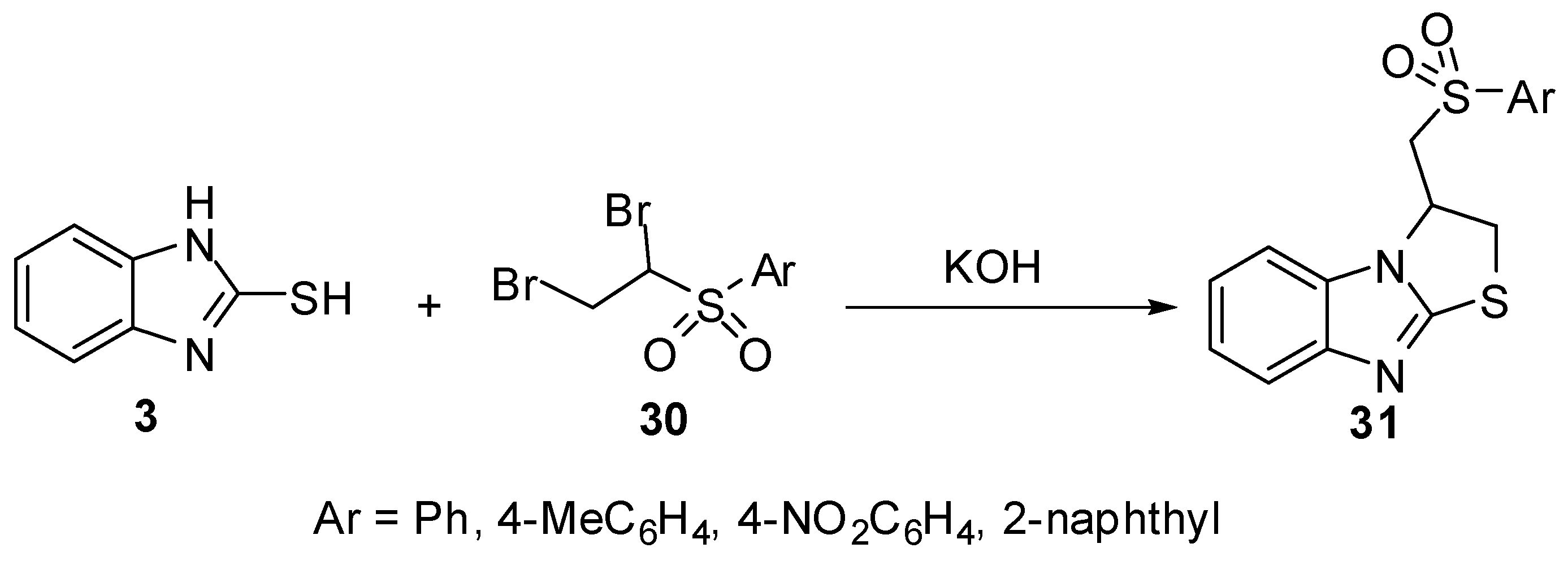
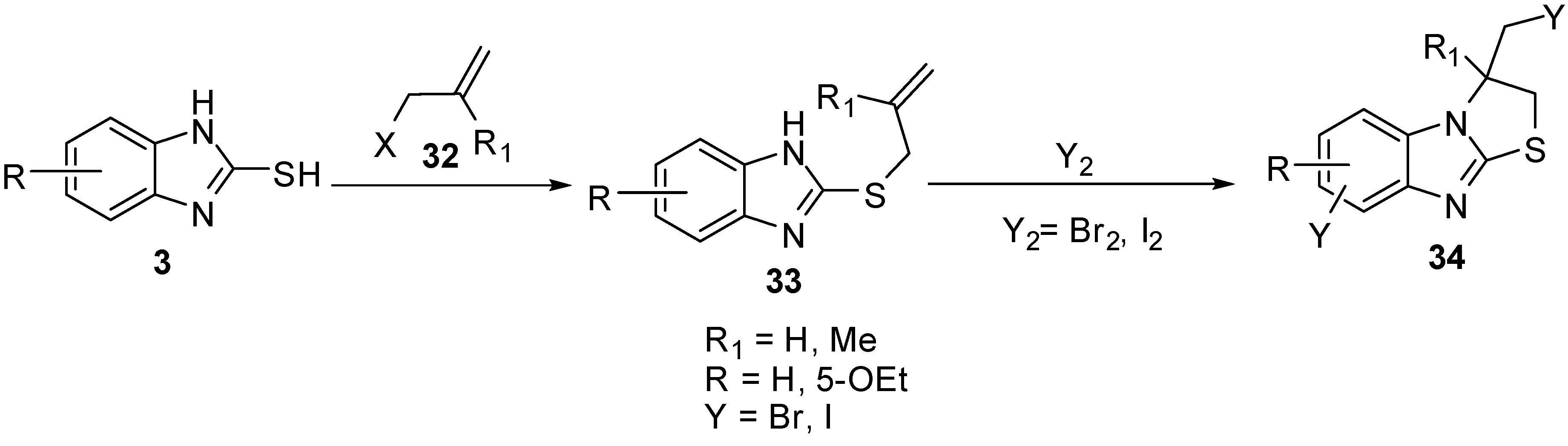
2-(Allylthio)-1H-benzimidazoles 33 were prepared by the reaction of 2-mercaptobenzimidazole (3) with allyl halides 32. Cyclization of 33 with iodine or bromine [40,45] gives 3-(halomethyl)-2,3-dihydro-3-methyl-thiazolo[3,2-a]benzimidazole derivatives 34(Scheme 10). The bromination of 33 5-ethoxy-2-alkenylthiobenzimidazole (R = -OEt) proceeds on the C6 site of benzimidazole ring in parallel with heterocyclization [45] (Scheme 10).
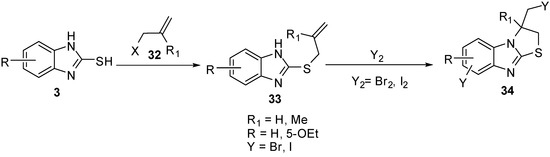
Scheme 10.
Reaction of 2-mercaptobenzimidazoles 3 with allyl halides 32.
Scheme 10.
Reaction of 2-mercaptobenzimidazoles 3 with allyl halides 32.
2-Mercaptobenzimidazole (3) was reacted with propargyl bromide (35) in refluxing EtOH in the presence of NH4OH to yield 2-propargylmercaptobenzimidazole (36) [46]. When 36 was treated in DMF with aryl halides and triethylamine in the presence of bis(triphenylphosphine)palladium chloride and copper iodide at room temperature, 3-benzylthiazolo[3,2-a]benzimidazoles 38 were obtained via the intermediate 37 (Scheme 11).
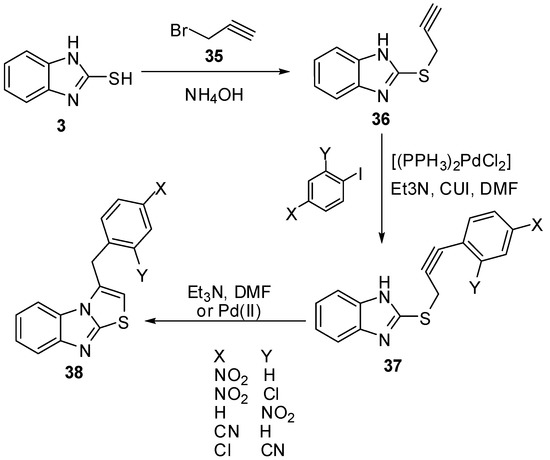
Scheme 11.
Reaction of 2-mercaptobenzimidazole (3) with propargyl bromide (35).
Scheme 11.
Reaction of 2-mercaptobenzimidazole (3) with propargyl bromide (35).
3-Aminothiazolo[3,2-a]benzimidazol-2-carbonitrile (41) was prepared by the reaction of 2-mercabtobenzimidaziole (3) with bromomalononitrile (39) in ethanol followed by cyclization reaction of product 40via anhydrous sodium acetate [11,47,48] (Scheme 12).
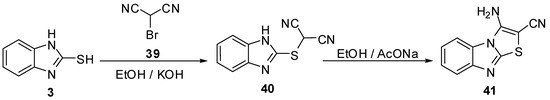
Scheme 12.
Reaction of 2-mercaptobenzimidazole (3) with bromomalononitrile (39).
Scheme 12.
Reaction of 2-mercaptobenzimidazole (3) with bromomalononitrile (39).
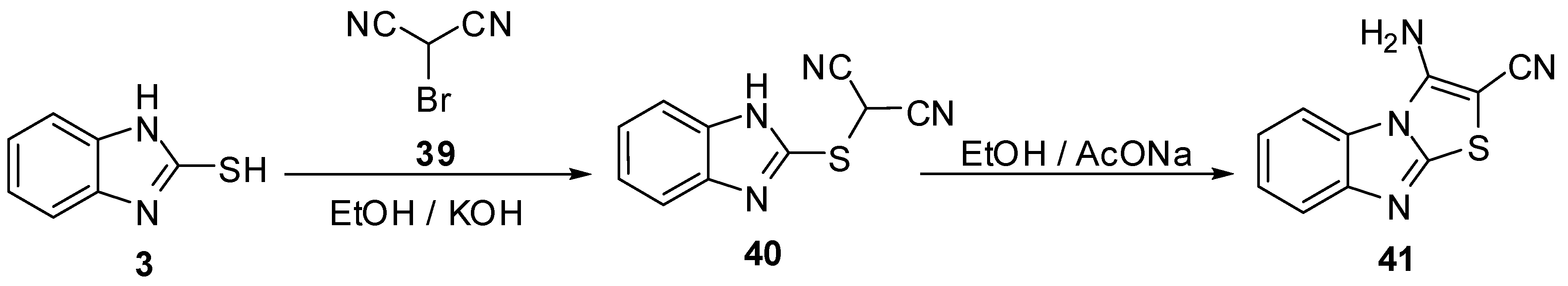
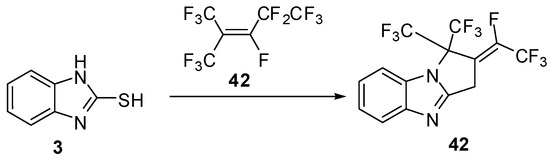
The reaction of 2-mercaptobenzimidazole (3) with fluoro ethylene derivative 42 [49] gives the functionally fluoro thiazolo[3,2-a]benzimidazole derivatives 43 (Scheme 13).
Scheme 13.
Reaction of 2-mercaptobenzimidazole (3) with fluoro ethylene derivative 42.
Scheme 13.
Reaction of 2-mercaptobenzimidazole (3) with fluoro ethylene derivative 42.
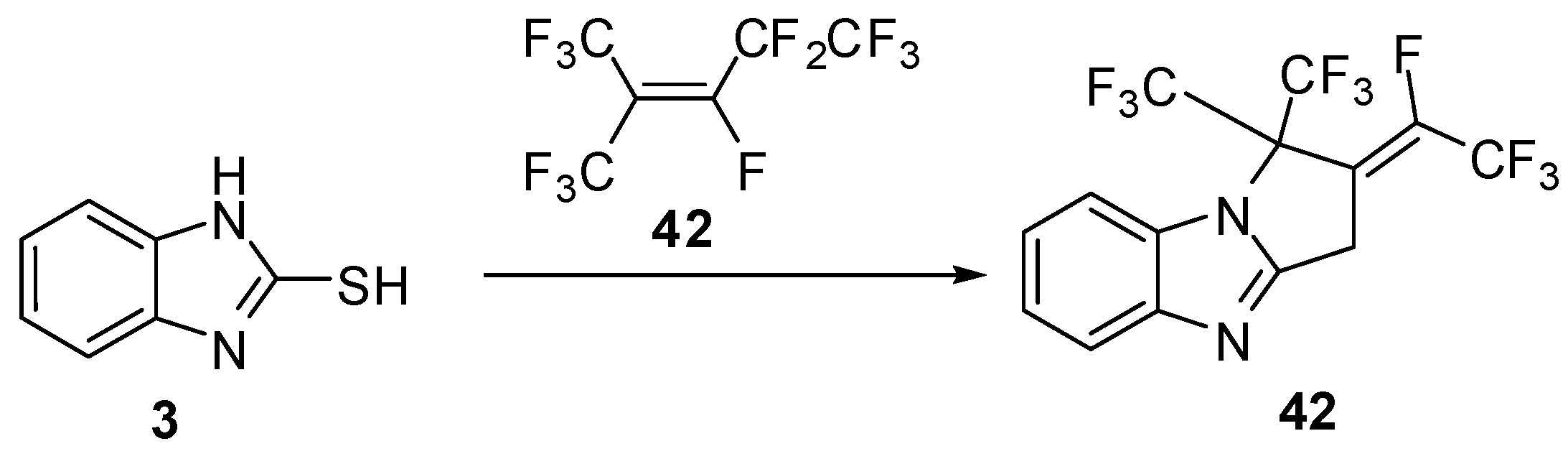
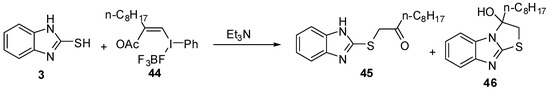
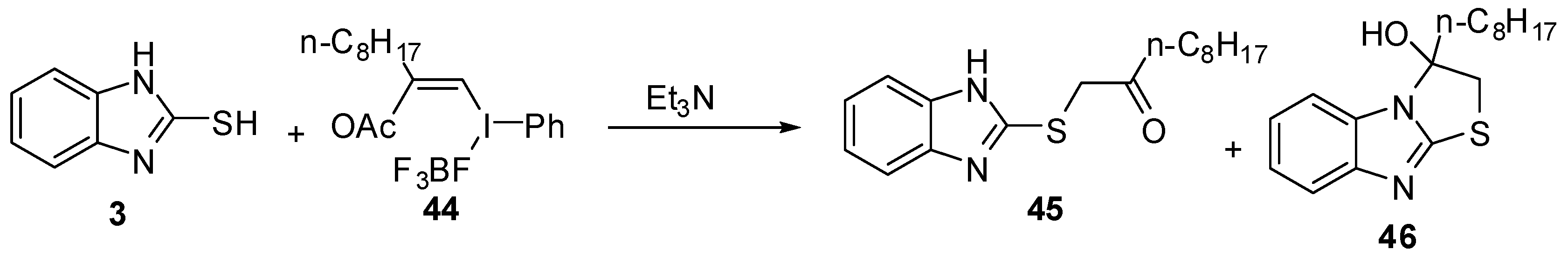
Recently, Masahito et al. [50] reported the reaction of 2-mercabtobenzimidazole (3) with (Z)-(2-acetoxy-1-decenyl)phenyl-λ3-iodanes (44) to afford the α-thio ketone 45 in equilibrium with the cyclized alcohol 46(Scheme 14).
Scheme 14.
Reaction of 2-mercaptobenzimidazole (3) with iodanes 44.
Scheme 14.
Reaction of 2-mercaptobenzimidazole (3) with iodanes 44.
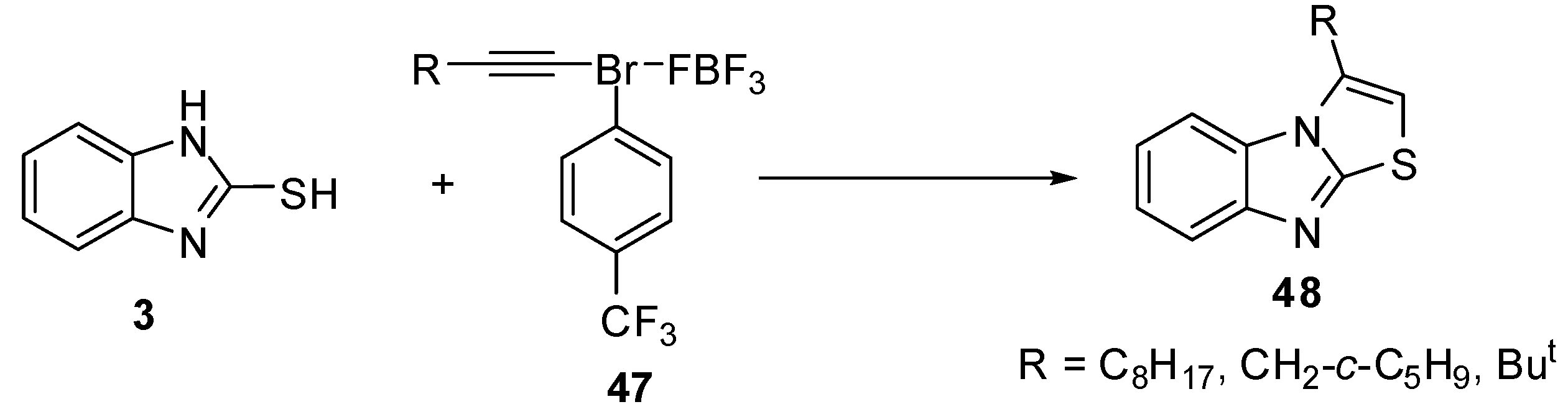
Exposure of 1-decynyl-λ3-bromane 47a to benzimidazole 3 resulted in the formation of 3-octylthiazolo[3,2-a]benzimidazole (48a). Similar results were obtained by the reactions of 3-(cyclopentyl)-1-propynyl 47b and 3,3-dimethyl-1-butynyl-3-bromane 47c to produce 3-(cyclopentylmethyl)- (48b) and 3-tert-butylthiazolo[3,2-a]benzimidazole (48c) [51] (Scheme 15).
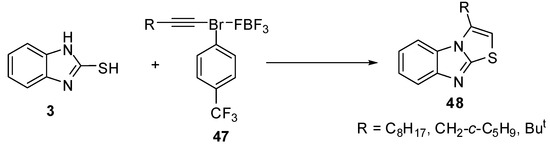
Scheme 15.
Reaction of 2-mercaptobenzimidazole (3) with bromanes 47.
Scheme 15.
Reaction of 2-mercaptobenzimidazole (3) with bromanes 47.
Moreover, the reaction of compound 3 with dimethyl acetylenedicarboxylate (49) gave 3-methoxy-4-oxo-4H-1-thia-4a,9-diaza-fluorene-2-carboxylic acid methyl ester (50) and benzo[4,5]imidazo[2,1-b]thiazole-2,3-dicarboxylic acid dimethyl ester (51) [52] as shown in Scheme 16.
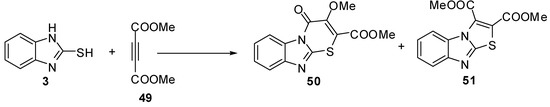
Scheme 16.
Reaction of 2-mercaptobenzimidazole (3) with dimethyl acetylenedicarboxylate (49).
Scheme 16.
Reaction of 2-mercaptobenzimidazole (3) with dimethyl acetylenedicarboxylate (49).
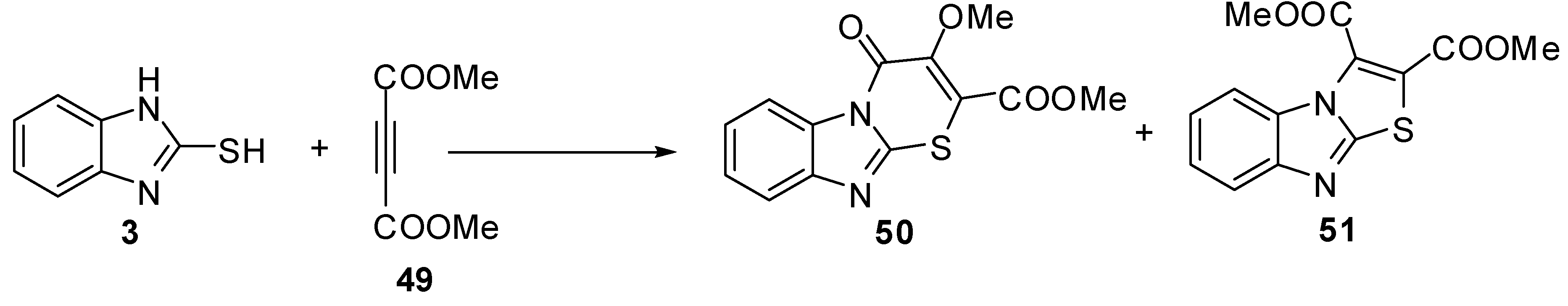
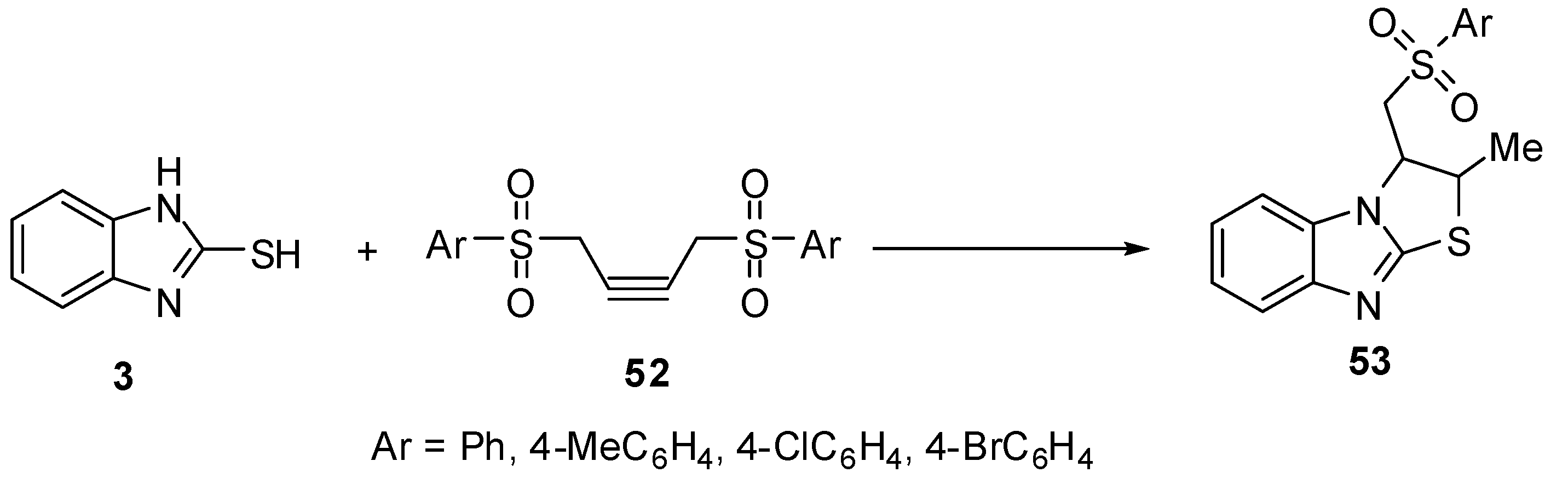
Treatment of 2-mercaptobenzimidazole (3) with 1,4-diarylsulfonyl-2-butynes 52 gives 2,3-dihydro-3-[(arylsulfonyl)methyl]-2-methylthiazolo[3,2-a]benzimidazoles 53 [53] (Scheme 17).
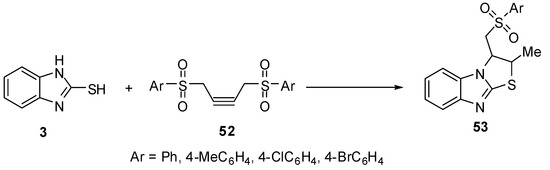
Scheme 17.
Reaction of 2-mercaptobenzimidazole (3) with 1,4-diarylsulfonyl-2-butynes 52.
Scheme 17.
Reaction of 2-mercaptobenzimidazole (3) with 1,4-diarylsulfonyl-2-butynes 52.
2-Arylidinethiazolo[3,2-a]benzimidazol-3(2H)-ones 55 were developed using a phosphine-catalyzed tandem addition and intramolecular cyclization of 2-mercaptobenzimidazole (3) on arylpropiolates 54 [54] (Scheme 18).
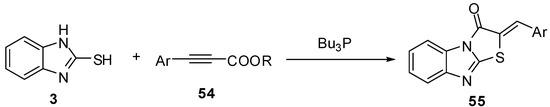
Scheme 18.
Reaction of 2-mercaptobenzimidazole (3) with arylpropiolates 54.
Scheme 18.
Reaction of 2-mercaptobenzimidazole (3) with arylpropiolates 54.
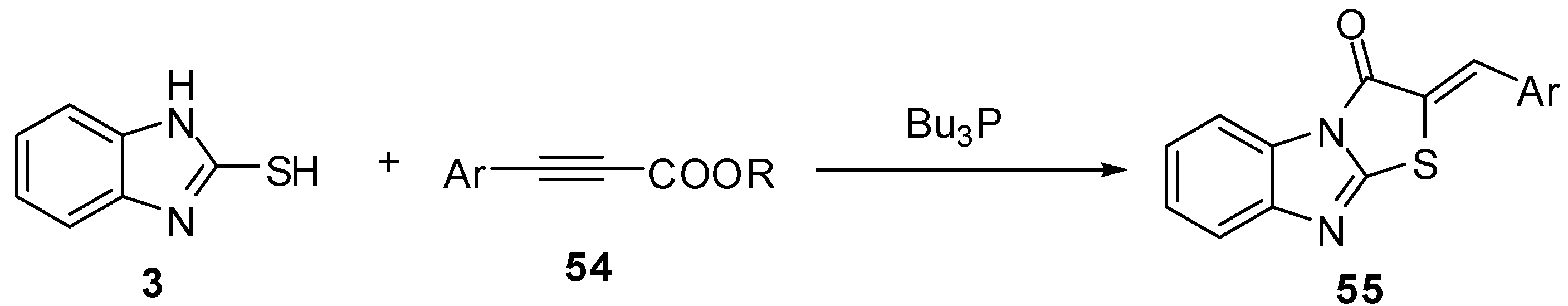
Treatment of compound 3 with the epoxyphosphorus derivative 56 [55] in ethanol gives isolable intermediate 57. Rearrangement of 2,3-dihydrothiazolo[3,2-a]benzimidazole 57 takes place in ethanol to give the acetyl derivative 58, while the reaction of compound 57 with triethyl orthoformate in acidic medium afforded the ethoxy derivative 59 (Scheme 19).
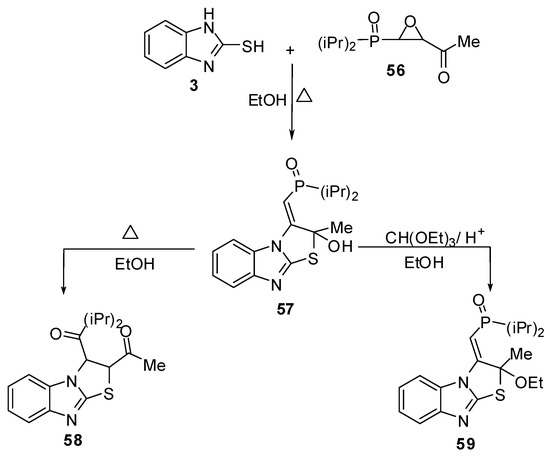
Scheme 19.
Reaction of 2-mercaptobenzimidazole (3) with epoxyphosphorus derivative 56.
Scheme 19.
Reaction of 2-mercaptobenzimidazole (3) with epoxyphosphorus derivative 56.
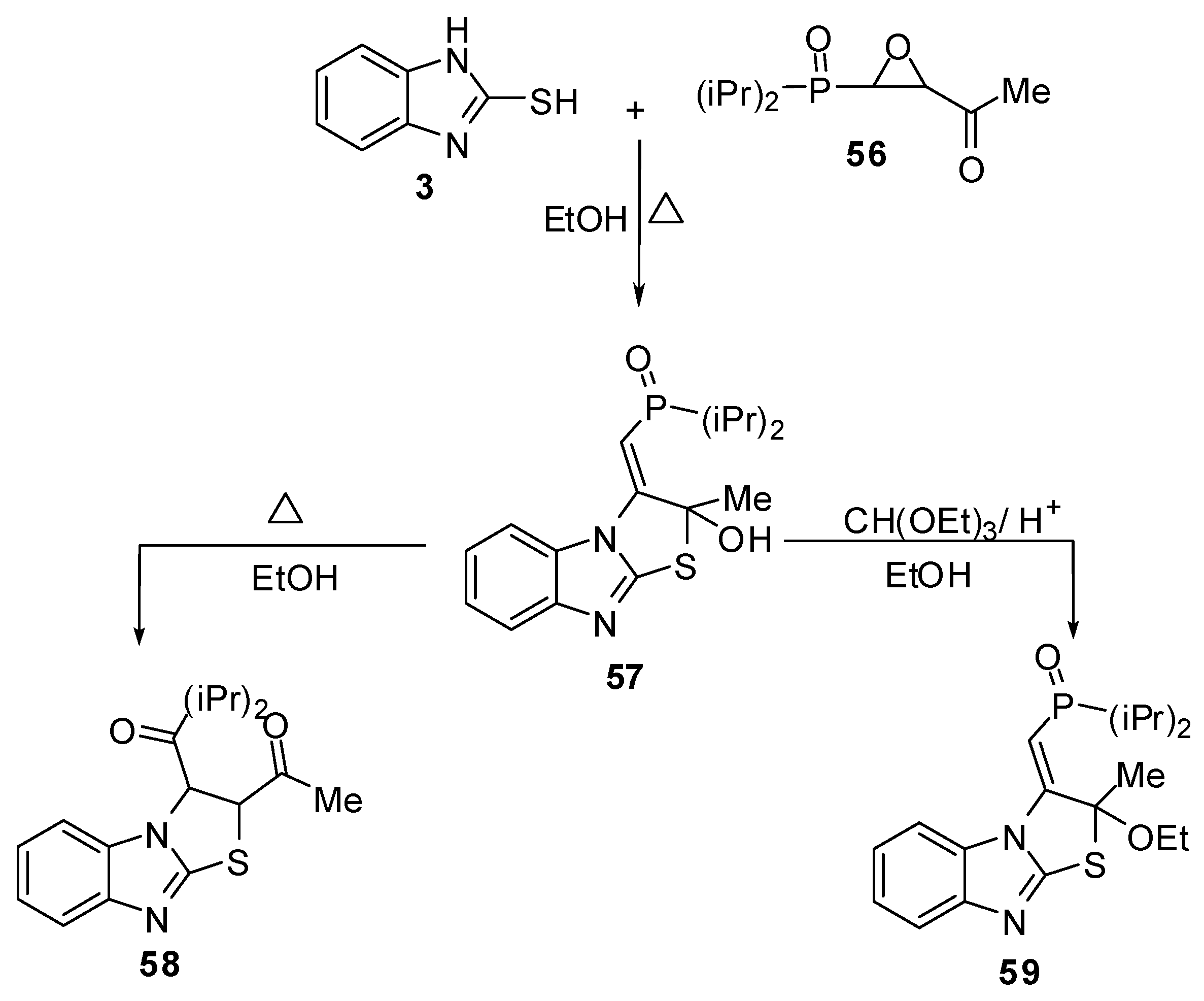
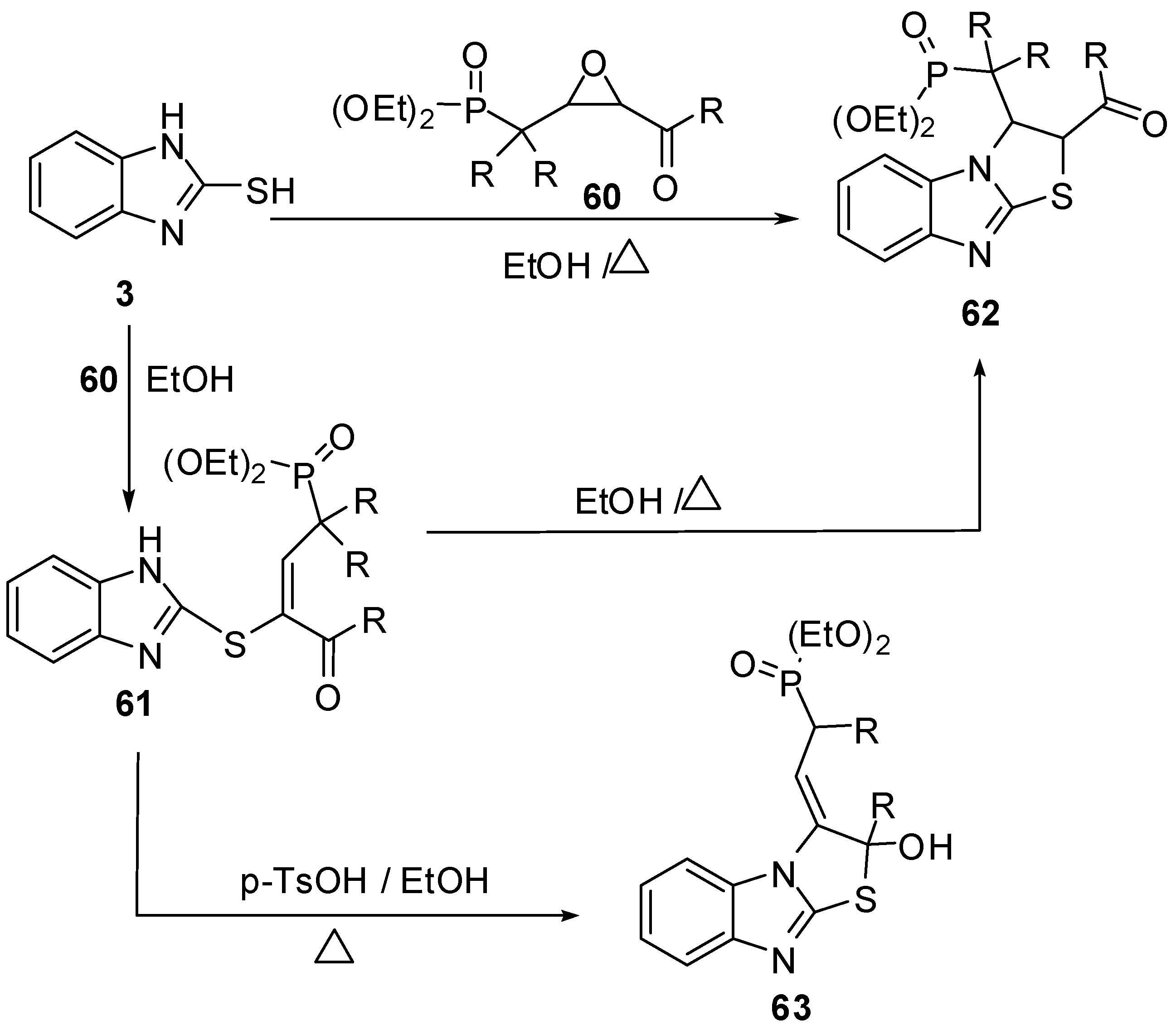
Furthermore, 2-mercaptobenzimidazole (3) reacts with epoxide derivative 60 to give compound 61 which cyclized in refluxing ethanol to give 2,3-dihydrothiazolo[3,2-a]benzimidazole derivative 62, or in the presence of p-toluenesulfonic acid to give compound 63. Compound 62 prepared directly by the reaction of compound 3 with epoxide 54 in refluxing ethanol [55] (Scheme 20).
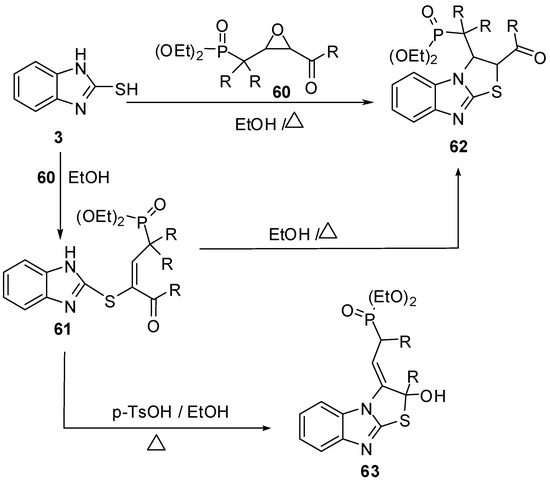
Scheme 20.
Reaction of 2-mercaptobenzimidazole (3) with epoxide derivative 60.
Scheme 20.
Reaction of 2-mercaptobenzimidazole (3) with epoxide derivative 60.
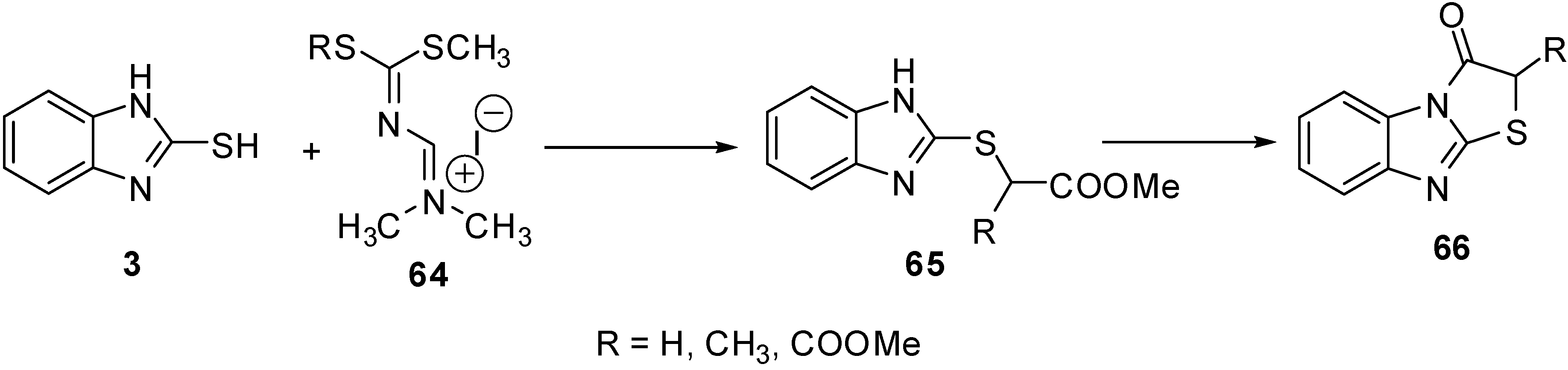
A series of 2-R-thiazolo[3,2-a]benzimidazol-3(2H)-ones 66 [56] were prepared from the intermediate 65. The latter intermediate was prepared by the reaction of amidinium salts 64 and 2-mercapto-benzimidazole (3) (Scheme 21).
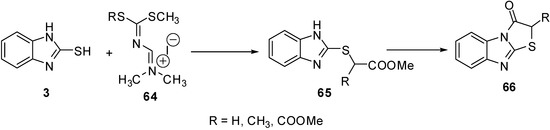
Scheme 21.
Reaction of 2-mercaptobenzimidazole (3) with amidinium salts 64.
Scheme 21.
Reaction of 2-mercaptobenzimidazole (3) with amidinium salts 64.
2.2. From 1-alkylbenzimidazoles
The interamolecular cyclization of 1-(dimethoxyethyl)-2-mercaptobenzimidazole derivative 67 [57] by diethyl ether-boron trifluoride 68 in dry methylene chloride furnished 2,3-dihydrothiazolo[3,2-a]benzimidazole derivative 69 (Scheme 22).
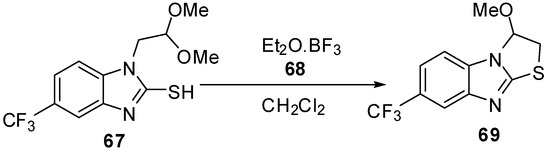
Scheme 22.
Reaction of compound 67 with diethyl ether-boron trifluoride 68.
Scheme 22.
Reaction of compound 67 with diethyl ether-boron trifluoride 68.
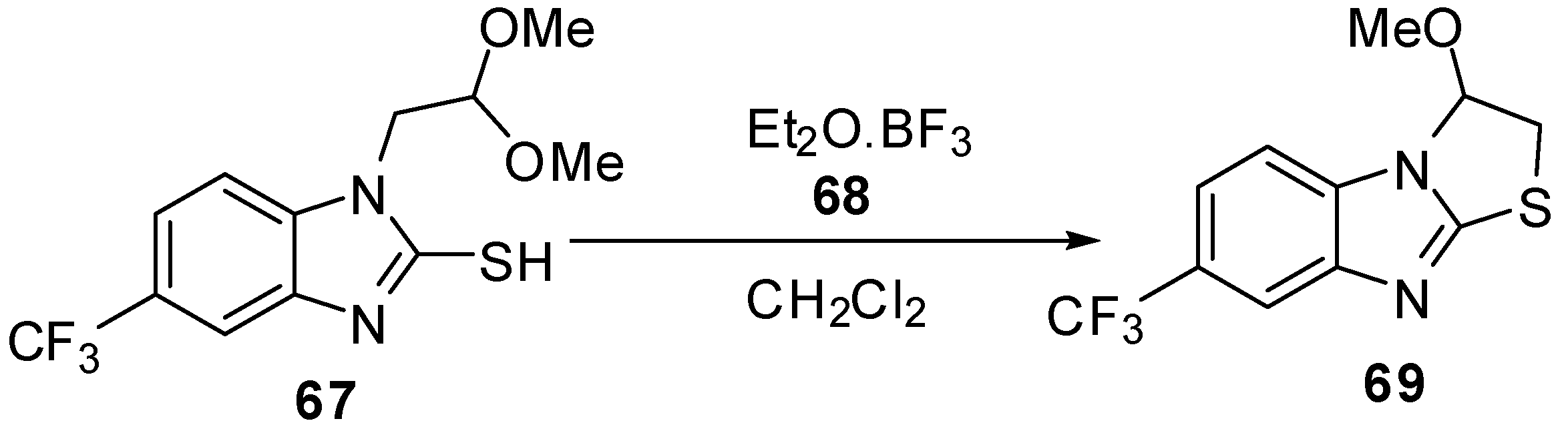
The cyclization reaction of compound 70 [58,59] gives 2,3-dihydro-3-(halomethyl)-3,9-dimethylthiazolo[3,2-a]benzimidazolium perchlorate derivatives 71 (Scheme 23).
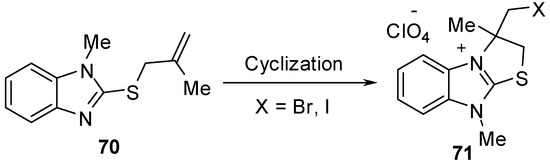
Scheme 23.
Cyclization of compound 70.
Scheme 23.
Cyclization of compound 70.
2.3. From 2-chlorobenzimidazoles
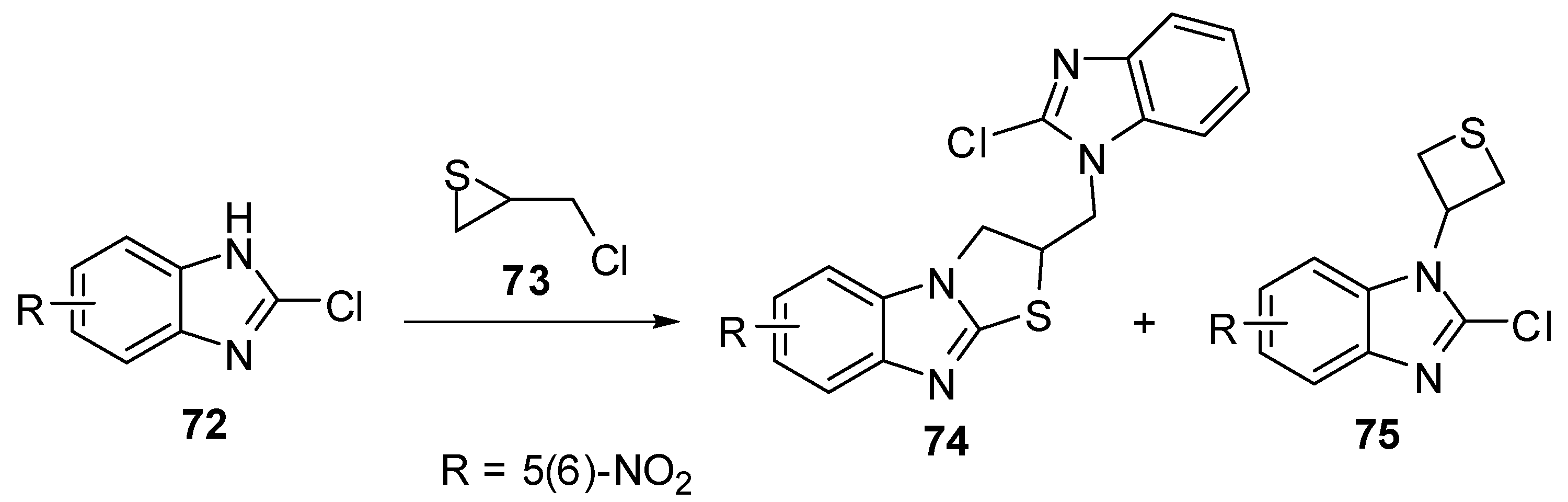
Treatment of 2-chloro-5(6)-nitrobenzimidazole 72 with (chloromethyl)thiirane (73) [60,61]afforded a mixture of 2,3-dihydro-2-[(2-chlorobenzimidazol-1-yl)methyl]thiazolo[3,2-a]-benzimidazoles 74 and 2-chloro-1-(3-thietanyl)benzimidazoles 75(Scheme 24).
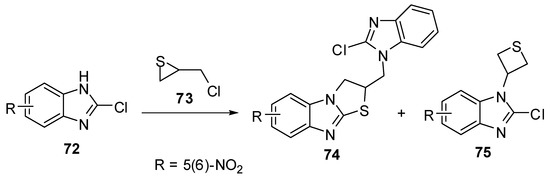
Scheme 24.
Reaction of benzimidazole 72 with (chloromethyl)thiirane (73).
Scheme 24.
Reaction of benzimidazole 72 with (chloromethyl)thiirane (73).
2.4. From 1,3-thiazoles
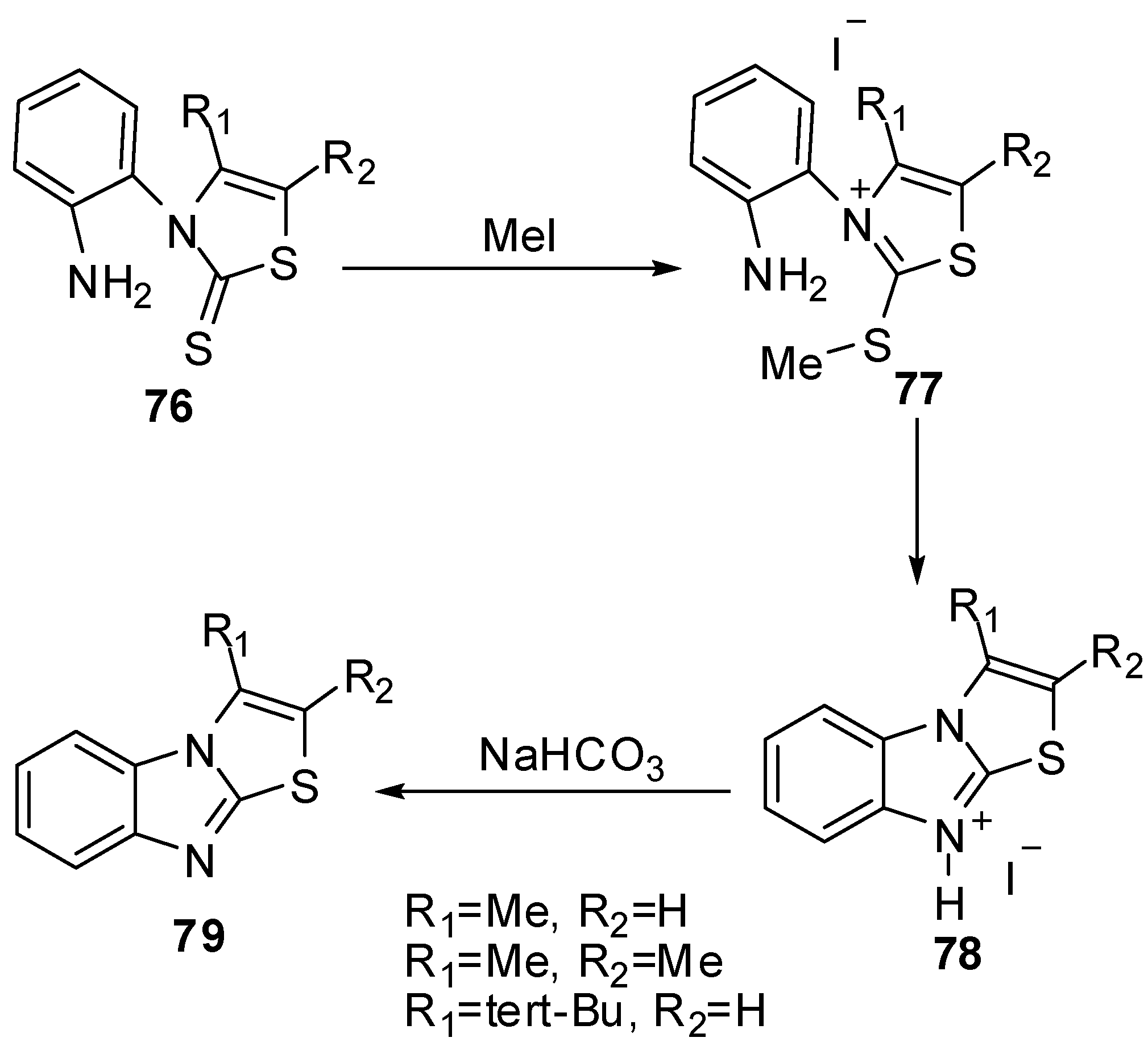
N-(2-Aminophenyl)-thiazoline-2-thione 76 reacted with methyl iodide in acetone at room temperature to afford quantitatively the thiazolium iodides 77 [62]. The latter thiazolium iodides were refluxed in methanol to afford thiazolo[3,2-a]benzimidazolium iodides 78 which treated with NaHCO3 to afford thiazolo[3,2-a]benzimidazole derivatives 79 (Scheme 25).
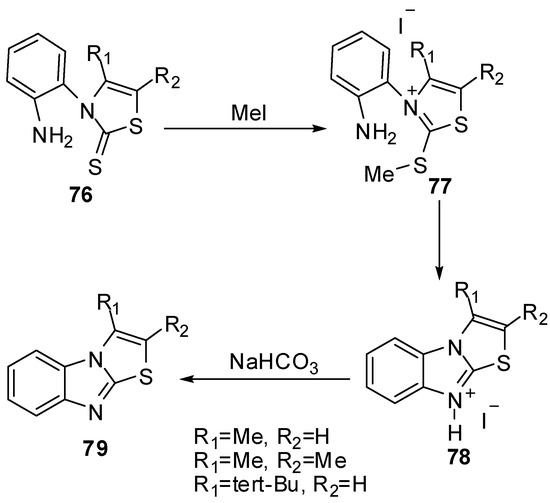
Scheme 25.
Reaction of thiazoline-2-thione 76 with methyl iodide.
Scheme 25.
Reaction of thiazoline-2-thione 76 with methyl iodide.
2.5. From other reagents
3-Aryl-2-N,N-dimethylaminothiazolo[3,2-a]benzimidazoles were prepared from diisothiocyanates [63], 2,3-dihydrothiazolo[3,2-a]benzimidazole of platinum amine complexes were also prepared [64]. Regioselective synthesis of 2-methoxy carbonyl-thiazolo[3,2-a]benzimidazole-6(7)-carboxylic acid were reported using crystallization induced region-isomerization [65].
3. Chemical Transformations
In this part, each sub-title was specified for the reaction(s) of certain atom and/or its substiuent(s) in thiazolo[3,2-a]benzimidazole ring system. Chemical Abstract numbering of thiazolo[3,2-a]benzimidazole atoms was considered.
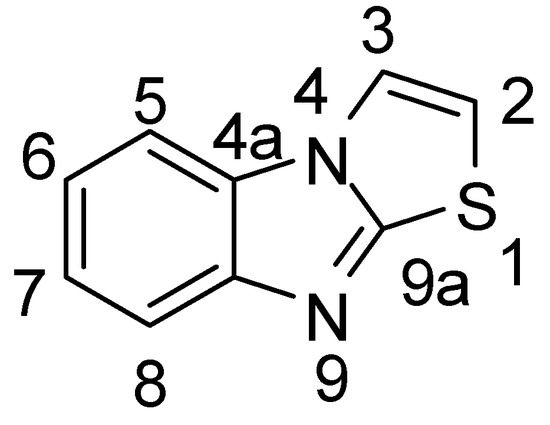
Figure 2.
Chemical Abstract numbering of thiazolo[3,2-a]benzimidazole atoms.
Figure 2.
Chemical Abstract numbering of thiazolo[3,2-a]benzimidazole atoms.
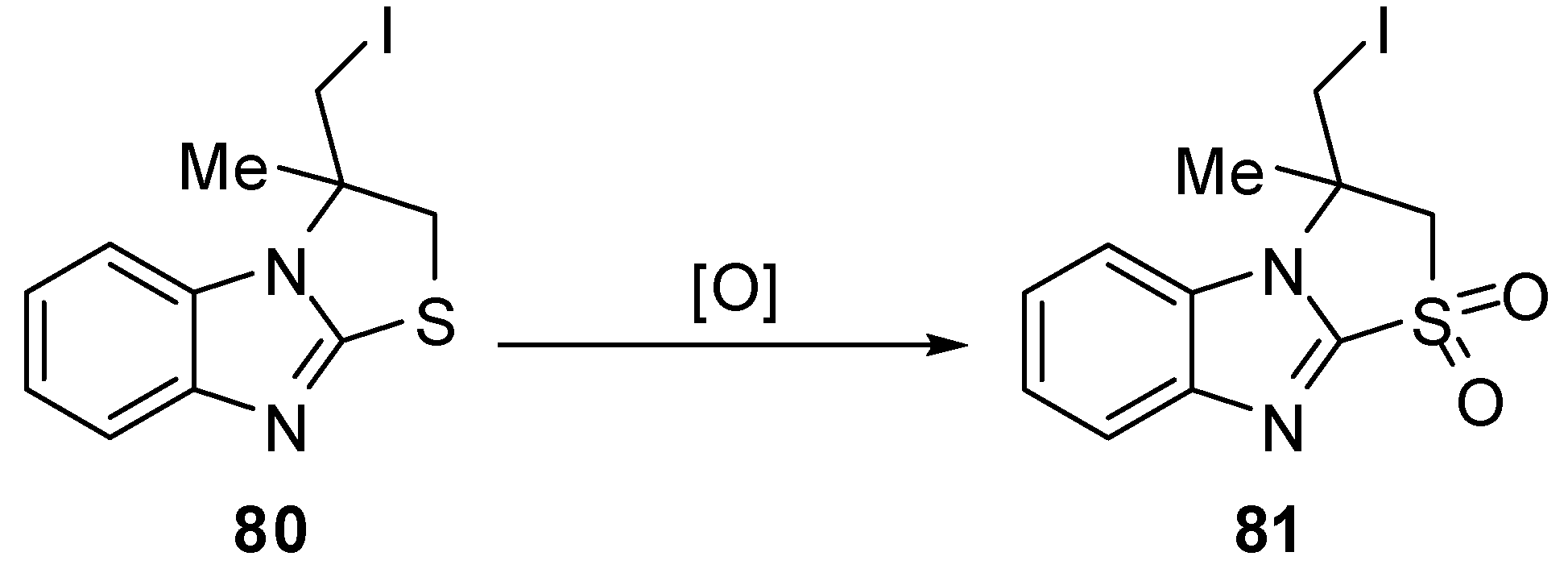
3.1. Reactions of S1
2,3-Dihydro-1,1-dioxthiazolo[3,2-a]benzimidazole 81 [66] was synthesized by oxidation of the corresponding thiazazolo[3,2-a]benzimidazole 80 with hydrogen peroxide in the presence of K2WO4 under mild conditions (Scheme 26).
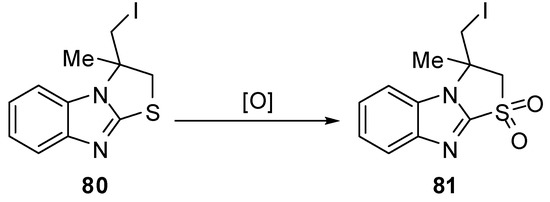
Scheme 26.
Oxidation of 2,3-dihydro-1,1-dioxthiazolo[3,2-a]benzimidazole 81.
Scheme 26.
Oxidation of 2,3-dihydro-1,1-dioxthiazolo[3,2-a]benzimidazole 81.
2-Benzimidazolylthioacetophenones 5 [11] were obtained when 3-aminothiazolo[3,2-a]-benzimidazole-2-carbonitrile (41) was allowed to react with aromatic ketones 4 using acidified acetic acid (Scheme 27).
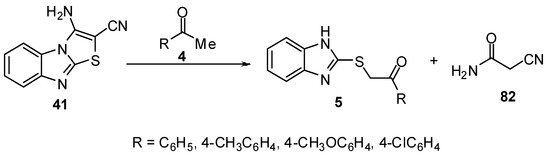
Scheme 27.
Reaction of 3-aminothiazolo[3,2-a]-benzimidazole-2-carbonitrile (41) with ketones 4.
Scheme 27.
Reaction of 3-aminothiazolo[3,2-a]-benzimidazole-2-carbonitrile (41) with ketones 4.
3.2. Reactions of S1-C1
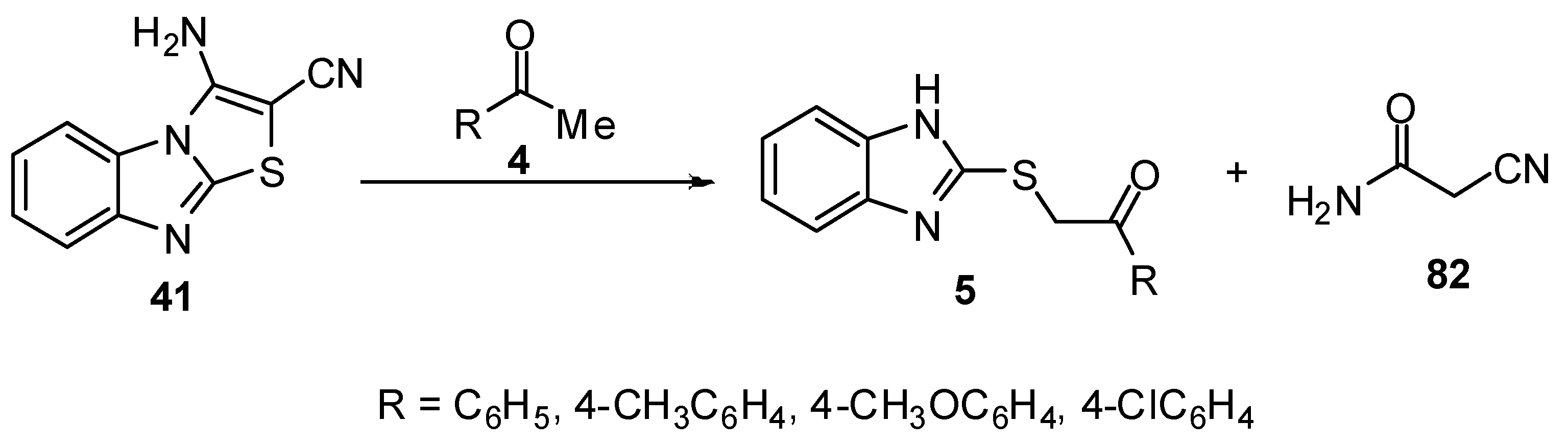
Microwave irradiation has been applied for a rapid and efficient synthesis of 2-arylidene-thiazolo[3,2-a]benzimidazol-3(2H)-ones 55 from 2-mercapto-1H-benzimidazole (3) [67]. Abdel-Aziz et al. reported the reaction of nitrilimine 83 with 2-arylidenethiazolo[3,2-a]benzimidazol-3(2H)-one 55 to afford pyrazoles 84. Spectroscopic analyses confirmed the regioselective 1,3-dipolar cycloaddition of the nitrilimine 83 to the exocyclic double bond of 55 to afford non-isolable spiro intermediate 84 which rearranged to the corresponding pyrazolylbenzimidazole derivatives 86 as shown in Scheme 28 [67].
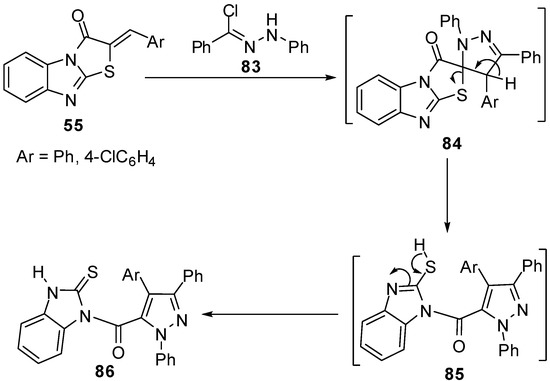
Scheme 28.
Reaction of 2-arylidenethiazolo[3,2-a]benzimidazol-3(2H)-one 55 with nitrilimine 83.
Scheme 28.
Reaction of 2-arylidenethiazolo[3,2-a]benzimidazol-3(2H)-one 55 with nitrilimine 83.
3.3. Reactions of S1-C9a
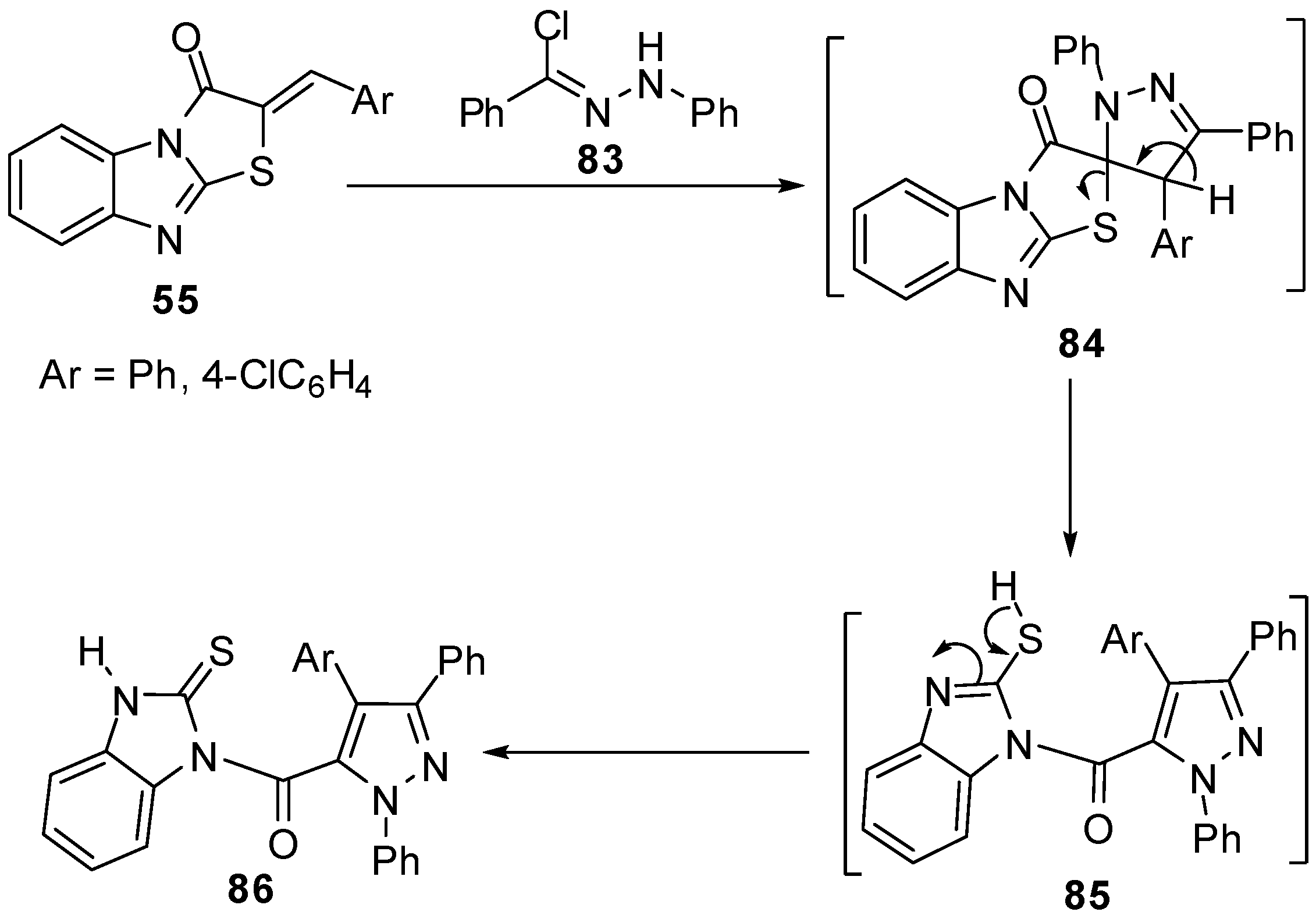
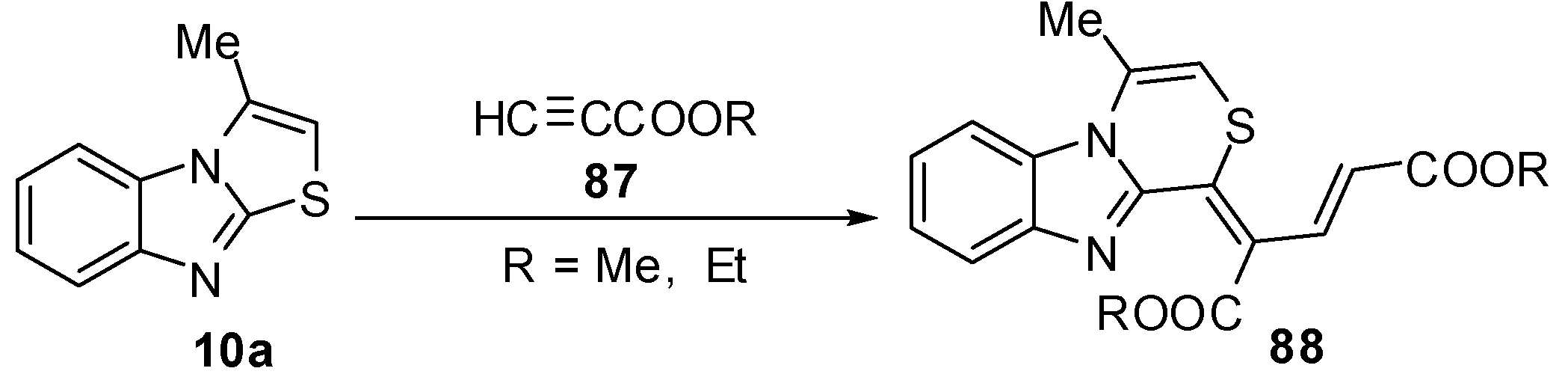
Abe et al. reported a re-investigation for the reaction of 3-methylthiazolo[3,2-a]benzimidazole (10a)with propiolic esteres 87 [68] which gave the thiazino[4,3-a]-benzimidazole rearrangement product derivative 88 (Scheme 29).
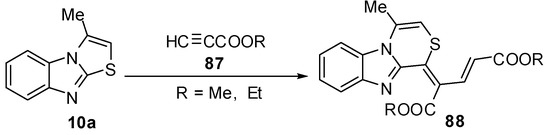
Scheme 29.
Reaction of 3-methylthiazolo[3,2-a]benzimidazole (10a) with propiolic esteres 87.
Scheme 29.
Reaction of 3-methylthiazolo[3,2-a]benzimidazole (10a) with propiolic esteres 87.
3.4. Reactions of C2
3.4.1. Reactions of 3-methylthiazolo[3,2-a]benzimidazole (10a)
Mannich reaction of 3-methylthiazolo[3,2-a]benzimidazole 10a with some secondary amines and paraformaldehyde in aqueous HCl gave the corresponding Mannich bases 89 [69] (Scheme 30).
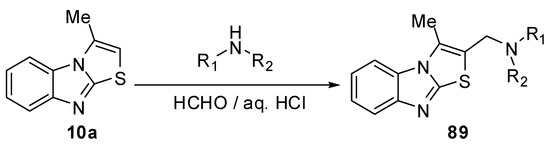
Scheme 30.
Mannich reaction of 3-methylthiazolo[3,2-a]benzimidazole 10a.
Scheme 30.
Mannich reaction of 3-methylthiazolo[3,2-a]benzimidazole 10a.
3.4.2. Reactions of 1-(3-methylthiazolo[3,2-a]benzimidazol-2-yl)ethanone (10b)
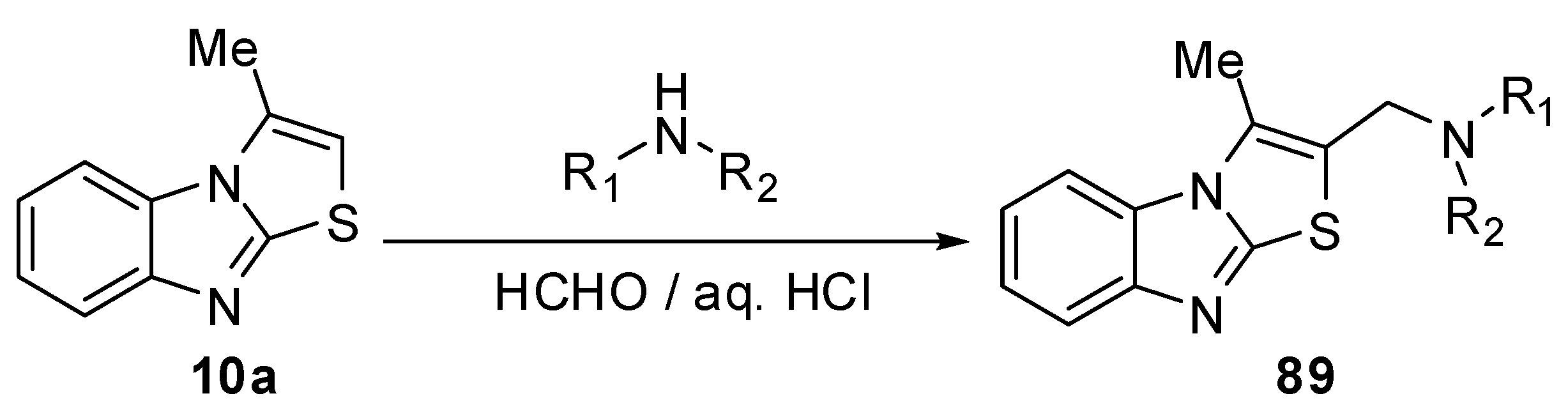
1-(3-Methylthiazolo[3,2-a]benzimidazol-2-yl)ethanone (10b) reacts with the aniline diazonium chlorideto afford 3-methyl-2-(phenylazo)thiazolo[3,2-a]benzimidazole (90) [13] (Scheme 31).
Scheme 31.
Reactions of ethanone derivative 10b.
Scheme 31.
Reactions of ethanone derivative 10b.
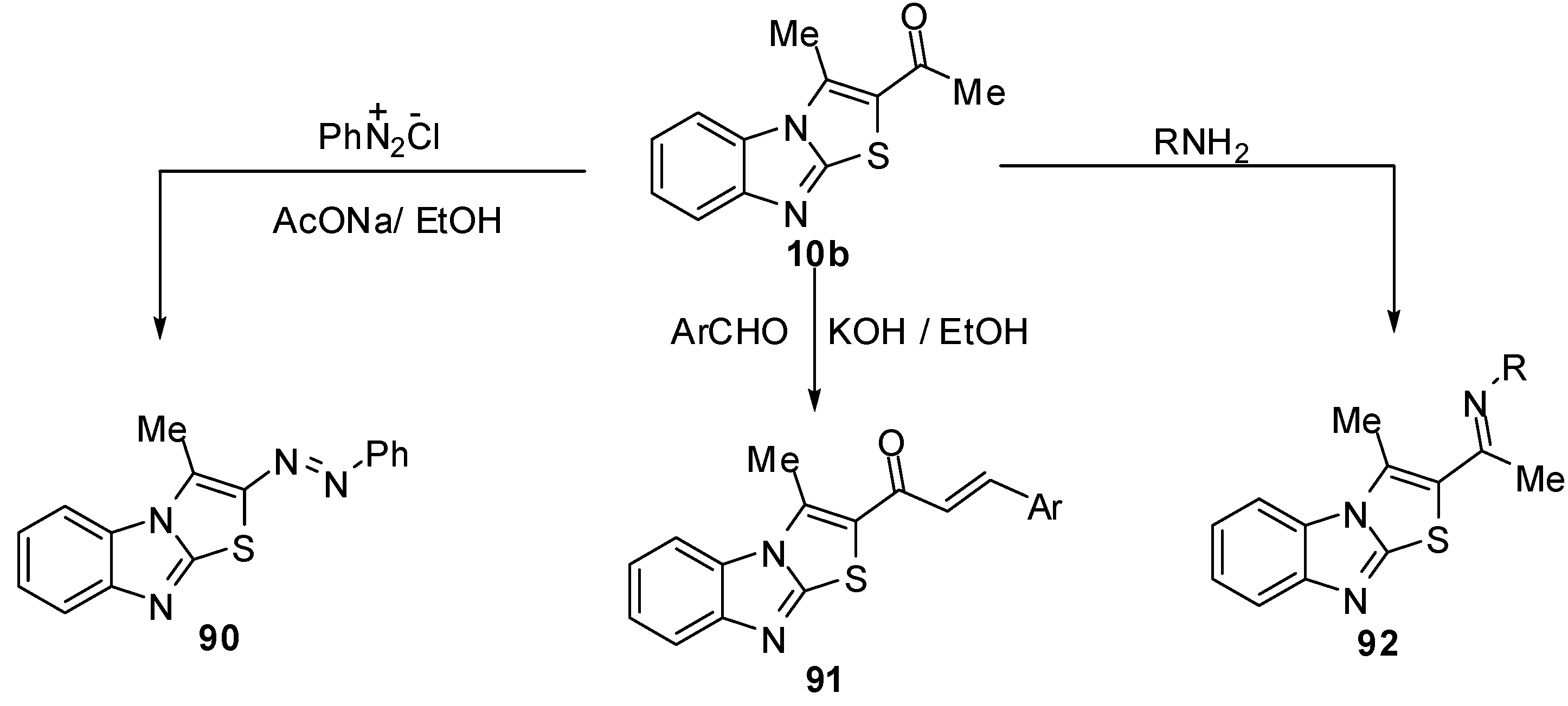
Moreover, condensation of 10b with some aromatic aldehydes in ethanolic potassium hydroxide gave the corresponding 2-(3-aryl-1-oxo-2-propenyl)-3-methylthiazolo[3,2-a]benzimidazole 91. The reactions of ethanone 10b with hydroxylamine, methyl amine or ethyl amine were reported [13] (Scheme 31).
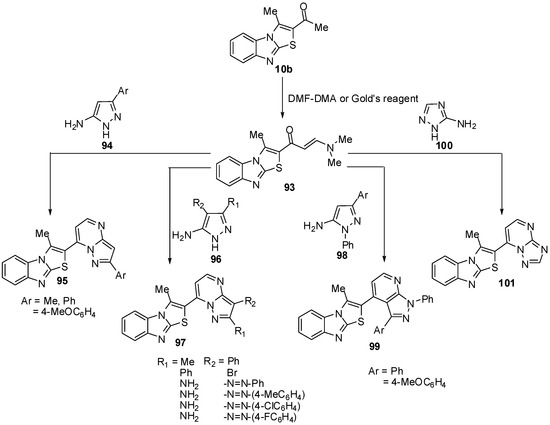
1-(3-Methylthiazolo[3,2-a]benzimidazol-2-yl)ethanone (10b) was treated with dimethylformamide-dimethylacetal (DMF-DMA), in dry xylene, at reflux temperature, it afforded E-3-(N,N-dimethylamino)-1-(3-methylthiazolo[3,2-a]benzimidazol-2-yl)prop-2-en-1-one (93) [70] (Scheme 32). Recently, Abdel-Aziz et al. reported an alternative synthesis of compound 93 using [3-(dimethyl-amino)-2-azaprop-2-en-1-ylidene]dimethylammonium chloride (Gold’s reagent) [71] where Gold's reagent reacted with ketone 10b in sodium methoxide to produce the enaminone 93 (Scheme 32). The reaction of compound 93 with aminopyrazoles 94 in refluxing pyridine [70] or in acetic acid presence of H2SO4 [71] afforded pyrazolo[1,5-a]pyrimidines 95.
Scheme 32.
Reaction of ethanone 10b with DMF-DMA.
Scheme 32.
Reaction of ethanone 10b with DMF-DMA.
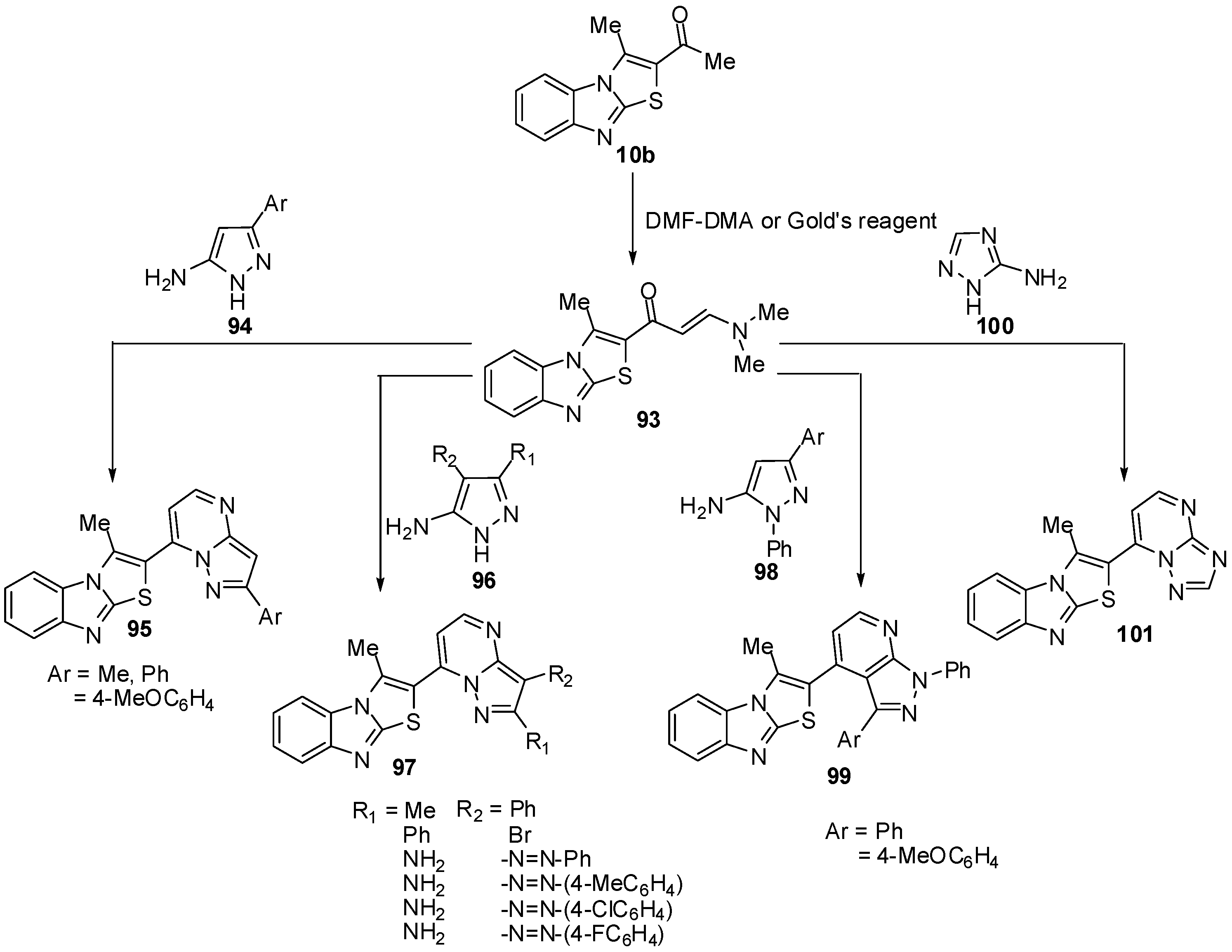
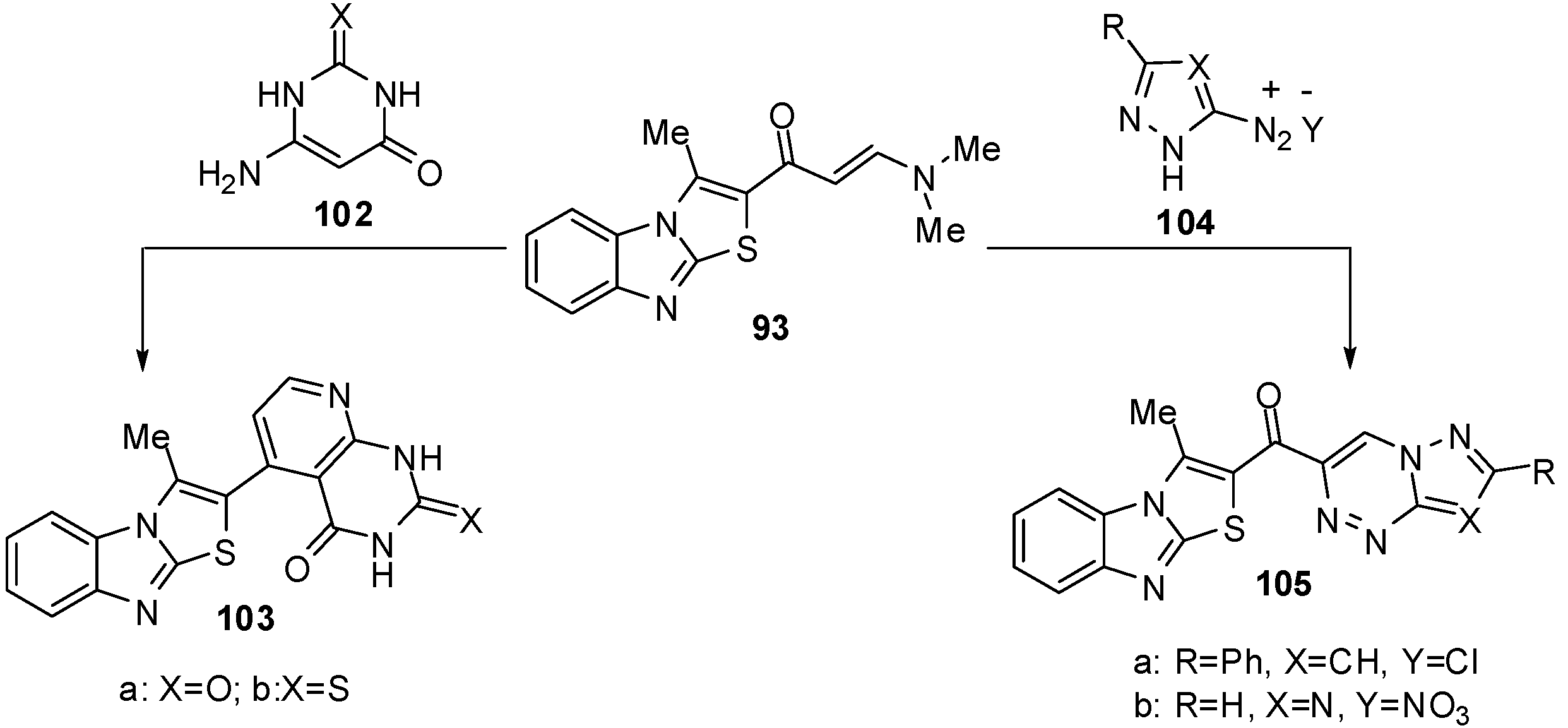
In a similar manner, treatment of compound 93 with 5-amino-3-methyl-4-phenyl-1H-pyrazole (96a), 4-bromo-5-amino-3-phenyl-1H-pyrazole (96b) or 4-(arylhydrazono)-3,5-diamino-1H-pyrazole 96c-f resulted in the formation of pyrazolo[1,5-a]pyrimidines 97a, 97b and 97c-f, respectively [71] (Scheme 32). Next, the reaction of enaminone 93 with 5-amino-3-aryl-1-phenylpyrazole 98a,b in refluxing glacial acetic acid, in the presence of sulphuric acid, yielded the corresponding pyrazolo[3,4-b]pyridine derivatives 99a,b (Scheme 32). When compound 93 was treated with 3-amino-1,2,4-(1H)-triazole (100) in refluxing pyridine, it furnished the 1,2,4-triazolo[1,5-a]pyrimidine derivative 101 (Scheme 32) [70].
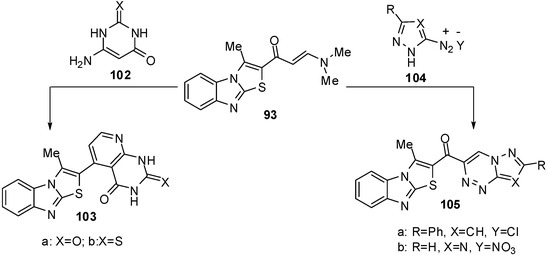
Furthermore, the reaction of enaminone 93 with 6-amino-1H-pyrimidin-2,4-dione (102a) or 6-amino-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one (102b) in refluxing acetic acid resulted in the formation of pyrido[2,3-d]pyrimidines 103a and 103b (Scheme 33). The enaminone 93 treated with the diazonium salt of 3-phenyl-5-amino-1H-pyrazole 104a or 5-amino-l,2,4-(1H)-triazole 104b to afford non-isolable azo-coupling intermediates which cyclized via dimethylamine elimination yielded the pyrazolo[5,1-c]-l,2,4-triazine and l,2,4-triazolo[5,1-c]-l,2,4-triazine derivatives 105a and 105b (Scheme 33) [70].
Scheme 33.
Reactions of enaminone 93.
Scheme 33.
Reactions of enaminone 93.
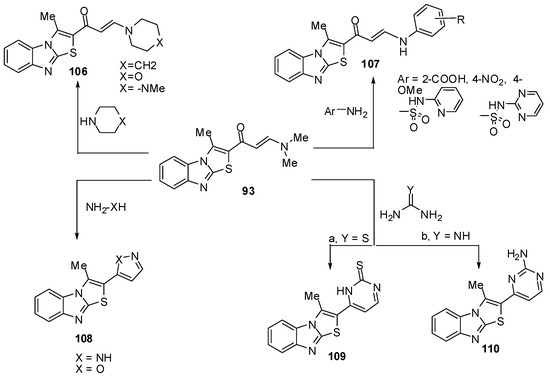
The enaminone 93 treated with some secondary amines like piperidine, morpholine or 1-methylpiperazine in refluxing ethanol to afford the corresponding tertiary amines 106, respectively (Scheme 34) while treatment of 93 with anilines, sulphapyridine and sulphapyrimidine in refluxing acetic acid gave the corresponding acyclic secondary amine derivatives 107 [72] (Scheme 34). The enaminone 93 reacted with hydrazine to afford 3-methyl-2-(2H-pyrazol-3-yl)thiazolo[3,2-a]benzimidazole (108a) while its reaction with hydroxylamine gave 2-(isoxazol-5-yl)-3-methylthiazolo[3,2-a]benzimidazole (108b) (Scheme 34).
Scheme 34.
Reactions of enaminone 93.
Scheme 34.
Reactions of enaminone 93.
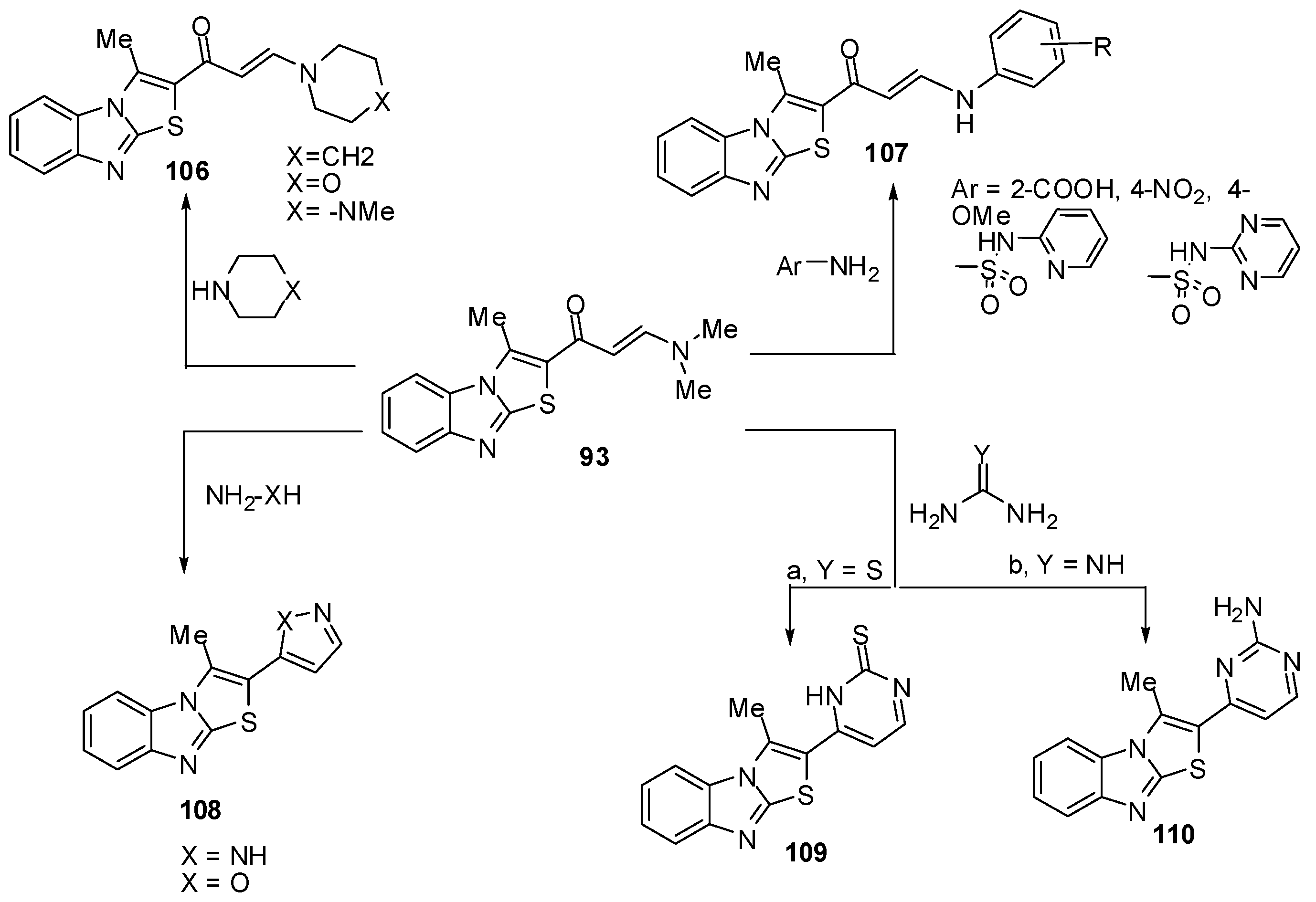
Furthermore, treatment of compound 93 with thiourea in refluxing ethanol, in the presence of sodium ethoxide, afforded pyrimidine-2-thione derivative 109. It reacted also with guanidine to give the corresponding pyrimidine derivative 110 (Scheme 34) [72].
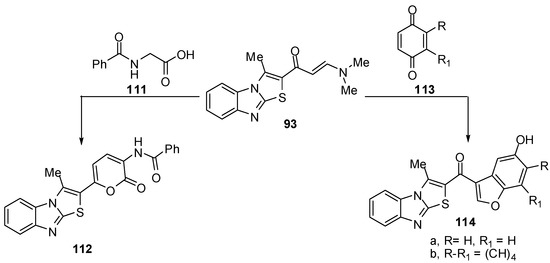
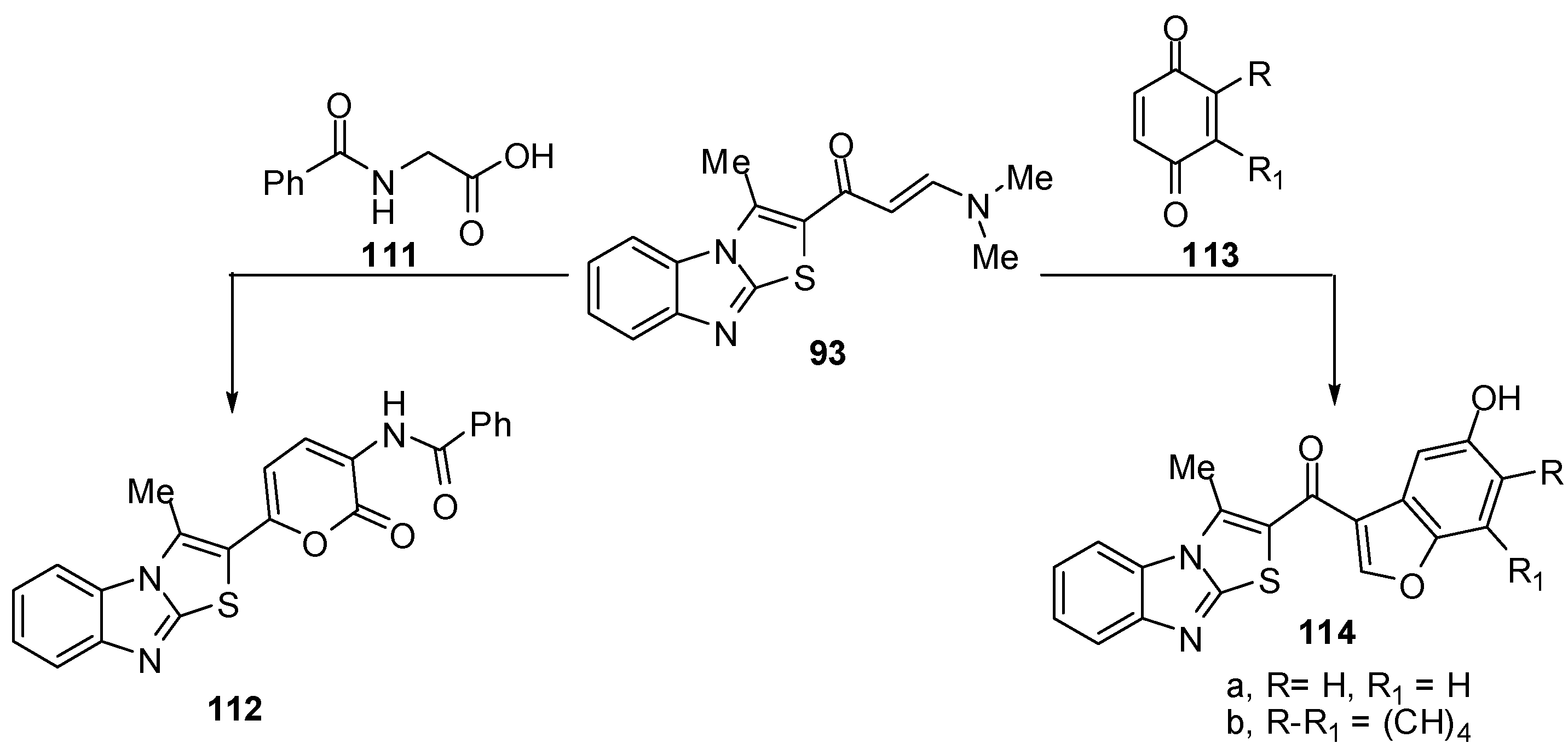
Treatment of compound 93 with 2-benzamidoacetic acid (111) in refluxing acetic anhydride to yield N-[6-(3-methylthiazolo[3,2-a]benzimidazol-2-yl)-2-oxo-2H-pyran-3-yl]benzamide (112) [72] (Scheme 35). Treatment of the enaminone 93 with p-benzoquinone (113a) in acetic acid at room temperature, afforded the benzo[b]furan derivative 114a. In a similar manner, the enaminone 93 reacted with 1,4-naphthoquinone (113b) and afforded 2-(5-hydroxy-naphtho[1,2-b]furan-3-oyl)-3-methylthiazolo[3,2-a]benzimidazole (114b) [72] (Scheme 35).
Scheme 35.
Reactions of enaminone 93.
Scheme 35.
Reactions of enaminone 93.
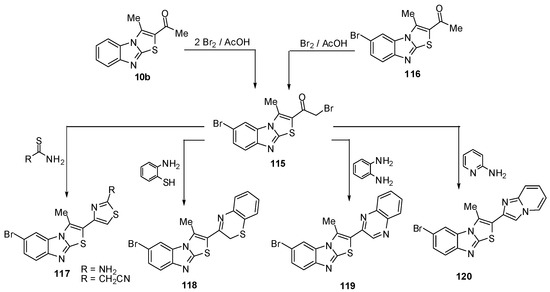
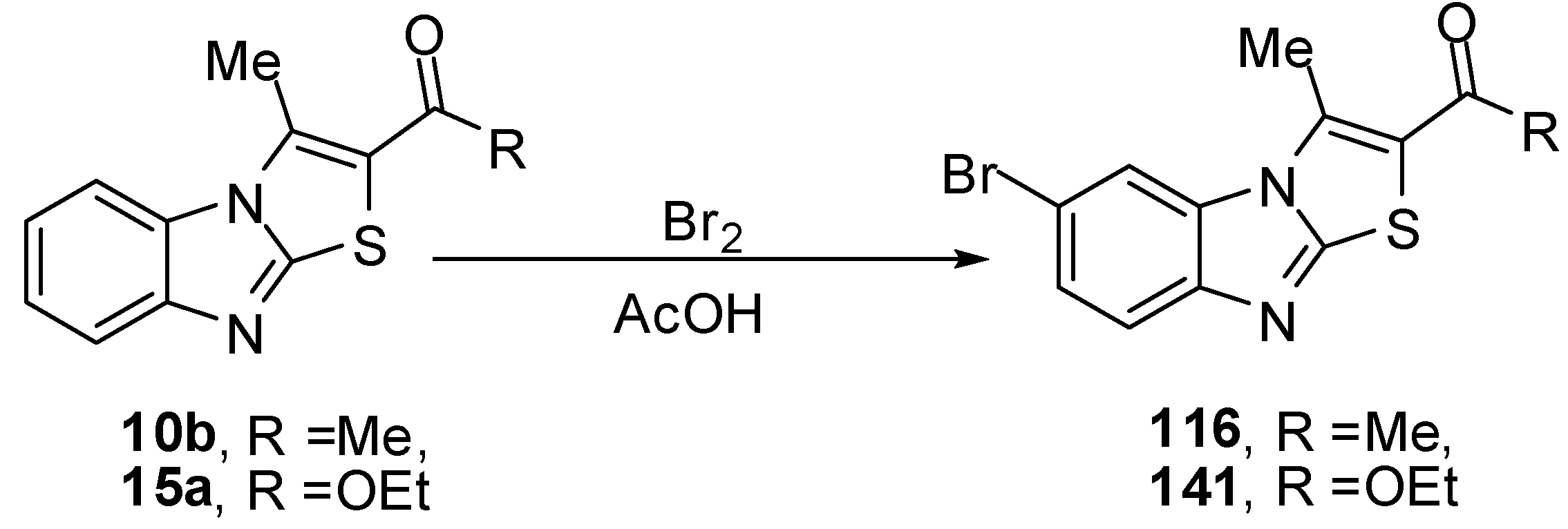
The reaction of ethanone 10b, using two molar equivalence of bromine in acetic acid at 90–100 ºC, resulted in the formation of bromoacetyl derivative 115. The structure of 115 was further confirmed by an independent synthesis outlined in Scheme 36 where compound 116 treated with equal molar quantity of bromine in acetic acid at 90–100 ºC resulted in the formation of compound 115. The reaction of compound 115 with thiourea in refluxing ethanol afforded the corresponding 1,3-thiazole derivative 117a. The reaction of 115 with cyanothioacetamide in refluxing ethanol furnished the cyanomethyl derivative 117b [73] (Scheme 36).
Scheme 36.
Bromination of ethanone 10b.
Scheme 36.
Bromination of ethanone 10b.
When the bromoacetyl 115 was treated with o-aminothiophenol in refluxing ethanol, it afforded 2-(2H-1,4-benzothiazin-3-yl)-6-bromo-3-methylthiazolo[3,2-a]benzimidazole (118). Similarly, 115 reacted with o-phenylenediamine to afford quinoxaline derivative 119. Furthermore, treatment of 115 with 2-aminopyridine in refluxing ethanol afforded 6-bromo-2-imidazo[1,2-a]pyridin-2-yl-3-methylthiazolo[3,2-a]benzimidazole (120) [73] (Scheme 36).
3.4.3. Reactions of ethyl 3-methyl-1,3-thiazolo[3,2-a]benzimidazole-2-carboxylate (15a)
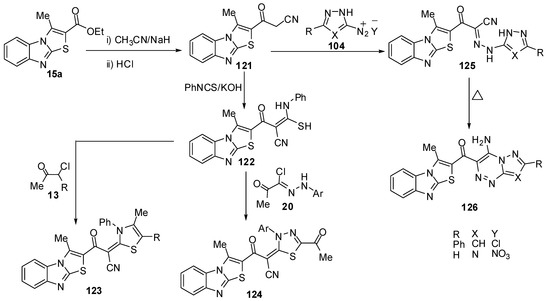
3-(3-Methylthiazolo[3,2-a]benzimidazol-2-yl)-3-oxopropionitrile (121) was synthesized by 3-methylthiazolo[3,2-a]benzimidazole-2-carboxylic acid ethyl ester (15a), with acetonitrile and sodium hydride [74] (Scheme 37). Treatment of compound 121 with phenyl isothiocyanate, in DMF and in the presence of KOH, at room temperature afforded the non-isolable potassium salt which was converted into the thioacetanilide derivative 122 upon treatment with dilute hydrochloric acid (Scheme 37). Compound 122 reacted with α-chloroacetylacetone (13a) and ethyl α-chloroacetoacetate (13b) in refluxing ethanol and in the presence of a catalytic amount of triethylamine resulted in the formation of 1,3-thiazole derivatives 123a and 123b (Scheme 37). Furthermore, the reaction of thioacetanilide derivative 122 with hydrazonyl chlorides 20 under the same reaction conditions afforded to form 1,3,4-thiadiazole derivatives 124. On the other hand, treatment of compound 121 with the diazonium salts of both 3-phenyl-5-amino-1H-pyrazole 104a and 5-amino-1H-l,2,4-triazole 104b afforded hydrazones 125a and 125b (Scheme 37). Compounds 125a and 125b underwent an intramolecular cyclization upon boiling in pyridine via Michael type addition of the endocyclic NH of the hydrazones 125a and 125b to the triple bond of a nitrile function to afford the corresponding pyrazolo[5,1-c]-1,2,4-triazine and 1,2,4-triazolo[5,1-c]-1,2,4-triazine derivatives 126a and 126b, respectively [74] (Scheme 37).
Scheme 37.
Reaction of ester 15a with acetonitrile.
Scheme 37.
Reaction of ester 15a with acetonitrile.
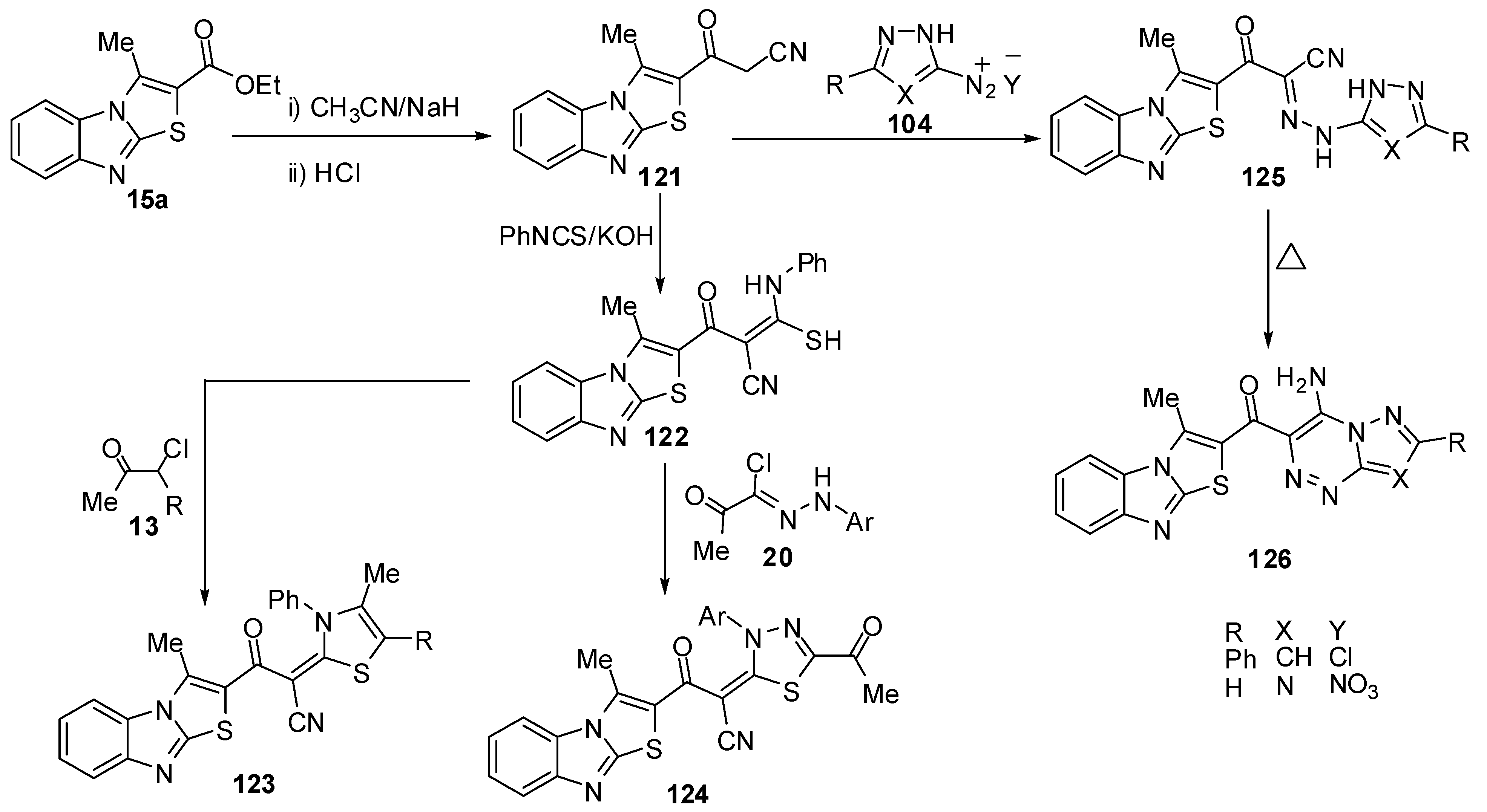
Abdel-Aziz et al. [75] reported the reaction of 3-methylthiazolo[3,2-a]benzimidazole-2-carboxylic acid ethyl ester (15a) with hydrazine hydrate in refluxing ethanol to give the hydrazide 127 (Scheme 38). Treatment of compound 127 with the appropriate aldehydes in refluxing ethanol yielded the corresponding hydrazones 128a-d (Scheme 38). On the other hand, the hydrazide 127 reacts with carbon disulfide in ethanol in the presence of potassium hydroxide to give the potassium salt 129, which reacts with l-aryl-2-bromoethanones to give the 1,3-thiazolidine derivatives 130. Heating of the potassium salt 129 in a aqueous solution of potassium hydroxide afforded 5-(3-methylthiazolo[3,2-a]benzimidazol-2-yl)-1,3,4-oxadiazole-2-thione (131) (Scheme 38). Moreover, treatment of the potassium salt 129 with hydrazine hydrate in a mixture of ethanol and water afforded 4-amino-5-(3-methylthiazolo[3,2-a]benzimidazol-2-yl)-4H-1,2,4-triazole-3-thione (132) [75] (Scheme 38). The structure of compound 132 was further confirmed by an independent synthesis outlined in Scheme 38. Thus, treatment of 1,3,4-oxadiazole-2-thione derivative 131 with hydrazine hydrate in refluxing ethanol resulted in the formation of a product identical to compound 132. On the other hand, treatment of compound 132 with l-aryl-2-bromoethanones in refluxing ethanol yielded the 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazine derivatives 133. Similarly, the treatment of triazole132 with hydrazonyl halides 20 in ethanol afforded the 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazine derivatives 134 [75] (Scheme 38).
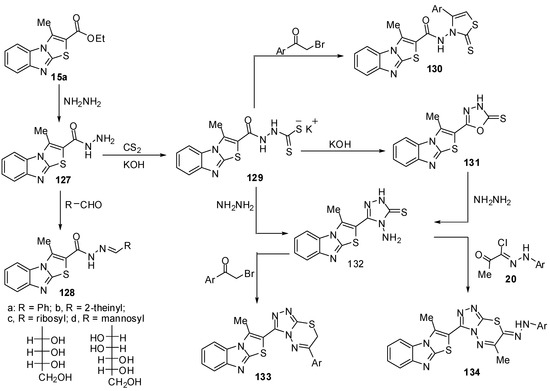
Scheme 38.
Reaction of ester 15a with hydrazine hydrate.
Scheme 38.
Reaction of ester 15a with hydrazine hydrate.
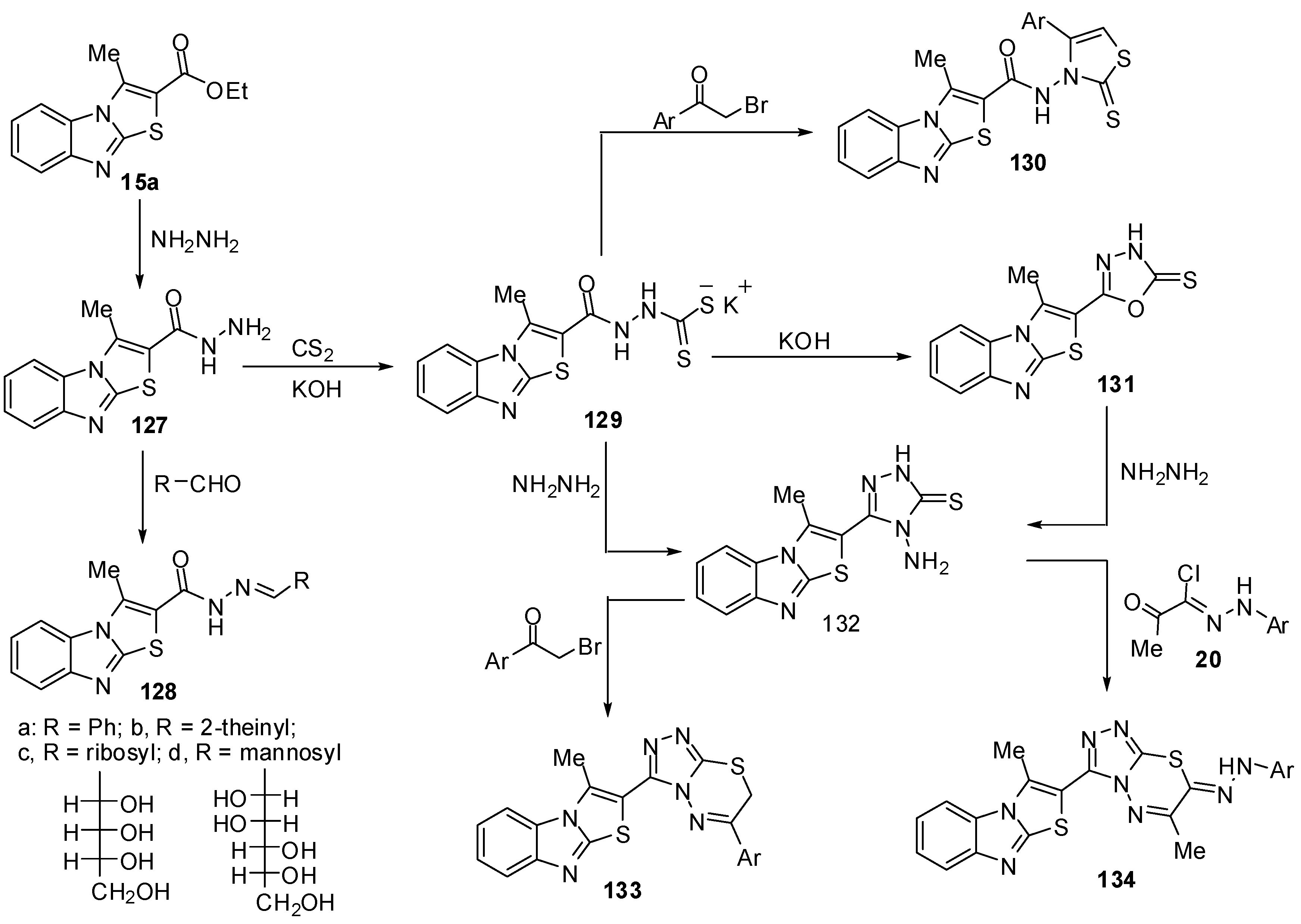
The reaction of hydrazide 127 with pentane-2,4-dione in refluxing ethanol afforded 2-(3,5-dimethylpyrazol-1-oyl)-3-methylthiazolo[3,2-a]benzimidazole (135), while the reaction of the hydrazide 127 with ethoxymethylene-malononitrile (136a) or with ethoxymethylene-ethyl cyanoacetate(136b) in ethanol afforded 5-amino-1-(3-methylthiazolo[3,2-a]benzimidazol-2-oyl)-1H-pyrazole-4-carbonitrile (137a) and 5-amino-1-(3-methylthiazolo[3,2-a]benzimidazol-2-oyl)-1H-pyrazole-4-carboxylic acid ethyl ester (137b), respectively [75] (Scheme 39).
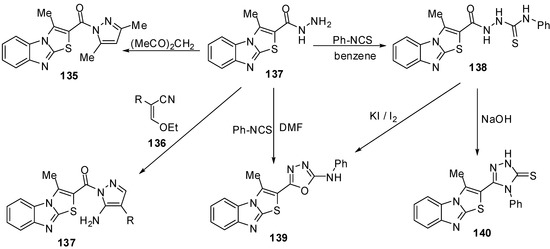
Scheme 39.
Reactions of hydrazide 137.
Scheme 39.
Reactions of hydrazide 137.
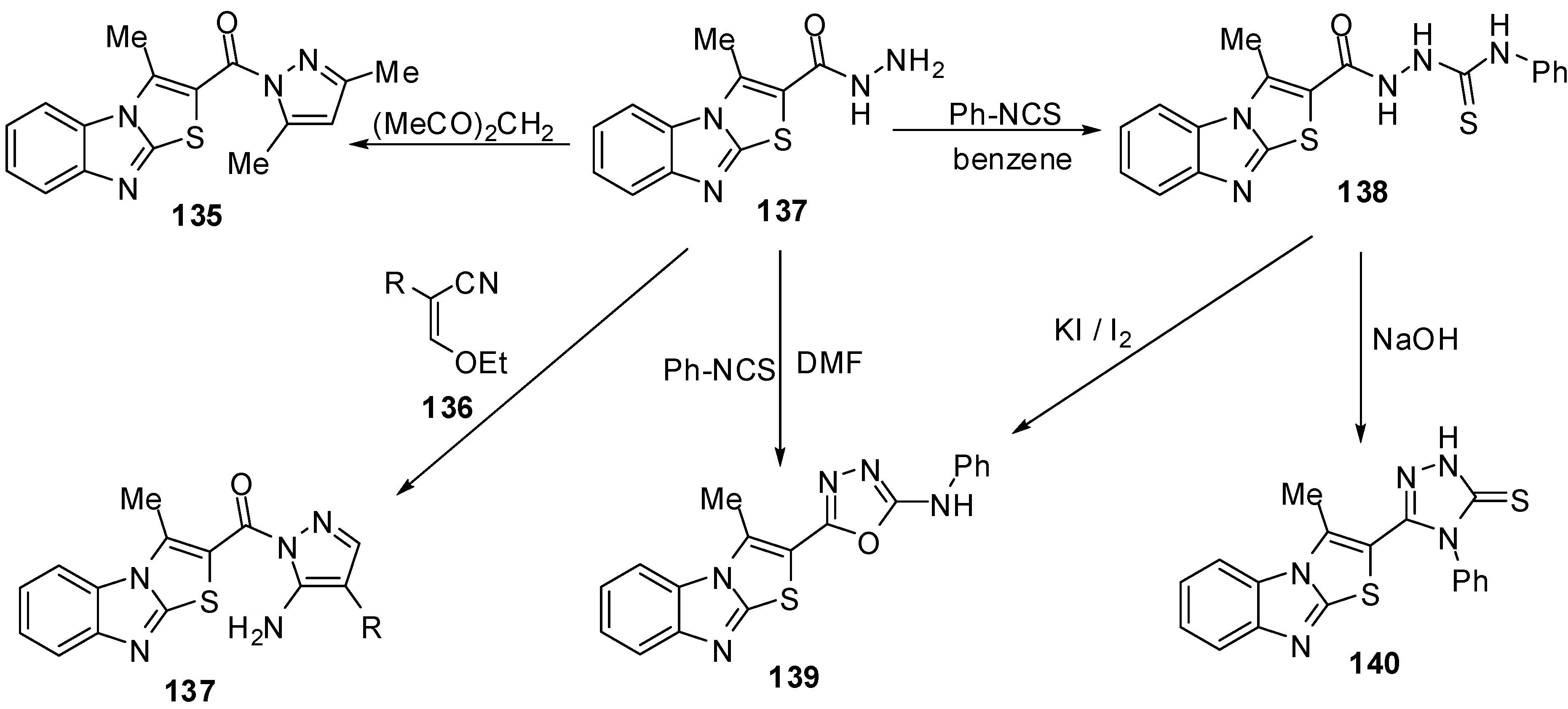
Treatment of the hydrazide 127 with phenyl isothiocyanate in refluxing benzene gave the thiosemicarbazide derivative 138 [75] (Scheme 39). When the latter reaction of the hydrazide 127 with phenyl isothiocyanate was carried out in refluxing DMF instead of benzene, the reaction gave 2-(3-methylthiazolo[3,2-a]benzimidazol-2-yl)-5-phenylamino-1,3,4-oxadiazole (139). On the other hand, the structure of compound 139 was further confirmed by an independent synthesis by treatment of the thiosemicarbazide derivative 138 with potassium iodide and iodine in the presence of sodium hydroxide. Furthermore, The intramolecular cyclization of thiosemicarbazide derivative 138 takes place upon heating with sodium hydroxide to produce the 1,2,4-triazole derivative 140 [75] (Scheme 39).
Scheme 40.
Reactions of hydrazide 142.
Scheme 40.
Reactions of hydrazide 142.
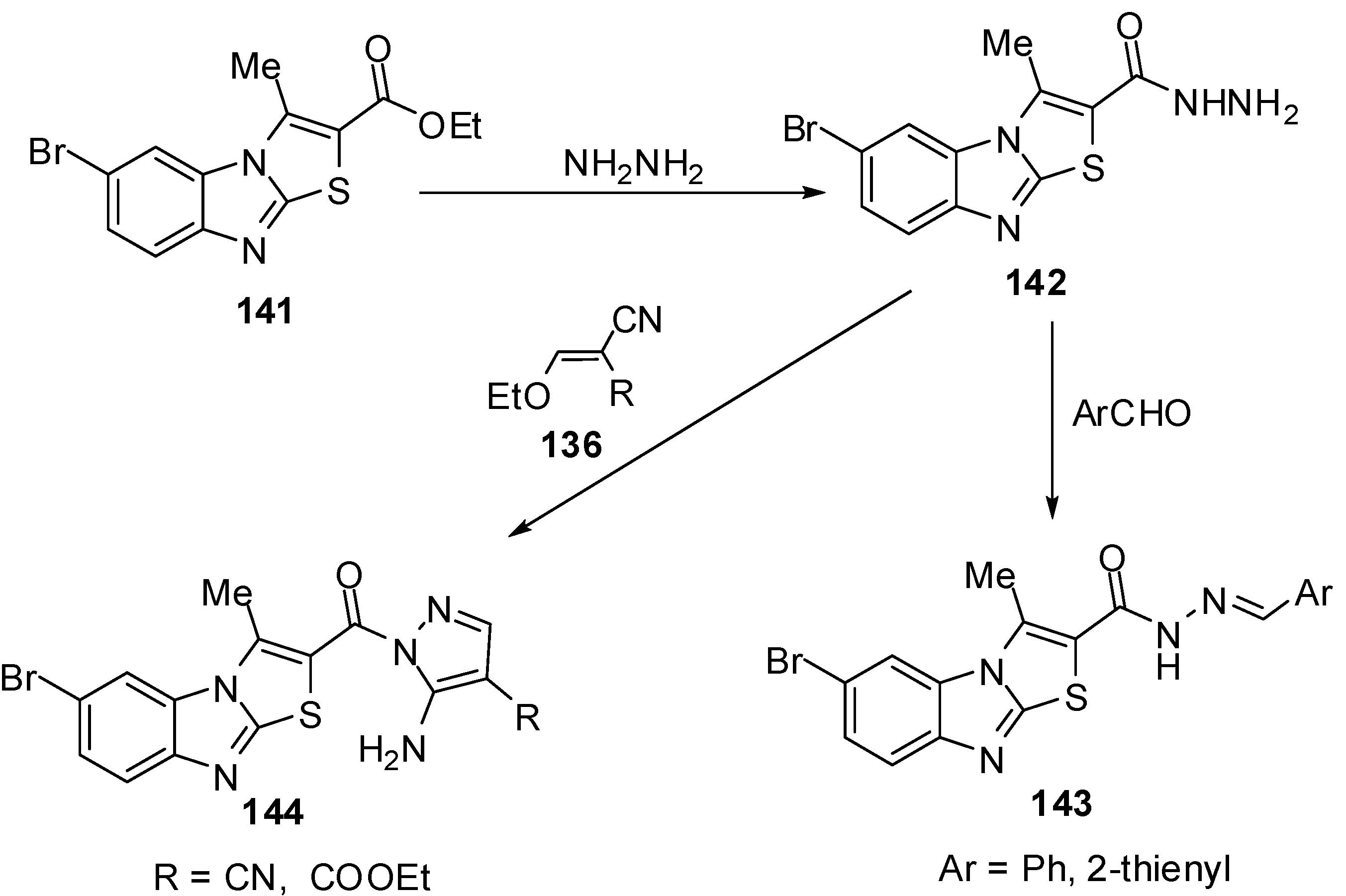
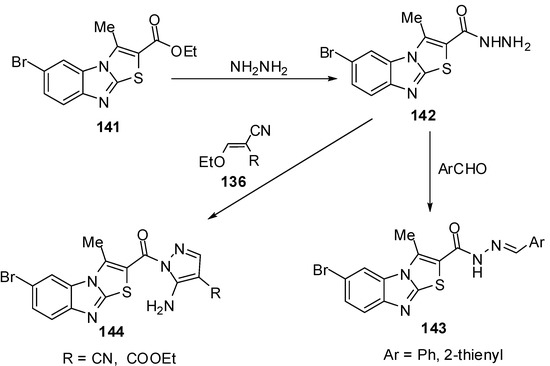
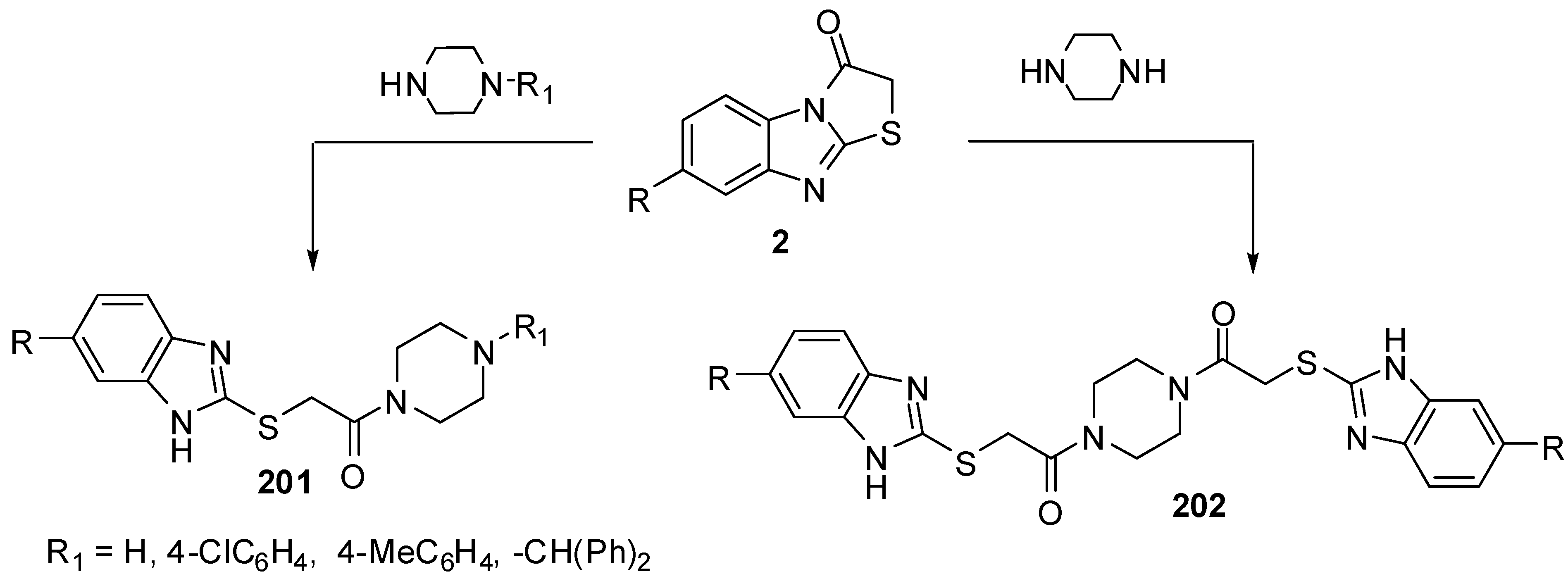
Abdel-Azia et al. [76] reported the synthesis of 6-bromo-3-methylthiazolo[3,2-a]benzimidazole-2-carboxylic acid ethyl ester (141) by the bromination of ester 15a. Compound 141 reacted with hydrazine hydrate in refluxing ethanol to give the hydrazide 142 (Scheme 40). The treatment of compound 142 with benzaldehyde or 2-thiophenaldehyde, in refluxing ethanol yielded the corresponding hydrazones 143a and 143b, respectively. On the other hand, the reaction of hydrazide 141 with ethoxymethylene malononitrile (136a) or with ethyl ethoxymethylene cyanoacetate (136b) in ethanol afforded pyrazole derivatives 144a and 144b, respectively [76] (Scheme 40).
3.4.4. Reactions of thiazolo[3,2-a]bezimidazol-3(2H)ones 2
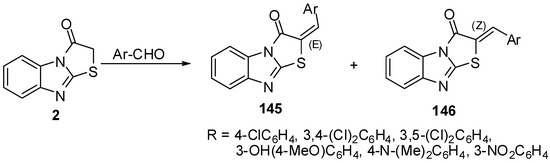
Refluxing of thiazolo[3,2-a]benzimidazol-3(2H)-one (2) with aromatic aldehydes in pyridine/ dicyclohexylcarbodiimide, EtOH/piperidine or in AcOH/AcONa, gave E/Z 2-arylidenethiazolo[3,2-a]benzimidazol-3(2H)-ones 145/146 [77,78,79] (Scheme 41).
Scheme 41.
Reaction of thiazolone 2 with aromatic aldehydes.
Scheme 41.
Reaction of thiazolone 2 with aromatic aldehydes.
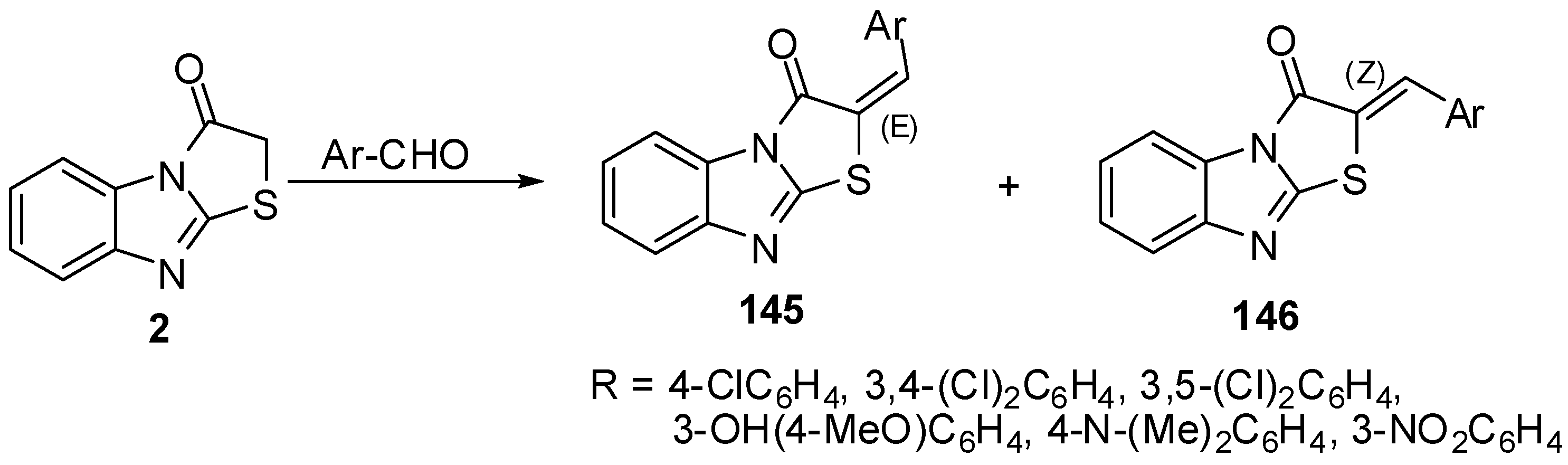
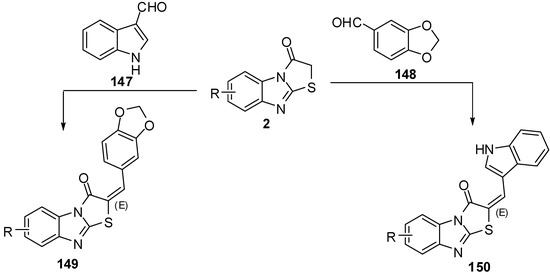
The condensation of thiazolones 2 with 1,3-benzodioxole-5-carbaldehyde (147) and indole-3-carbaldehyde (148), using pyridine as a catalyst resulted in products 149 and 150, respectively.
Scheme 42.
Reaction of thiazolone 2 with aldehydes.
Scheme 42.
Reaction of thiazolone 2 with aldehydes.
The one-pot synthesis of these compounds carried out by a cyclocondensation (a Knoevenagel condensation, followed by cyclization) of compounds 3, chloroacetic acid, aromatic or heteroaromatic aldehyde, acetic anhydride, and glacial acetic acid in the presence of sodium acetate (piperidine) led to 2-substituted thiazolo[2,3-a]benzimidazole-3(2H)-ones 149 and 150 in good yields [80] (Scheme 42).
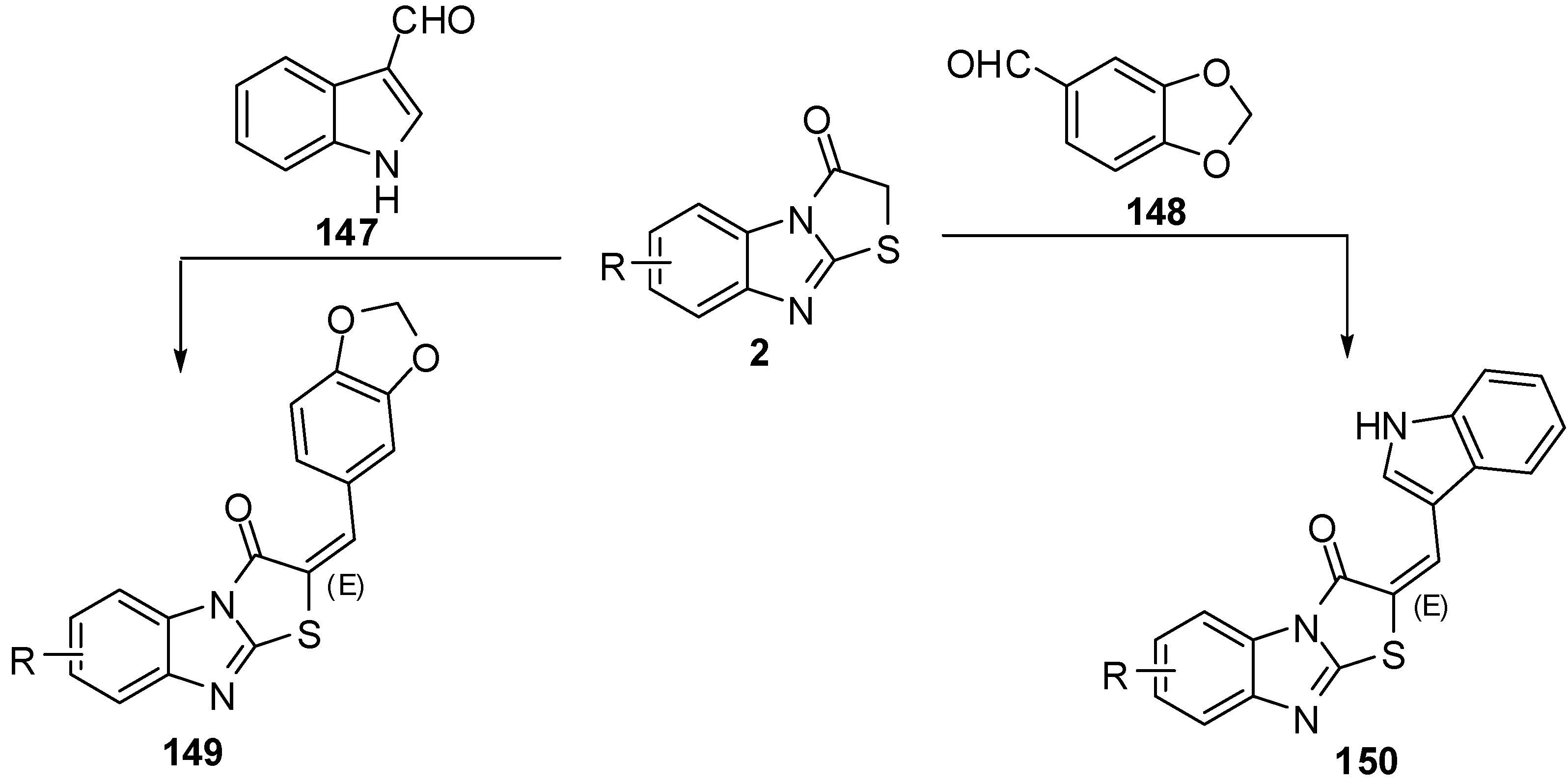
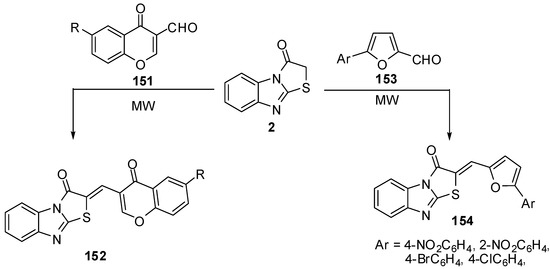
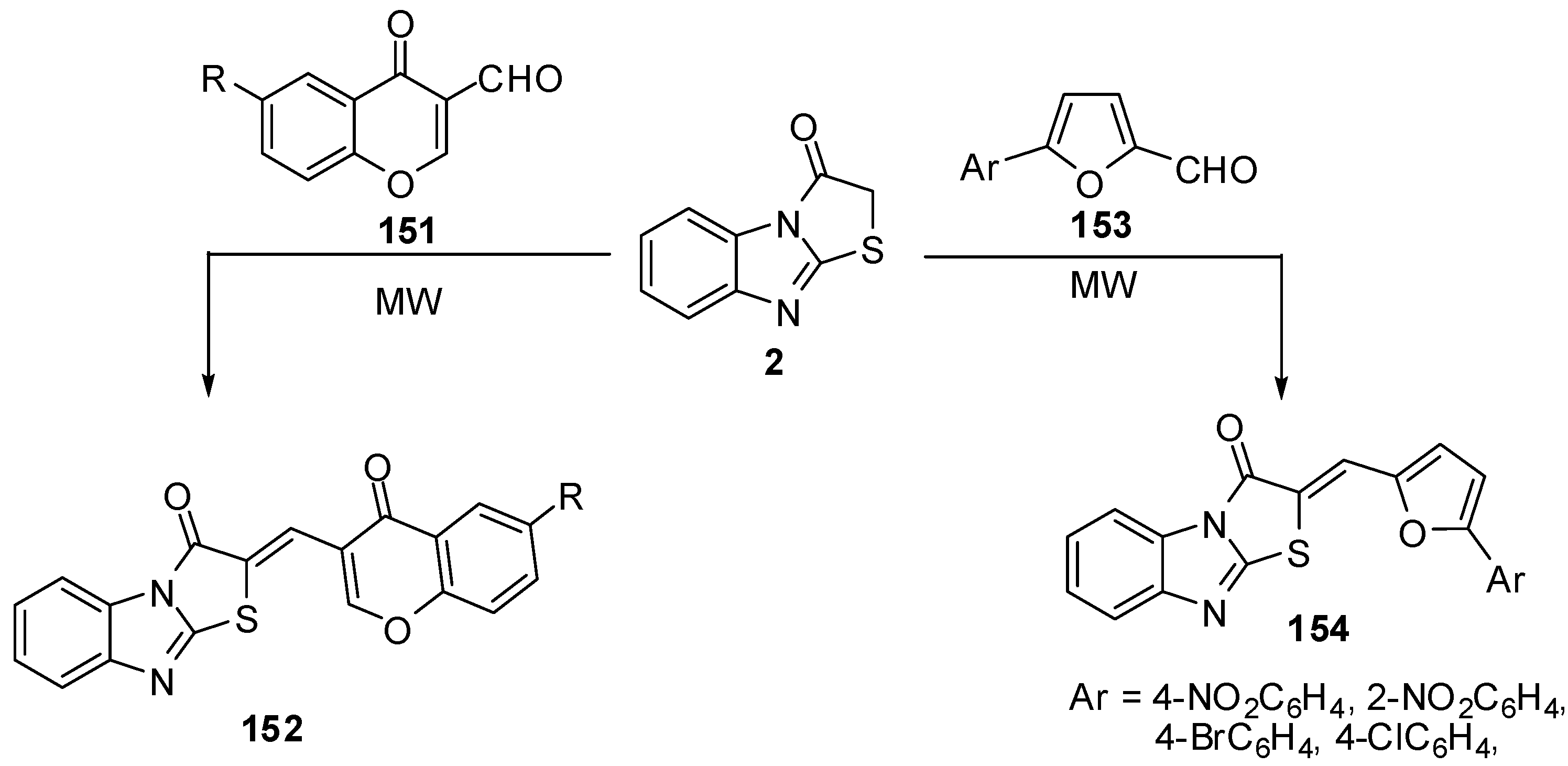
Condensation of 6-R-3-formylchromones 151 with thiazolone 2 by the classica method, as well as condensation in a microwave oven to synthesis compounds 152, has been studied [81] (Scheme 43). Synthesis of compounds 154 from 5-arylfuran-2-carboxaldehydes 153 have been studied in acetic anhydride, by both classical heating and under microwave assisted conditions. The beneficial effect of microwave irradiation on these condensations was found in a shortening of the reaction time and increase in the yields [82] (Scheme 43).
Scheme 43.
Reaction of thiazolone 2 with aldehydes.
Scheme 43.
Reaction of thiazolone 2 with aldehydes.
Several fused isoxazoles 155 were synthesized by the reaction of hydroxyl amine with benzylidines derivatives 55 [83] (Scheme 44).
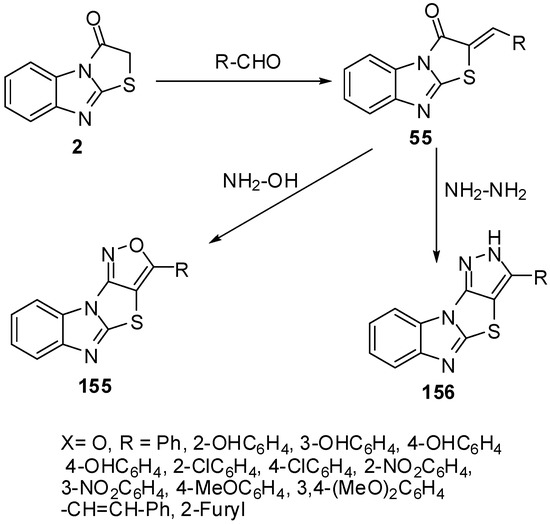
Scheme 44.
Reaction of thiazolone 2 with aldehydes.
Scheme 44.
Reaction of thiazolone 2 with aldehydes.
On the other hand, dihydropyrazoles 156 were synthesized by condensation of hydrazine hydrate with arylidines 55 under microwave irradiation and solvent free conditions [84] (Scheme 44).
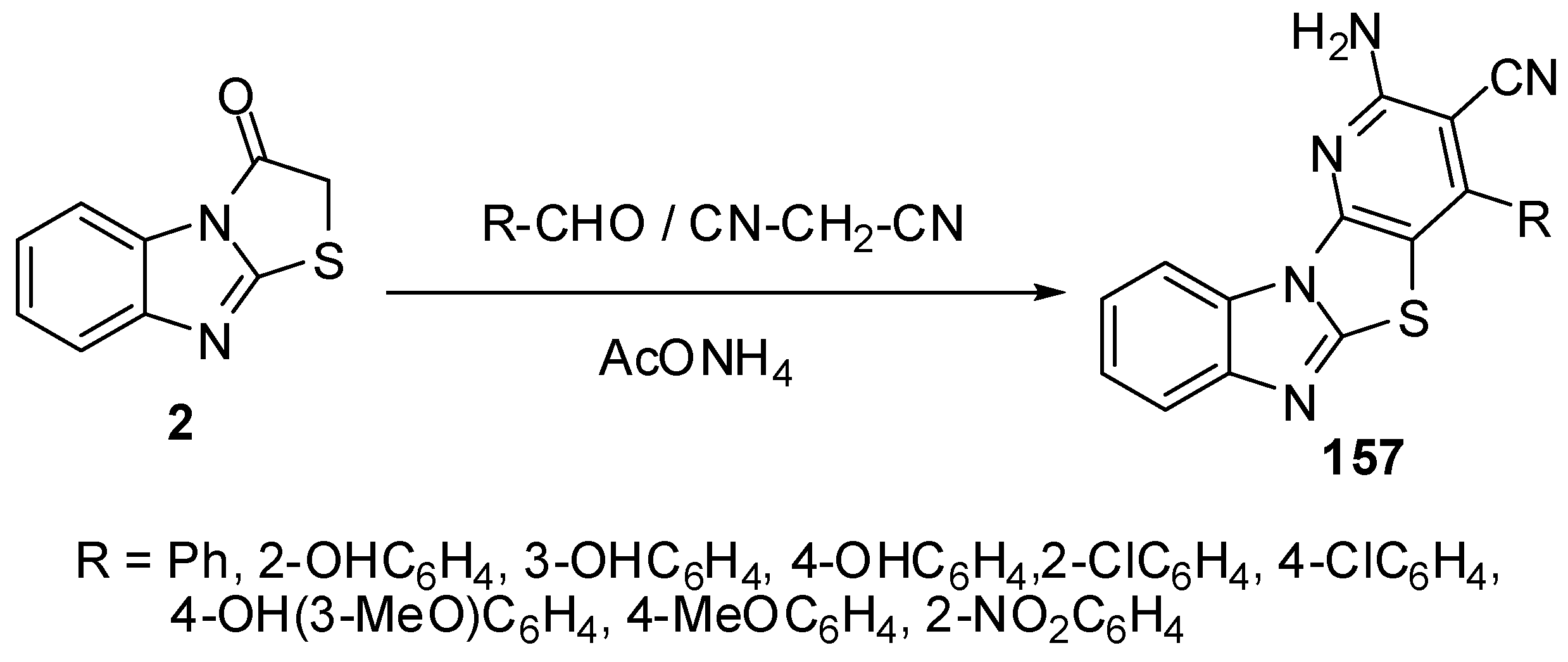
Furthermore, condensed pyridines 157 were prepared in one-pot three component reaction from thiazolone 2, aromatic aldehydes and malononitrile [83] (Scheme 45).
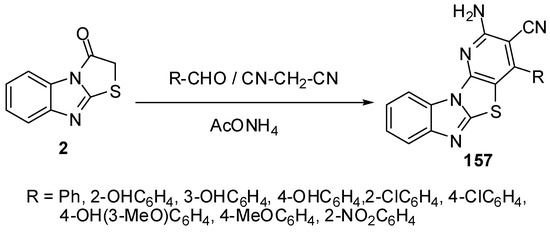
Scheme 45.
One-pot reaction of pyridines 157.
Scheme 45.
One-pot reaction of pyridines 157.
4'-(2,4-Dichlorophenyl)-1'-methyl-2,3,2'',3''-tetra-hydro-1H-indole-3-spiro-2'-pyrrolidine-3'-spiro-2'-(1,3-benzimidazo[2,1-b]thiazole)-2,3''-dione 161 was synthesized by the intermolecular [3+2]-cycloaddition of azomethine ylide, derived from isatin 159 and sarcosine 160 by a decarboxylative route, and 2-(2,4-dichlorobenzylidene)benzo[4,5]imidazo[2,1-b]thiazol-3-one 158 [85] (Scheme 46).
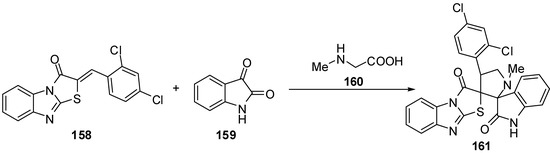
Scheme 46.
Synthesis of spiro-pyrrolidine 161.
Scheme 46.
Synthesis of spiro-pyrrolidine 161.
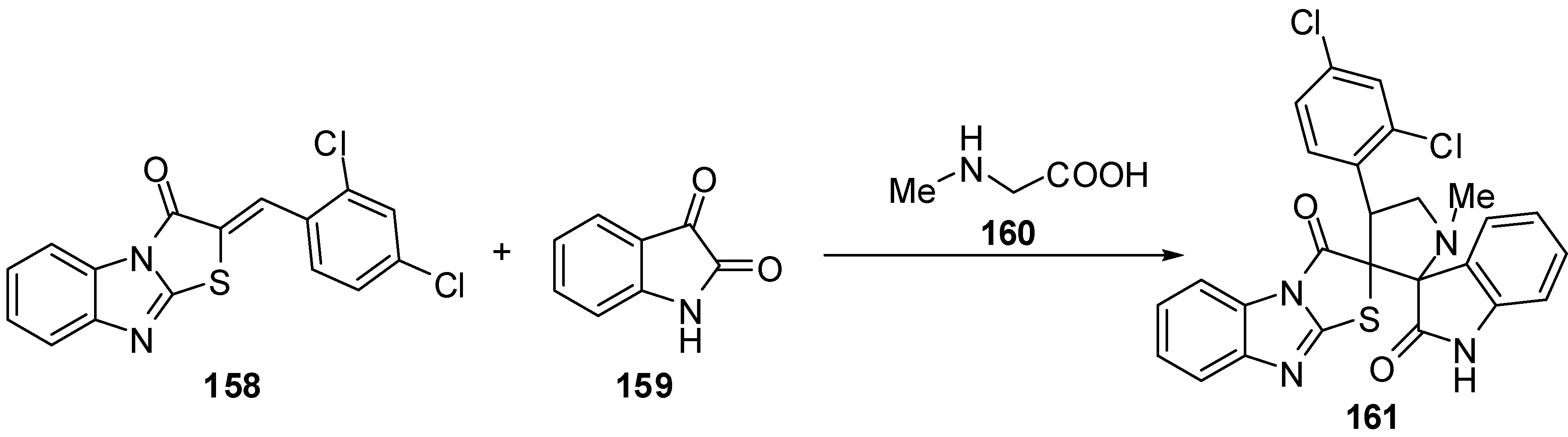
Compound 2 couples smoothly with 3-phenyl-1H-pyrazole-5-diazonium chloride (104a) and 1H-l,2,4-triazole-5-diazonium nitrate (104b) to afford the corresponding hydrazones 162a and 162b [74] (Scheme 47).
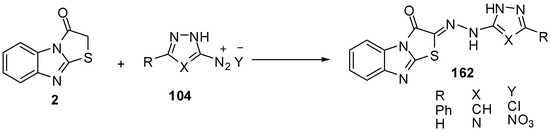
Scheme 47.
Coupling reaction of thiazolone 2.
Scheme 47.
Coupling reaction of thiazolone 2.
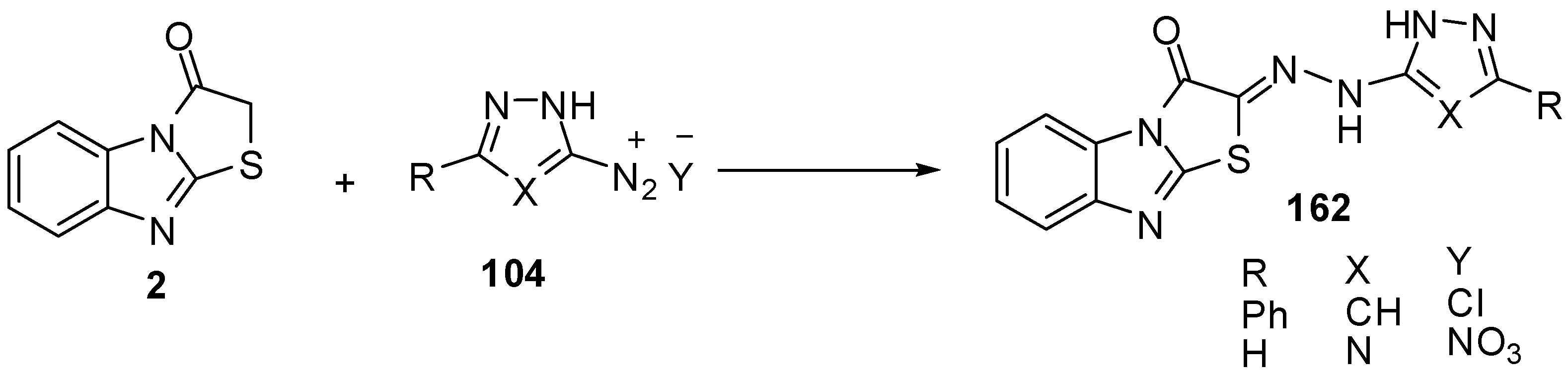
Treatment of thiazolo[3,2-a]benzimidazol-3(2H)-one (2) with phenyl isothiocyanate, in DMF and in the presence of KOH, at ambient temperature furnished the nonisolable potassium salt 163 which reacts in situ with α-chloroacetylacetone (13a) and ethyl α-chloroacetoacetate (13b) to give 1,3-thiazole derivatives 164a and 164b. In a similar manner, hydrazonyl chlorides 20 reacted with the non-isolable potassium salt 163 under the same reaction conditions, to afford 1,3,4-thiadiazole derivatives 165 [74] (Scheme 48).
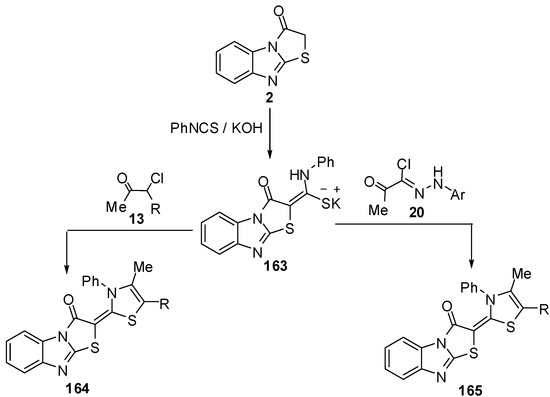
Scheme 48.
Reaction of thiazolone 2 with phenyl isothiocyanate.
Scheme 48.
Reaction of thiazolone 2 with phenyl isothiocyanate.
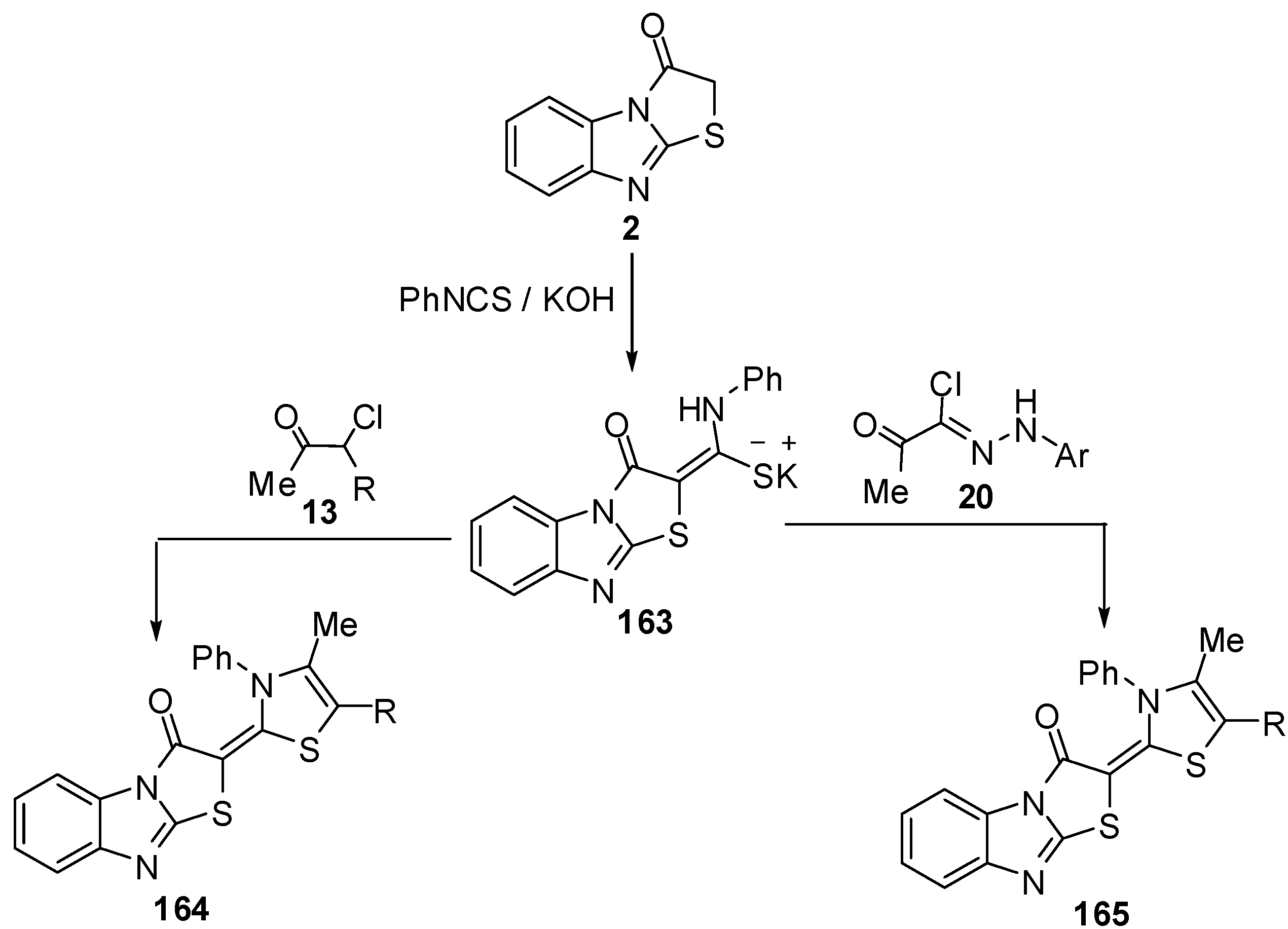
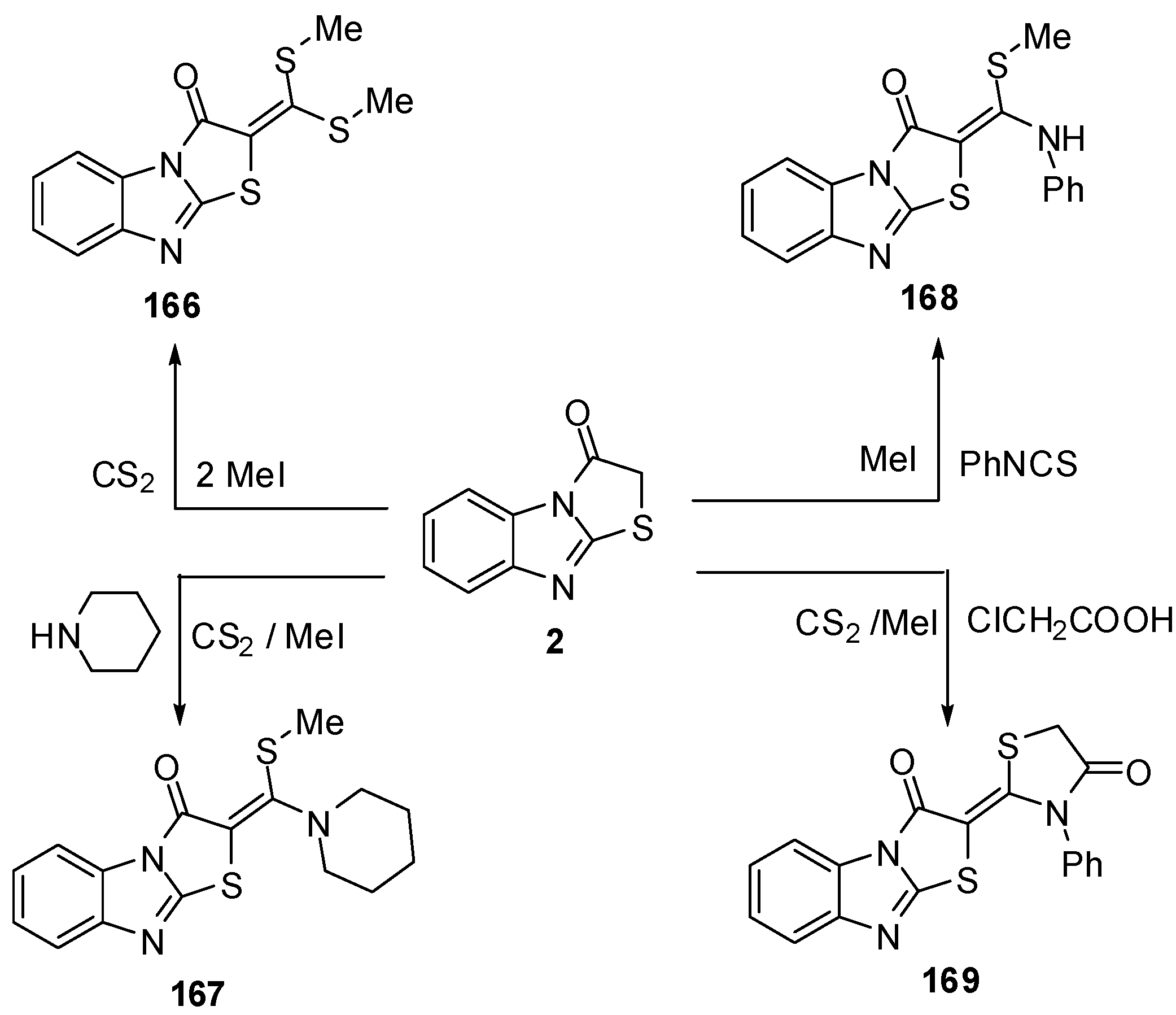
Treatment of thiazolone 2 with carbon disulphide followed by the reaction with methyl iodide or piperidine afforded compounds 166 and 167, respectively, while the reaction of thiazolone 16 with pheny isothicyanate and methyl iodide or chloroacetic acid gave compounds 168 and 169, respectively [79] (Scheme 49).
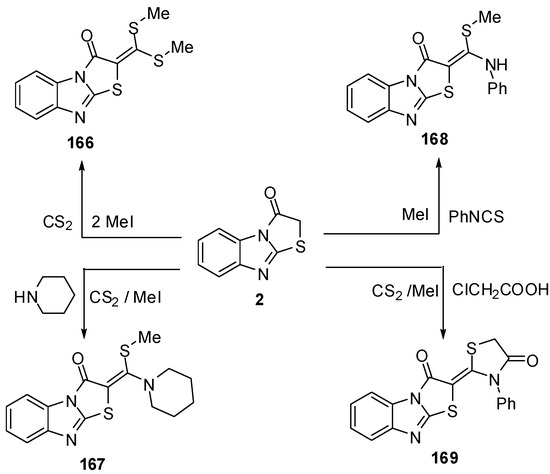
Scheme 49.
Reaction of thiazolone 2 with carbon disulphide.
Scheme 49.
Reaction of thiazolone 2 with carbon disulphide.
3.5. Reactions of C2-C3 [Reactions of 3-aminothiazolo[3,2-a]benzimidazol-2-carbonitrile (41)]
Synthesis and reactions of 3-aminothiazolo[3,2-a]bezimidazole-2-carbonitrile 41 were reported by Sarhan and his co-workers [11,47,48,86,87,88,89]. Condensation of compound 41 with aromatic aldehydes afforded the arylmethylidenes 170. Cycliztion of 41 with formic acicc, formamide and acetic anhydride gave fused pyrimidines 171, 172 and 173, respectively. Alkylation of 173 with alkyl halides gave the N-alkyl derivatives 174 (Scheme 50).
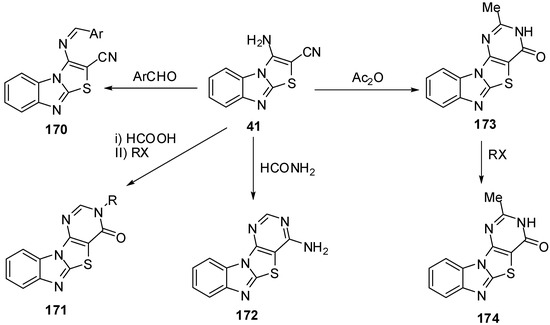
Scheme 50.
Reactions of 3-aminothiazolo[3,2-a]bezimidazole-2-carbonitrile 41.
Scheme 50.
Reactions of 3-aminothiazolo[3,2-a]bezimidazole-2-carbonitrile 41.
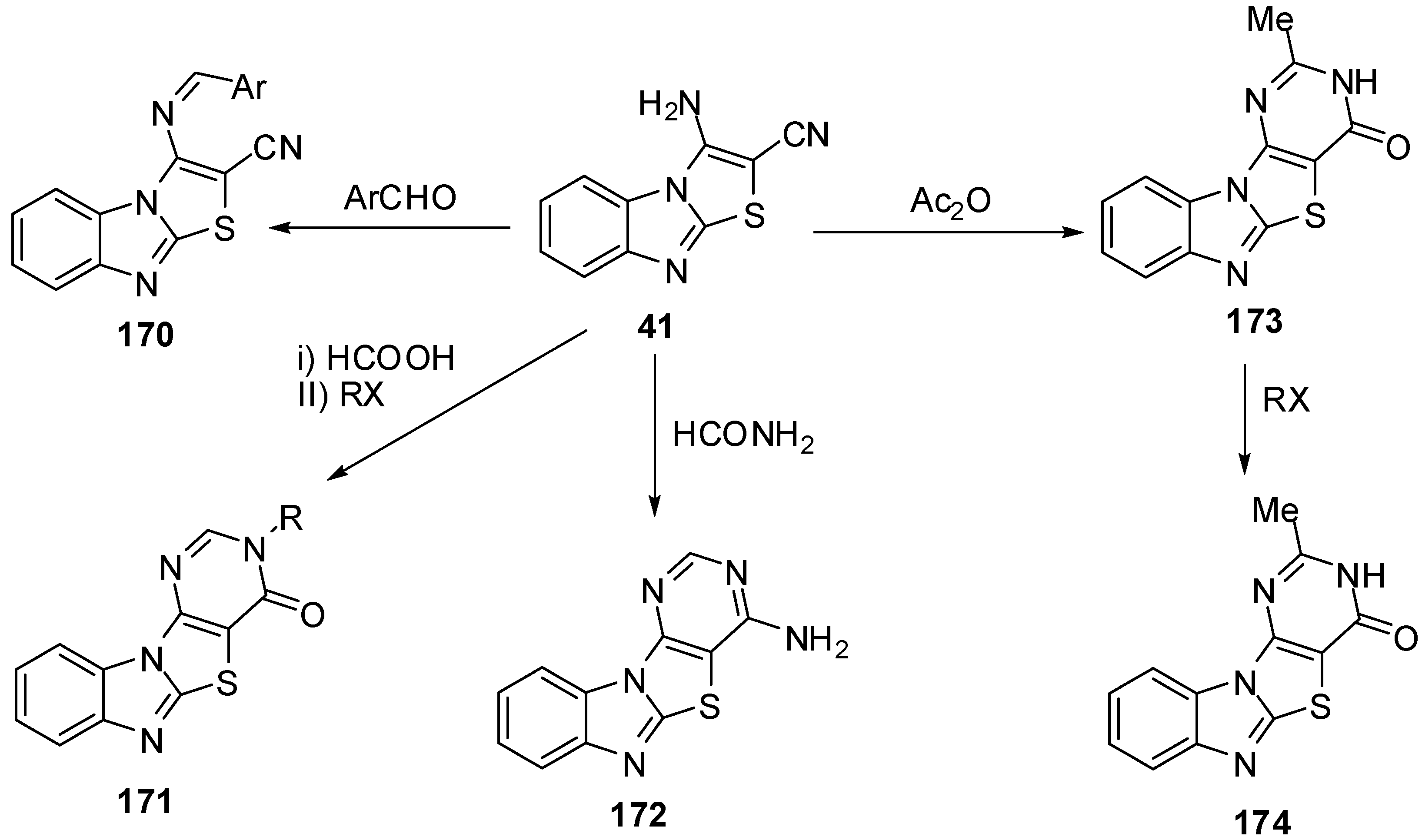
Reaction of 41 with triethyl ortho formate gave the ethoxymethyleneamino derivative 175, which cyclized with different amines to give the products 176 and 177. Hydrolysis of 41 by H2SO4 or H3PO4 afforded the amide derivative 178 (Scheme 51).
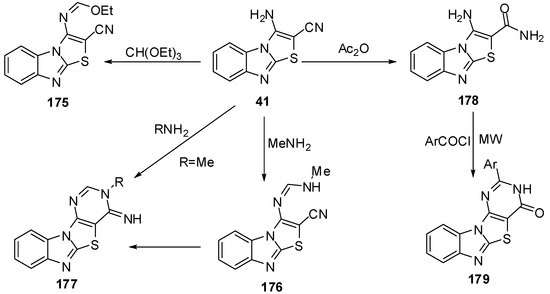
Scheme 51.
Reactions of 3-aminothiazolo[3,2-a]bezimidazole-2-carbonitrile 41.
Scheme 51.
Reactions of 3-aminothiazolo[3,2-a]bezimidazole-2-carbonitrile 41.
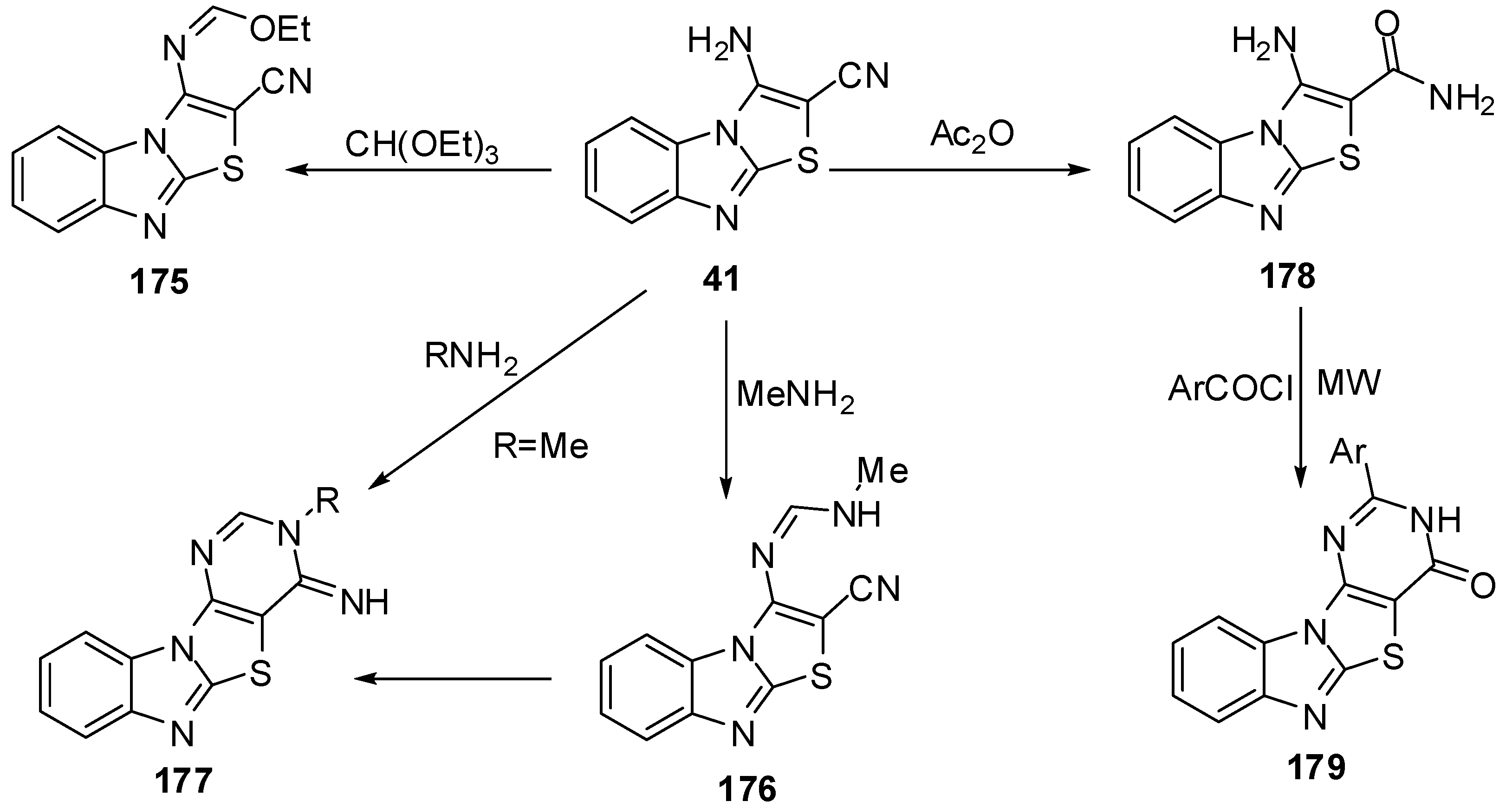
On the other hand, Davoodnia et al. [90] reported the reaction of compound 178 with aroyl halides under microwave irradiation in solvent-free condition at 800 W to afford 2-arylpyrimido[4',5':4,5]-thiazolo[3,2-a]benzimidazol-4(3H)-ones 179 (Scheme 51).
4-Chloro-2-methylpyrimidino[4',5':4,5]-thiazolo[3,2-a]benzimidazole (180) was prepared by chlorination of pyrimidine 173. Nucleophilic substitution of 180 with alcohols, phenols, primary amines, secondary amines, sodium azide, and mercaptoacetic acid gave the corresponding derivatives 181. Thination of fused pyrimidine 173 afforded 2-methylpyrimidino[4',5':4,5]-thiazolo[3,2-a]benzimidazol-4-thiol (182). The thiol derivative 182 was reacted with alkyl/aralkyl halides, phenacyl bromide derivatives, bromoacetone, chloroanilides, bromomalonic ester, and ethyl bromoacetate to afford sulphides 183 [88] (Scheme 52).
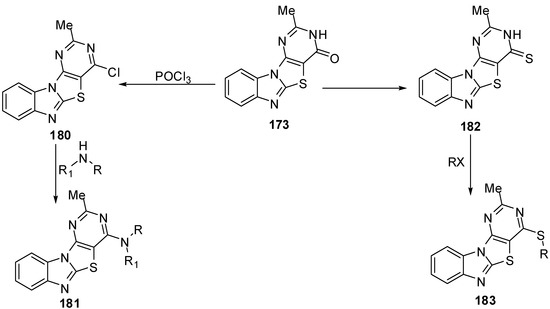
Scheme 52.
Reactions of pyrimidinone 173.
Scheme 52.
Reactions of pyrimidinone 173.
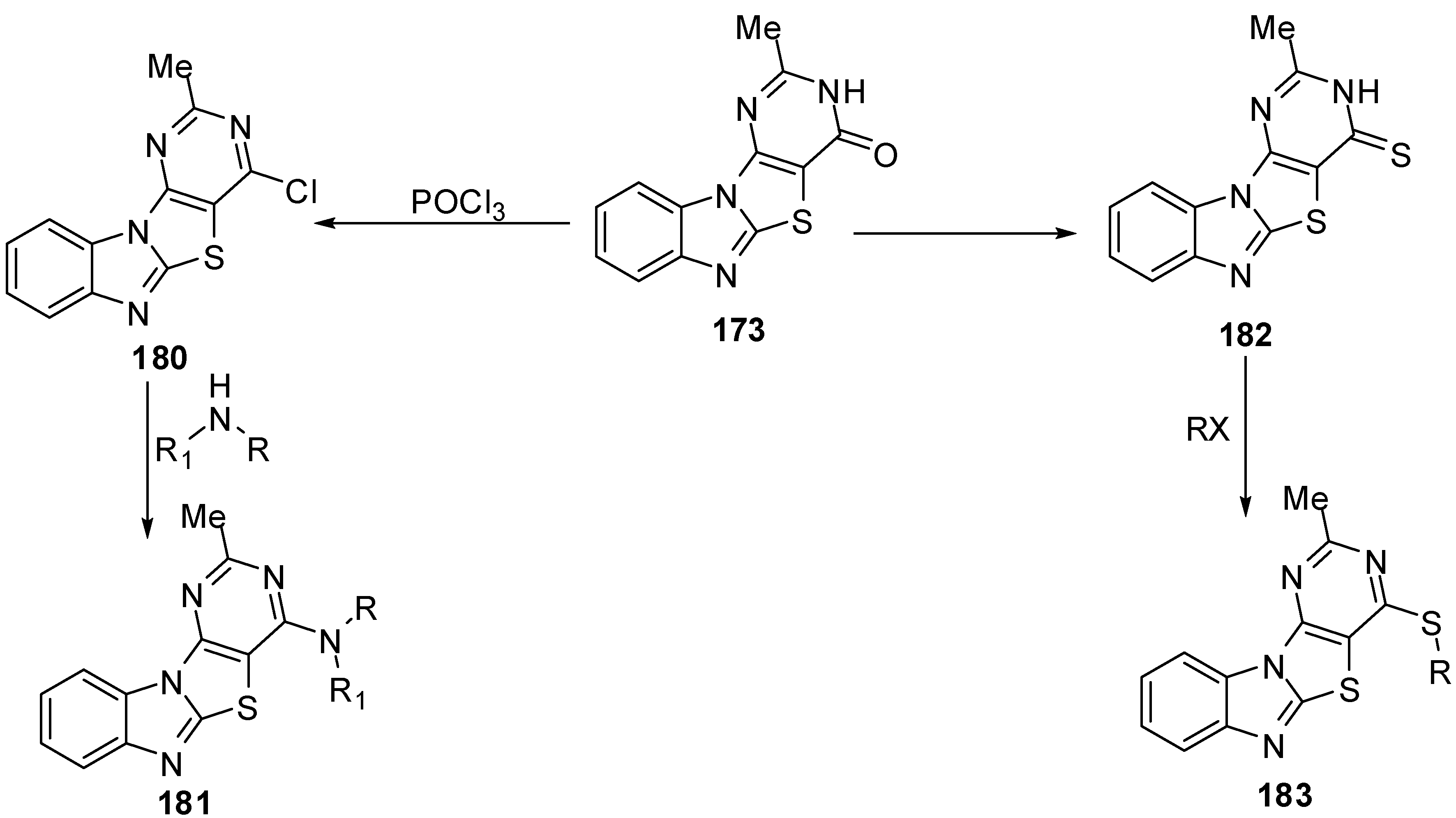
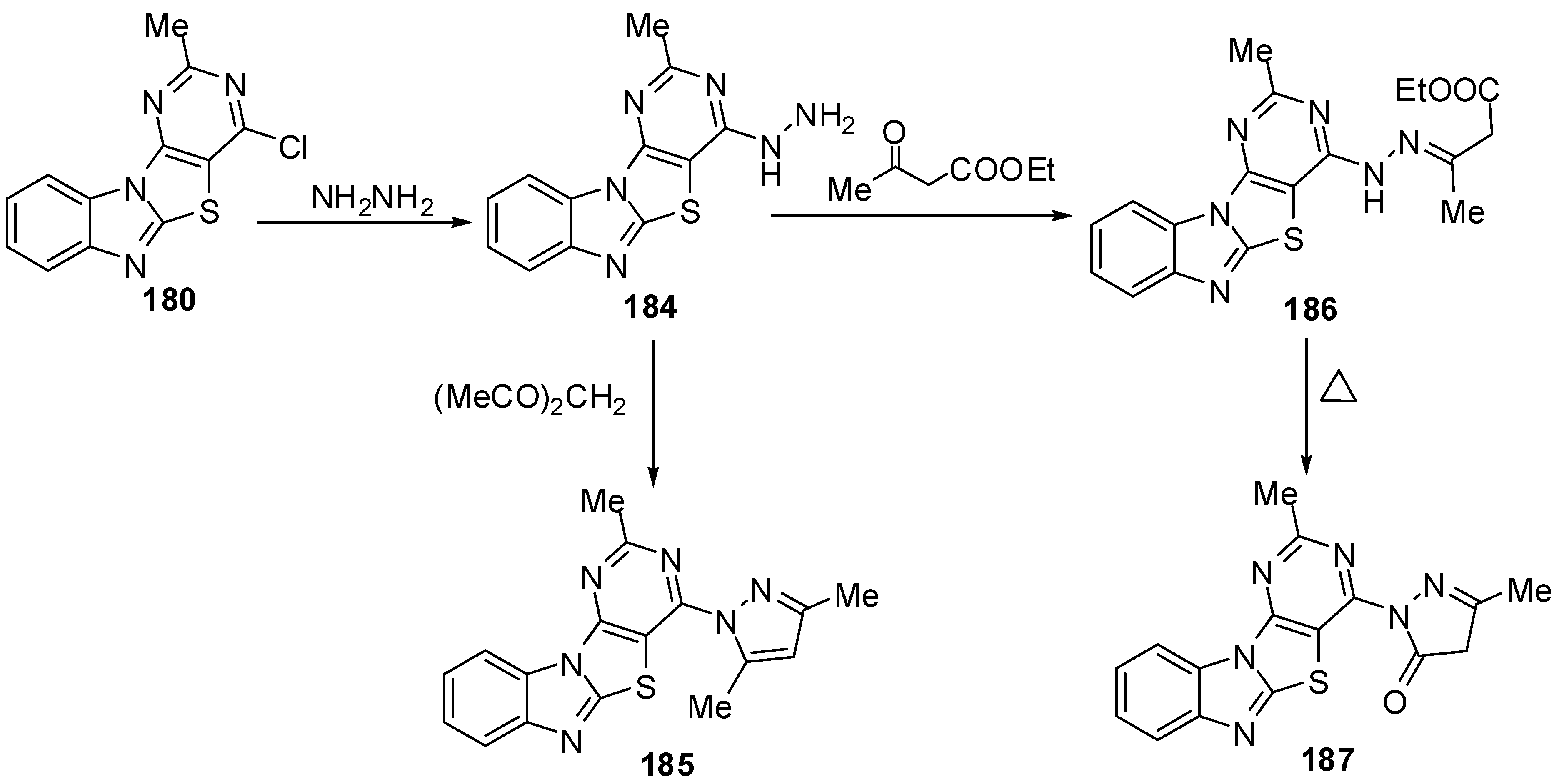
In addition, the reaction of chloro compound 180 with hydrazine hydrate in refluxing ethanol gave 4-hydrazino-2-methylpyrimidino[4',5':4,5]thiazolo[3,2-a]benzimidazole (184) as showed in Scheme 53. Condensation of 4-hydrazino derivative 184 with acetyl acetone under neat conditions gave the corresponding 2-methyl-4-(3,5-dimethylpyrazolyl)pyrimidino[4',5':4,5]-thiazolo[3,2-a]benzimidazole (185) [88] (Scheme 53).
Condensation of 184 with ethyl acetoacetate in refluxing ethanol afforded the uncyclized derivative 186 which, on heating over its melting point, resulted in the formation pyrazolone derivative 187 [88](Scheme 53).
The reaction of hydrazino derivative 184 with triethyl orthoformate gave the corresponding 4-ethoxymethylidenehydrazino-2-methylpyrimidino[4',5':4,5]-thiazolo[3,2-a]benzimidazole (188). On heating of 188 under neat conditions afforded the triazolo[2,3-c] isomer 189. In addition, the triazolo[2,3-c] isomer 189 was also obtained by refluxing 184 in formic acid or formic acid/glycerol mixture at refluxing temperature (Scheme 53).
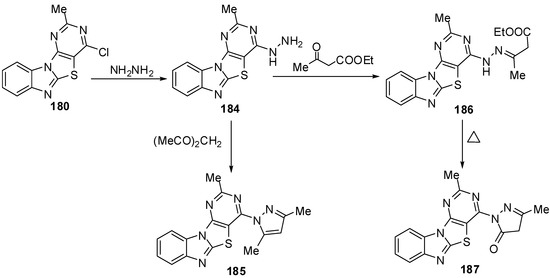
Scheme 53.
Reactions of chloro pyrimidine 180.
Scheme 53.
Reactions of chloro pyrimidine 180.
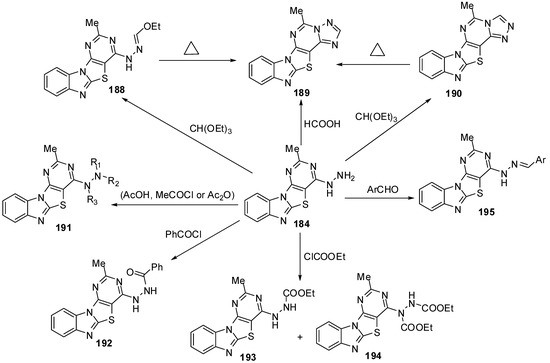
Refluxing the hydrazino compound 184 with triethyl orthoformate gave the triazolo[4,3-c] isomer 190 which was isomerized on heating over its melting point to give the isomer 189. The triazolo[2,3-c] derivatives undergo isomerization to [3,4-c] isomers [88] (Scheme 54).
Scheme 54.
Reactions of hydrazino pyrimidine 184.
Scheme 54.
Reactions of hydrazino pyrimidine 184.
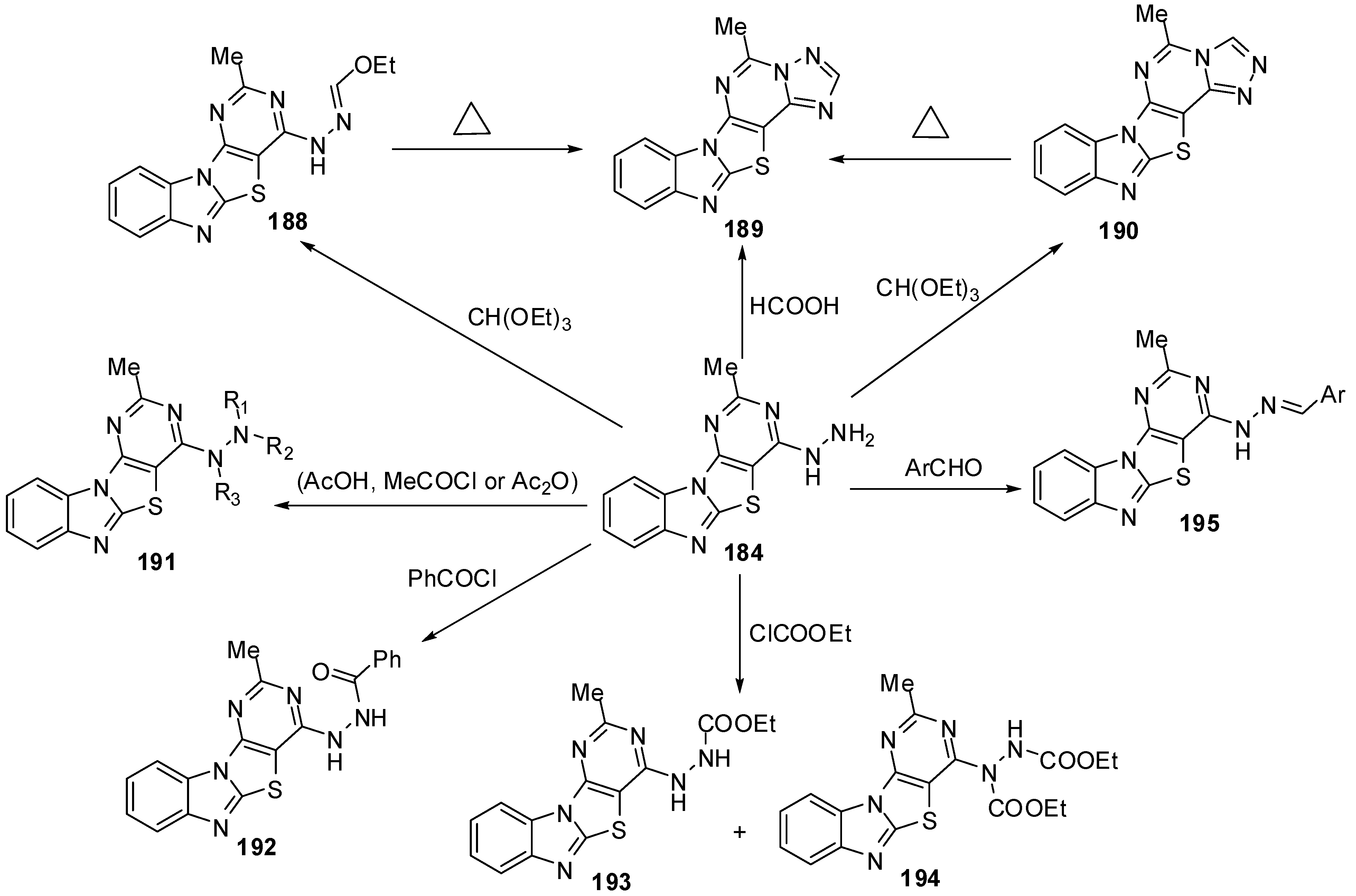
The reaction of 184 with acetic acid afforded the mono acetyl derivative 191a. While, when acetyl chloride was used as acetylating agent the diacetyl derivative 191b was obtained be sides the triacetyl derivative 191c. Moreover the triacetyl derivative 192c was obtained independently in pure form by refluxing 184 with acetic anhydride. Benzoylation of the hydrazino compound 184 was carried out in CHCl3 containing K2CO3 with benzoyl chloride to afford the mono benzoyl derivative 192. Reaction of 184 with ethylchloroformate afforded a mixture of separable carbamates 193 and 194. Moreover, condensation of hydrazino derivative 184 with aromatic aldehydes obtained the E-form of arylmethylideneamino derivatives 195 [88] (Scheme 54).
3.6. Reactions of C3
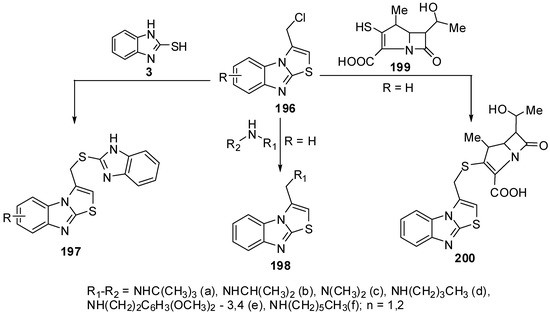
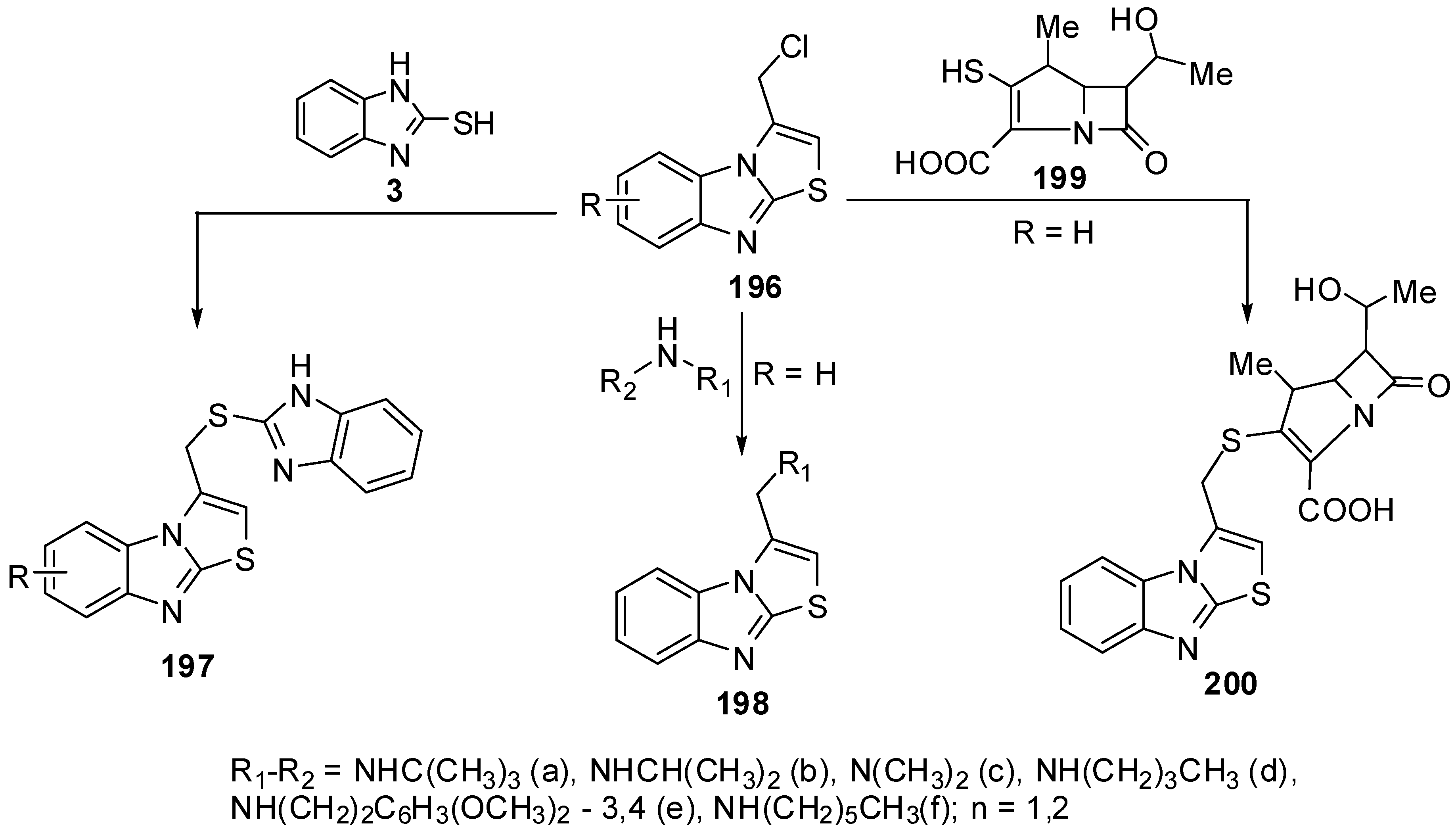
Compounds 197 were prepared from the reaction of 3-chloromethythiazolo[3,2-a]benzimidazole (196) with 2-mercaptobenzimidazole 3 [91], while 3-aminomethylthiazolo[3,2-a]benzimidazoles 198 were synthesized by reacting 3-(chloromethyl)thiazolo[3,2-a]benzimidazole 196 with primary and secondary amines [92] Recently, β-methyl carbapenem incorporating thiazolobenzimidazole moiety compound 200 [93] was prepared from lactame 199 (Scheme 55).
Scheme 55.
Reactions of 3-chloromethythiazolo[3,2-a]benzimidazole (196).
Scheme 55.
Reactions of 3-chloromethythiazolo[3,2-a]benzimidazole (196).
3.7. Reactions of C3-N4
The hydrolysis of thiazolo[3,2-a]benzimidazol-3(2H)-ones 2 with piperazine or an appropriated N-monosubstituted piperazine in ethanol under refluxing conditions resulted in the formation of piperazine derivatives 201 and 202, respectively [94] (Scheme 56).
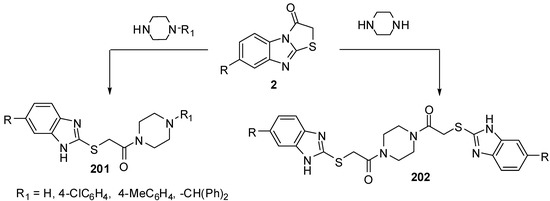
Scheme 56.
Reactions of thiazolones 2 with piperazines.
Scheme 56.
Reactions of thiazolones 2 with piperazines.
3.8. Reactions of C6
Compound 116 was prepared from ethanone 10b and bromine in acetic acid at ambient temperature [76]. Compound 141 was prepared by the treatment of 1-(3-methylthiazolo[3,2-a]benzimidazol-2-yl)ethanone (15a) under the same reaction conditions. The structure 141 was assigned for the reaction product on the basis of its single crystal X-ray diffraction [73] (Scheme 57).
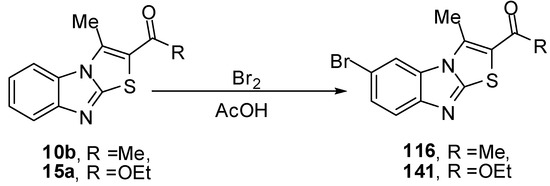
Scheme 57.
Bromination of ethanone 10b and ester 15a.
Scheme 57.
Bromination of ethanone 10b and ester 15a.
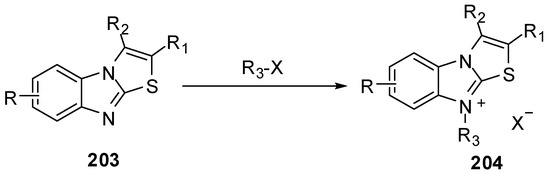
3.9. Reactions of N9
Treatment of compounds 203 with alkyl or aryl halides [95] gave 9-substituted thiazolo[3,2-a]benzimidazolium salts 204 (Scheme 58).
Scheme 58.
Reaction of compounds 203 with alkyl or aryl halides.
Scheme 58.
Reaction of compounds 203 with alkyl or aryl halides.
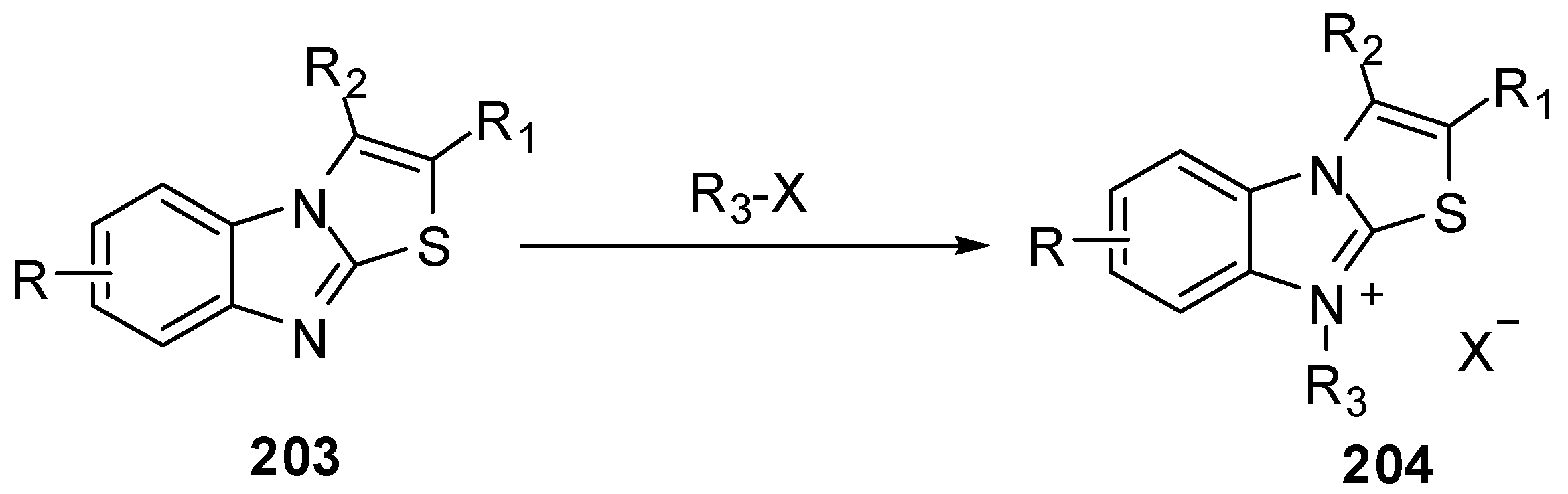
3.10. Reactions of N9-C9a
Acid hydrolysis of arylidene derivatives 55 lead to the formation of hydrochlorides of the corresponding thiazolid-2,4-ones 205via the rupture of endocyclic C=N bonds in imidazole ring [96,97] (Scheme 59).
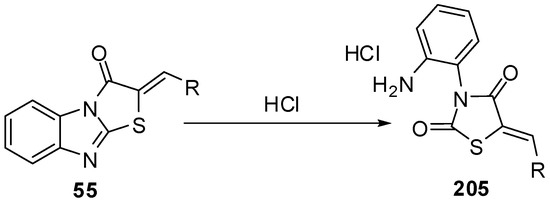
Scheme 59.
Acid hydrolysis of arylidene derivatives 55.
Scheme 59.
Acid hydrolysis of arylidene derivatives 55.
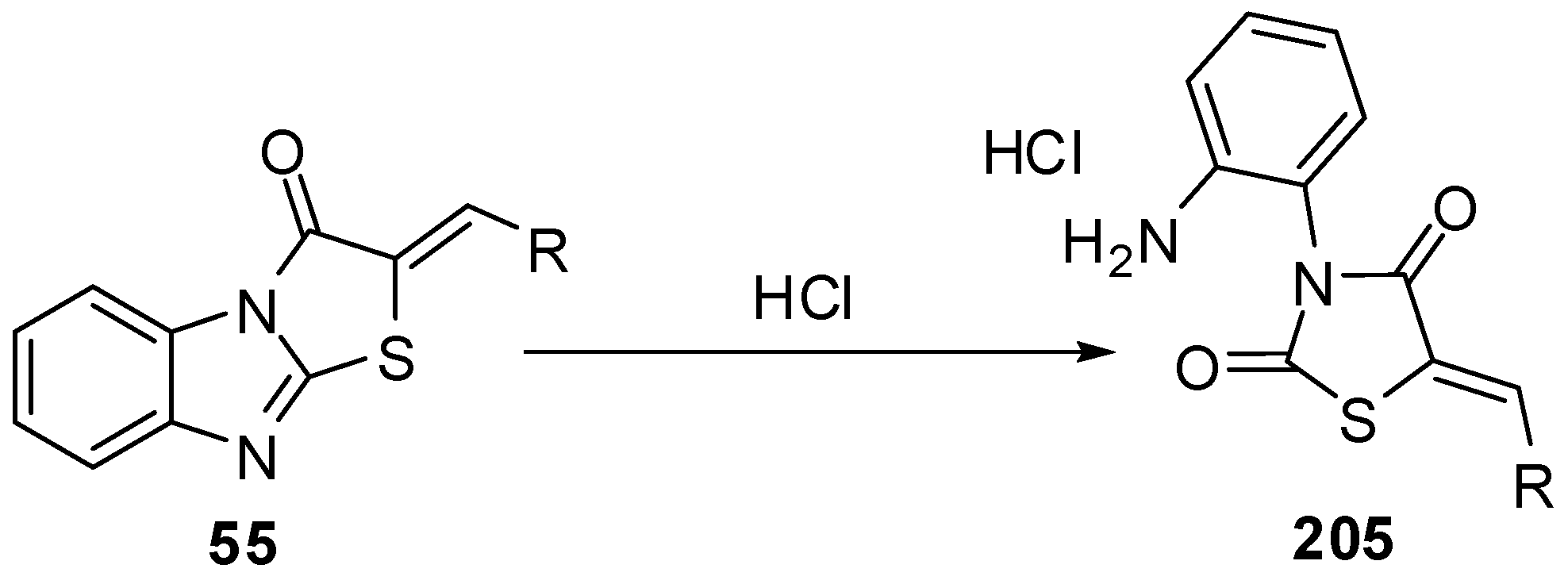
4. Spectral characteristics
Recently, Abdel-Aziz et al. reported the single crystal X-ray diffractions of 2-[2-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidin-7-yl]-3-methylthiazolo[3,2-a]benzimidazole 95b [71] and 1-(6-bromo-3-methylthiazolo[3,2-a]benzimidazol-2-yl)ethanone 141 [73]. Crystallography studies of 4'-(2,4-dichlorophenyl)-1'-methyl-2,3,2'',3''-tetra-hydro-1H-indole-3-spiro-2'-pyrrolidine-3'-spiro-2'-(1,3-benzimidazo[2,1-b]thiazole)-2,3''-dione 161 [85], 4-(2-chlorophenyl)-3-(2,6-dichlorophenyl)-spiroisoxazoline-5,2`-thiazolo[3,2-a]benzimidazol-3(2H)-one dioxane hemisolvate [98] and 5`-(2-chlorophenyl)-1`-methyl-2"-(thiazolo[3,2-a]benzimidazol-3(2H)-one)-2,3"-dione dioxane hemisolvate [99] were reported. The electron impact mass spectra of β-D-glucopyranuronic acid of thiazolo[3,2-a]benzimidazole derivatives [100] and IR spectra of some thiazolo[3,2-a]benzimidazole derivatives [101] were reported.
5. Biological Activities
This section highlights the biological activities of thiazolo[3,2-a]benzimidazoles published in the last 22 years, as other biological activity appeared up to 1988 were collected in the review of Chimirri et al. [9]. Diverse biological properties have been associated with thiazolo[3,2-a]benzimidazole derivatives in last two decades, including antibacterial [77,93,102], antifungal [69], anti-inflammatory [95,103], antiulcer [104,105,106,107], antiviral [108,109], anthelmintic [18,110] and anticancer activity [71,73,76]. The parasitological study in vitro showed that the heterocyclic benzylidines of thiazolo-[3,2-a]benzimidazoles exhibited higher activity than albendazole against T. spiralis [80].
Moreover, thiazolo[3,2-a]benzimidazole derivatives are well known as platelet activating factor antagonists [111] and neoplasm inhibitors [112]. Some thiazolo[3,2-a]benzimidazole derivatives inhibit H+/K+-ATPase and gastric secretion and are thus useful as antiulcer agents [113]. Furthermore, thiazolo[3,2-a]benzimidazol-1-oxide (WY-26,769) shows gastric antisecretory activity [114].
3-Amino-derivatives of thiazolobenzimidazole inhibited, to different extents, the oxidation of adrenaline to adrenochrome, thus preventing formation of the superoxide radical [92]. Some synthesized thiazolobenzimidazoles showed antiparasitic activity on the helminth Trichinella spiralis in infected white mice in vitro as well as in vivo [115]. In addition, many thiazolo[3,2-a]benzimidazole derivatives are of great importance due to their antidiabetic [16], broncholytic [91], immunotropic [17] and antitrichomonal activities [116]. On the other hand, several thiazolo[3,2-a]benzimidazole derivatives are used for cancer treatment [117] or prevention of cerebral infarction [118], and the treatment and/or prevention of bone diseases [119].
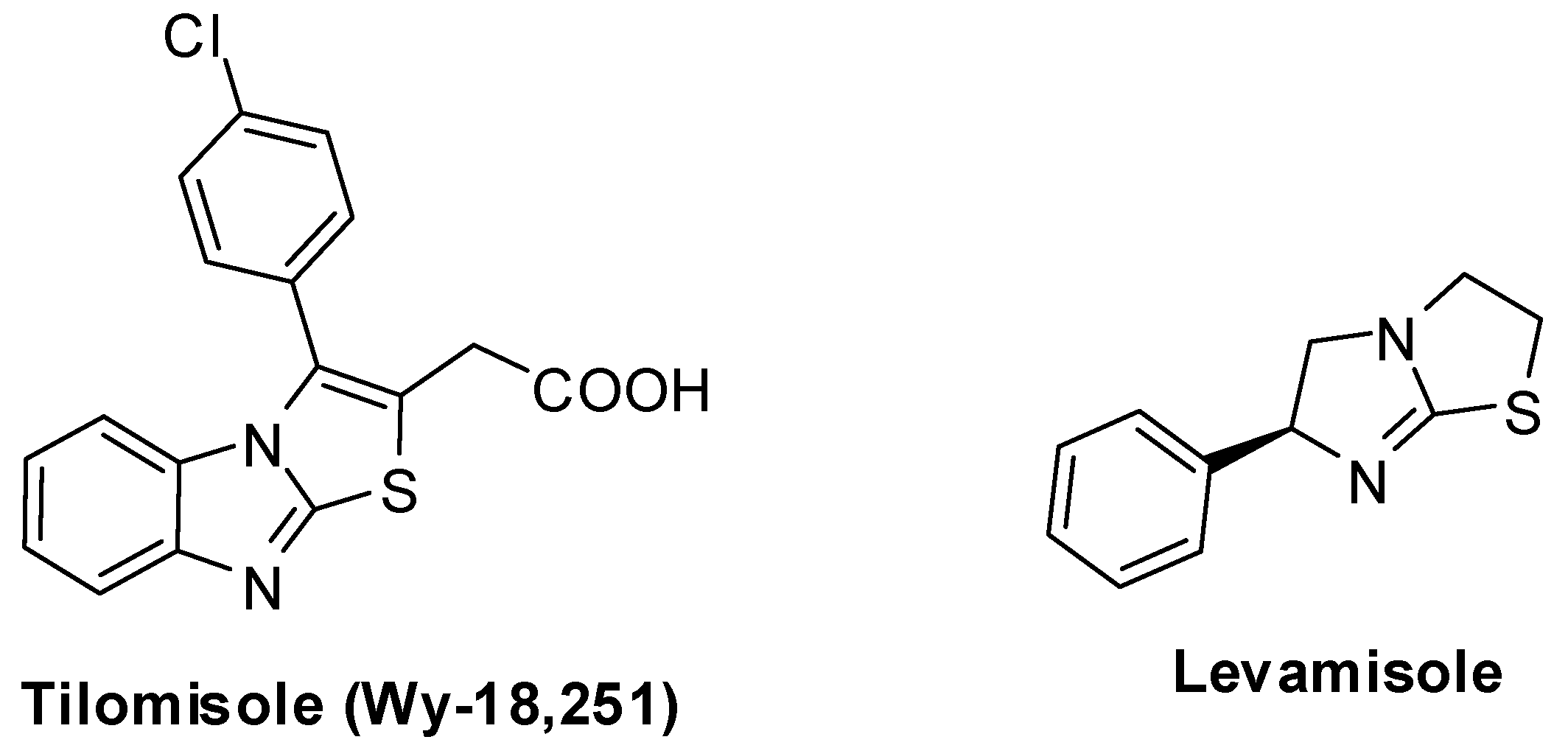
Tilomisole (WY-18,251) [120,121,122,123,124,125,126,127,128] (Figure 3) has been widely studied. It showed several potent activities, such as antinflammatory activity [123,124,125,126]. It has been reported to possess remarkable anticancer activity since it could be considered an analog of levamisole, a well-known immunomodulator which is used for the adjuvant treatment of the colon cancer. However, tilomisole (Wy-18,251) has favorable biological response effects in-vivo and it is a suitable alternative to levamisole in cancer treatments [127,128].
Figure 3.
Chemical structure of tilomisole (WY-18,251) and levamisole.
Figure 3.
Chemical structure of tilomisole (WY-18,251) and levamisole.
The combination effect of tilomisole (Wy-18,251) with aspirin or naproxen was studied in rats with carrageenan-induced paw edema and established adjuvant arthritis was reported [126]. Tilomisole was found effective in inhibiting of alpha-interleukin 1 (IL-1)-induced cartilage proteoglycan resorption in-vitro [129].
Pharmacological characterization of 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimidazole-2-carboxamide (YM-298198) (Figure 4), a high-affinity, selective, and noncompetitive antagonist of metabotropic glutamate receptor type 1 was reported [130,131,132,133,134].
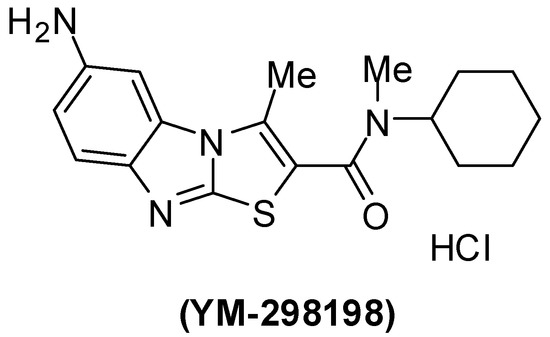
Figure 4.
Chemical structure of YM-298198.
Figure 4.
Chemical structure of YM-298198.
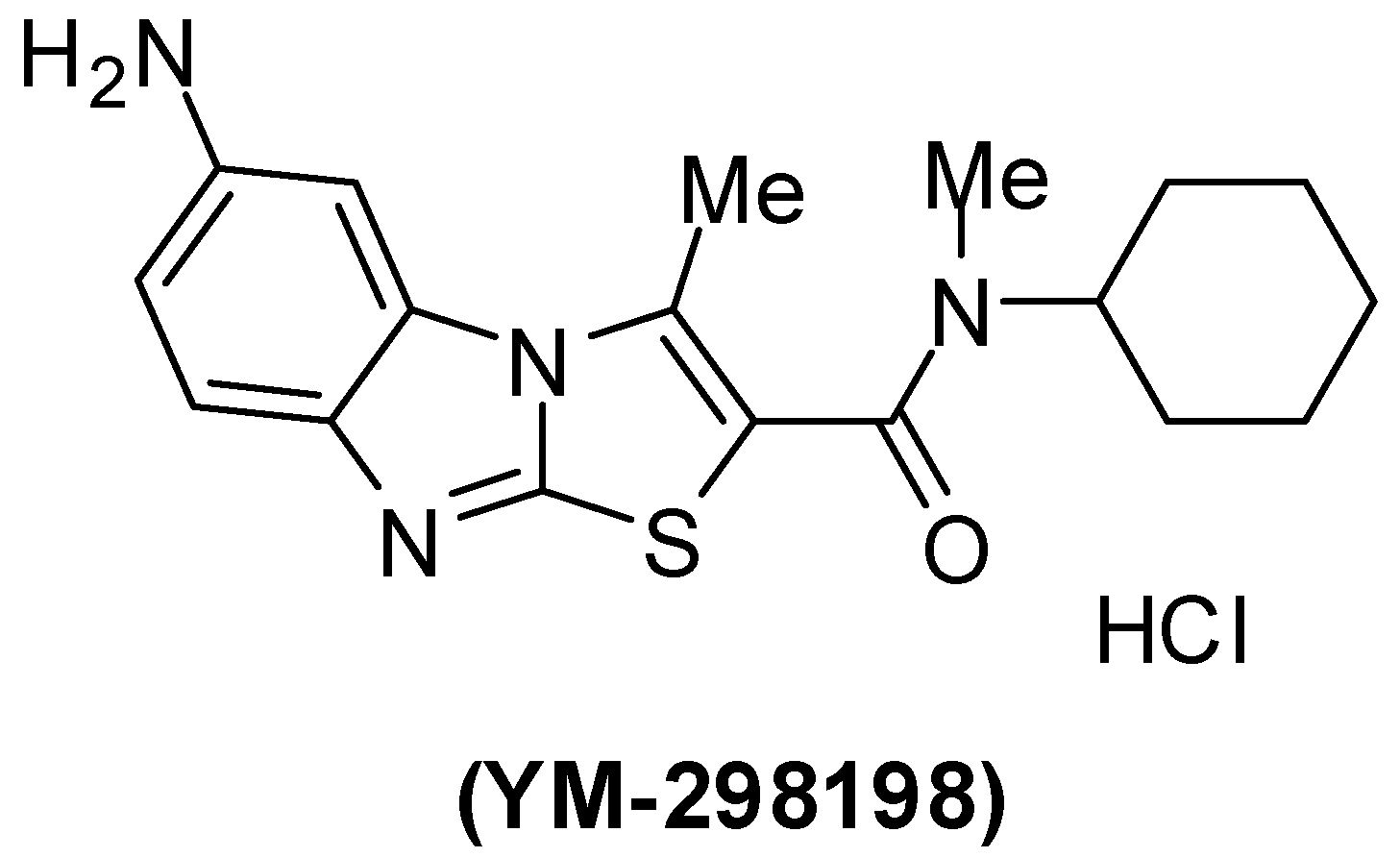
The mGlu1 antagonist YM-298198 in a physiological functional assay facilitates elucidation of this receptor's role in brain function and as a potential drug target. This compound is more potent than previously available compounds [131,132,133]. However, YM-298198 represented as the most potent known blocker of type I mechanoreceptors [134]. It also used in the treatment of neurogenic pain [135].
6-Aminomethyl-substituted thiazolobenzimidazole derivatives (R = H) (Figure 5) act as remedies for schizophrenia [136]. These compounds used in treatment or prevention of mGluR1 related diseases [137] (epilepsy, inhibition of nerve cell death, Parkinson's disease, migraine headache, anxiety disorder, cerebral infarction and neurogenic pain). 6-Aminomethyl-substituted fluorothiazolobenzimidazole derivative (R = F) as a metabotropic glutamate receptor, has excellent activity in oral administration, and is useful as a medicine [138].
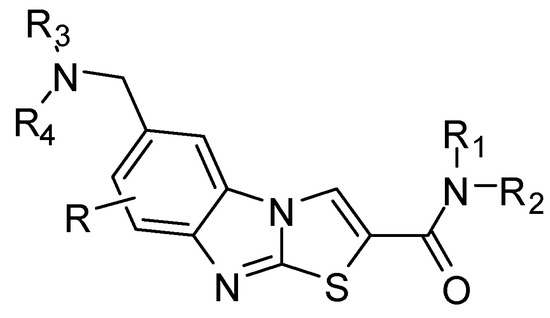
Figure 5.
6-Aminomethyl-substituted thiazolobenzimidazole derivatives.
Figure 5.
6-Aminomethyl-substituted thiazolobenzimidazole derivatives.
The activities of thiazolo[3,2-a]benzimidazole derivatives also extend into fields other than the medicinal one; for example, they are used in magnetic recording disks [139] and in photographic fields [140,141,142].
6. Conclusions
In light of the literature reports cited herein, the synthetic strategies and subsequent chemical transformations of the resulting thiazolo[3,2-a]benzimidazoles provides several important classes of functionalized compounds. The simplicity and flexibility of the experimental procedures in the generation of these classes, together with the diversity of thiazolo[3,2-a]benzimidazole chemistry, make these synthetic methodologies a highly efficient and practical method for preparation of various biologically active derivatives. The investigations in the pharmaceutical filed and medicinal applications are developing quite rapidly and we hope it will bring new and useful results.
References and Notes
- Stephen, H.W.; Wilson, F.J. Some thiazole derivatives. Part I. J. Chem. Soc. 1926, 2531–2538. [Google Scholar] [CrossRef]
- Kochergin, P.M.; Krasovskii, A.N. Synthesis of thiazolo[3,2-a]benzimidazole. Khimiya Geterotsiklicheskikh Soedinenii 1966, 6, 945–946. [Google Scholar]
- Alper, A.E.; Taurins, A. Thiazolo[3,2-a]benzimidazoles. Can. J. Chem. 1967, 45, 2903–2912. [Google Scholar] [CrossRef]
- Andersag, H.; Westphal, K. Über die synthese des antineuritischen vitamins. Ber. Deut. Chem. Ges. 1937, 70, 2035–2054. [Google Scholar] [CrossRef]
- Todd, A.R.; Bergel, F.; Karimullah. Über aneurin II. Mitteil über die synthese von N-arylthiazoliumsalzen; über einzelheiten in der konstitution des aneurins und thiochroms. Ber. Deut. Chem. Ges. 1936, 69, 217–223. [Google Scholar] [CrossRef]
- D’Amico, J.J.; Campbell, R.N.; Guinn, E.C. Derivatives of 3-methylthiazolo[3,2-a]benzimidazole. J. Org. Chem. 1965, 29, 865–869. [Google Scholar]
- DeStevens, G.; Halamanaris, A. Investigations in heterocycles III. Imidazo and imidazolino[2,1-b]thiazolium compounds. J. Am. Chem. Soc. 1957, 79, 5710–5711. [Google Scholar] [CrossRef]
- Rudner, B. Heterocyclic fused ring phenols. U.S. Patent 2,790,172,1957.
- Chimirri, A.; Grasso, S.; Romeo, G.; Zappala, M. Thiazolobenzimidazole. Heterocycles 1988, 27, 1975–2003 and References cited therein. [Google Scholar] [CrossRef]
- Kotovskaya, S.K.; Perova, N.M.; Baskakova, Z.M.; Remanova, S.A.; Charushin, V.N.; Chupakhin, O.N. Fluoro-containing heterocycles. IV. Synthesis of benzimidazole derivatives. Russ. J. Org. Chem. 2001, 37, 564–569. [Google Scholar] [CrossRef]
- Sarhan, A.A.O.; El-Shereif, H.A.H.; Mahmoud, A.M. A convenient one-pot synthesis of 2-benzimidazolyl-thioacetophenones and thiazolo[3,2-a]benzimidazoles. Tetrahedron 1996, 52, 10485–10496. [Google Scholar]
- Barchéchath, S.D.; Tawatao, R.I.; Corr, M.; Carson, D.A.; Cottam, H.B. Inhibitors of apoptosis in lymphocytes: Synthesis and biological evaluation of compounds related to pifithrin-α. J. Med. Chem. 2005, 48, 6409–6422. [Google Scholar] [CrossRef]
- Preakash, O.; Rani, N.; Goyal, S. Hypervalent iodine in the synthesis of bridgehead heterocycles: novel and facile syntheses of 3-substituted-5,6-dihydroimidazo[2,1-b]thiazoles and 3-phenylthiazolo[3,2-a]benzimidazole from acetophenones using [hydroxy(tosyloxy)iodo]benzene. J. Chem. Soc. Perkin Trans. 1 1992, 1, 707–710. [Google Scholar]
- Badr, M.Z.A.; Mahmoud, A.M.; Mahgoub, S.A.; Hozine, Z.A. Condensation and cyclization reactions of 2-hydrazinobenzimidazole, -benzoxazole, and -benzothiazole. Bull. Chem. Soc. Jpn. 1988, 61, 1339–1344. [Google Scholar] [CrossRef]
- Khaliulin, F.A.; Kataev, V.A.; Alekhin, E.K.; Volkova, S.S.; Nasyrov, K.M.; Strokin, Y.V. Synthesis of biologically active derivatives of xanthine and benzimidazole. Bashk. Khim. Zh. 1997, 4, 59–62, Chem. Abstr. 1998, 129, 49337m. [Google Scholar]
- Shorbagi, A.A.; Hayallah, A.A.; Omar, N.M.; Ahmed, A.N. Design and synthesis of some thiazolo[3,2-a]benzimidazole quaternary salts of potential antidiabetic activity. Bull. Pharm. Sci. Assiut. Univ. 2001, 24, 7–20, Chem. Abstr. 2002, 136, 151102n. [Google Scholar]
- Dianov, V.M.; Sibiryak, S.V.; Sadykov, R.F.; Strokin, Yu.V.; Khaibullina, S.F. Synthesis and immunotropic activity of thiazolo[3,2-a]benzimidazole derivatives. Khim. Farm. Zh. 1991, 25, 40–42, Chem.Abstr.1991, 115, 29202w. [Google Scholar]
- Rached, A.; Baziard-Mouysset, G.; Payard, M.; Bellan, J.; Bonnafous, R.; Tisne-Versailles, J.; Bories, C.; Loiseau, P.; Gayral, P. Synthèse et approche pharmacologique de nouveaux hétérocycles azotés et soufrés apparentés au fostedil. Eur. J. Med. Chem. 1992, 27, 425–429. [Google Scholar]
- Bercin, E.; Eroglu, Y.; Cakir, B. Synthesis and anthelmintic activities of some acetophenone and thiazolo[3,2-a]benzimidazole derivatives II. J. Fac. Pharm. Gazi. Univ. 1993, 10, 25–34, Chem. Abstr. 1994, 121, 9244g. [Google Scholar]
- D’Amico, J.J.; Bollinger, F.G.; Thompson, M.; Freeman, J.J.; Dahl, W.E.; Pustinger, J.V. Synthesis of heterocyclic compounds from 3-acetyl-3-chloropropyl acetate. J. Het. Chem. 1988, 25, 1193–1198. [Google Scholar] [CrossRef]
- Peseke, K.; Bohn, I. Preparation of substituted (polyhydroxyalkyl) thiazole analogs. Ger (East) DD 269,853, 1989. Chem.Abstr. 1990, 112, 119333g. [Google Scholar]
- Mohan, J.; Anjaneyulu, G.S.R. Heterocyclic systems containing bridgehead nitrogen atom: Synthesis of thiazolo[3,2-a]benzimidazol-3(2H)-ones and thiazolo[3,2-a]benzimidazoles. Indian J. Chem. 1989, 28B, 631–634. [Google Scholar]
- Koos, M. Acetylation and cyclization reactions of some imidazole derivatives containing 1,3-dicarbony system. Chem. Pap. 1994, 48, 108–110, Chem. Abstr.1995, 122, 55911r. [Google Scholar]
- Bercin, E.; Eroglu, Y.; Noyanalpan, N. Synthesis and structure elucidation of some acetophenone and thiazolo[3,2-a]benzimidazole derivatives III. J. Fac. Pharm. Gazi. Univ. 1993, 10, 93–104, Chem. Abstr. 1994, 121, 179548b. [Google Scholar]
- Pujari, H.K.; Shrma, B.R.; Dahiya, R.; Kumar, S.; Murakami, Y.; Tani, M. Heterocyclic systems containing bridgehead nitrogen atoms. Part LXVIII. Reaction of 5-fluorobenzimidazolyl-2-thione with chloroacetic acid: studies of orientation of cyclization in the syntheses of 6-fluoro- and 7-fluorothiazolo[3,2-a]benzimidazol-3(2-H)-ones. J. Flu. Chem. 1990, 46, 343–355. [Google Scholar] [CrossRef]
- Dahiya, R.; Pujari, H.K. Heterocyclic systems containing bridgehead nitrogen atom: Part LXVI. Studies of orientation of cyclization in the synthesis of 8-fluorothiazolo[3,2-a]benzimidazol-3(2H)one. J. Flu. Chem. 1989, 42, 245–255. [Google Scholar] [CrossRef]
- Pujari, H.K.; Murakami, Y.; Watanabe, T. Cyclization of [(5-bromo-2-benzimidazolyl)thio]acetic acid. Indian J. Chem. 1989, 28(B), 90–91. [Google Scholar]
- Dianov, V.M.; Zeleev, K.M.; Enikeev, D.A.; Timirkhanova, L.V. Reaction of mercaptoazoles with chloroacetopropyl acetate. Bashk. Khimi. Zh. 2004, 11, 32–33, Chem. Abstr. 2005, 143, 7639v. [Google Scholar]
- Abdelhamid, A.O.; Metwally, N.H.; Bishai, N.S. Reactions with hydrazonoyl halides. Part XXIX. Synthesis of some new 1,2,4-triazolo[4,3-a]benzimidazole, thiazolo[3,2-a]benzimidazole, and unsymmetrical azine derivatives. J. Chem. Res. 2000, (S), 462–463, (M), 1144-1154. [Google Scholar]
- Abdel-Mohdy, F.A.; Abdelhamid, A.O. Reactions with hydrazidoyl halides VIII: Synthesis of thiazolo[3,2-a]benzimidazoles, imidazo[2,1-b]thiazoles and imidazo[2,1-b]benzthiazoles. Arch. Pharm. Res. 1992, 15, 9–13. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Attaby, F.A. Reactions with hydrazidoyl halides. IV. Synthesis of thiazolo[3,2-a]benzimidazoles, imidazo[2,1-b]thiazoles and pyrazolo[4,3-b]thiazines. J. Het. Chem. 1991, 28, 41–44. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Abdelmegeid, F.F.; Hassan, N.M.; Zohdi, H.F. Synthesis and reactions of 1-bromo-2-(4-cyano-5-phenyl-1-p-tolylpyrazol-3-yl)-ethananedione-1-phenylhydrazone. J. Chem. Res. 1995, (S), 942–943, (M) 3036. [Google Scholar]
- Hassan, N.M.; Abdelhamid, A.O. Coupling of organotin reagents with aryl, acyl and heteroaryl halides: synthesis of pyridazine and quinoxalne derivatives. J. Chem. Res. 1995, (S), 350–351, (M) 2422-2433. [Google Scholar]
- Backert, R.; Gruner, M. 1,3-Acyl rearrangments in the cyclization of thioureas with oxalimidoyl chlorides. An approach to interesting thiocarbonyl system. J. Prakt. Chem. 1992, 334, 611–618. [Google Scholar] [CrossRef]
- Kumar, S.; Dahiya, R.; Pujari, H.K. Heterocyclic systems containing bridgehead nitrogen atom. Part LXV. Synthesis of thiazolo- and thiazino[3,2-a]benzimidazole derivatives from 4,6-dimethylbenzimidazole-2-thione. Indian J. Chem. 1990, 29(B), 989–991. [Google Scholar]
- Sharma, B.R.; Pujari, H.K. Heterocyclic systems containing bridgehead nitrogen atom. Part LXII. Synthesis of thiazolo[3,2-a]benzimidazol-3(2H)-ones. Indian J. Chem. 1988, 27(B), 121–127. [Google Scholar]
- Shawali, A.S.; Abdallah, M.A.; Zayed, M.E.M. Regioselectivity in reactions of bis-hydrazonoyl halides with some bifunctional heterocycles. J. Chin. Chem. Soc. 2002, 49, 1035–1040. [Google Scholar]
- Dawood, K.M.; Raslan, M.A.; Farag, A.M. Synthesis of 3,3′-bi-1,2,4-Triazolo[4,5-a]- benzimidazole, 5,5′-bi-1,3,4-Thiadiazole, and Thiazolo[3,2-a]benzimidazole Derivatives. Syn. Comm. 2003, 33, 4079–4086. [Google Scholar] [CrossRef]
- Shklyarenko, A.A.; Yakovlev, V.V.; Chistokletov, V.N. Aryl 2,3-dibromopropyl sulfones in S,N-tandem heterocyclizations. New synthesis of benzimidazothiazolidines. Russ. J. Org. Chem. 2004, 40, 591–592. [Google Scholar] [CrossRef]
- Korotkikh, N.I.; Aslanov, A.F.; Raenko, G.F.; Shvaika, O.P. Halocyclization and recyclization reactions: Synthesis of thiirane, thietane, and selenetane derivatives of azolones. Rus. J. Org. Chem. 1999, 35, 730–740. [Google Scholar]
- Korotkikh, N.I.; Raenko, G.F.; Aslanov, A.F. Heterocyclization of 2-(allylthio)benzimidazoles with bromine. Zh. Org. Khim. 1996, 32, 632–640, ChemAbstr. 1997, 126, 18833d. [Google Scholar]
- Korotkikh, N.I.; Raenko, G.F.; Shvaika, O.P. Heterocyclization of 2-(allylthio)benzimidazoles into benzidazo[2,1-b]-1,3-thiazine derivatives. Khim. Geterotsikl. Soedin. 1995, 410–415, Chem. Abstr. 1995, 123, 198717p. [Google Scholar]
- Popov, I.I. Study of unsaturated derivatives of azoles II. Reaction of benzimidazol-2-thione with 1,2,3-tribromopropane. Khim. Geterotsikl. Soedin. 1994, 567–570, Chem. Abstr. 1995, 123, 339886g. [Google Scholar]
- Kim, G.D.; Avdin, V.V.; Gavrilova, L.V. Reaction of 2-alkenylthiobenzimidazoles with iodine. Chem. Heterocycl. Compd. 1997, 33, 986–988. [Google Scholar] [CrossRef]
- Slivka, N.Yu.; Gevaza, Y.I.; Staninets, V.I.; Turov, A.V. Chemoselectivity and regioselectivity in halogen cyclization reactions of substituted 2-alkenylthiobenzimidazoles. Ukrainskii. Zh. 2003, 69, 104–110, Chem. Abstr. 2004, 141, 23506p. [Google Scholar]
- Heravi, M.M.; Keivanloo, A.; Rahimizadeh, M.; Bakavoli, M.; Ghassemzadeh, M. Pd-Cu catalyzed heterocyclization during Sonogashira coupling: synthesis of 3-benzylthiazolo[3,2-a]benzimidazole. TetrahedronLett. 2004, 45, 5747–5749. [Google Scholar]
- Sarhan, A.A.O.; Hozien, Z.A.; El-Sherief, H.A.H.; Mahmoud, A.M. Cyclocondensation of novel thiazolo[3,2-a]benzimiazoles. Pol. J. Chem. 1995, 69, 1479–1483. [Google Scholar]
- Sarhan, A.A.O.; El-Sherief, H.A.H.; Mahamoud, A.M. Synthesis and reactions of 3-aminothiazolo[3,2-a]benzimidazole-2-carbonitrile. J. Chem. Res. 1996, (S), 4–5, (M)1996, 116-135. [Google Scholar]
- Chi, K.; Kim, H.; lee, W.; Park, T.; Lee, U.; Furin, G. ynthesis of novel heterocycles containing perfluoroalkyl groups:Reaction of perfluoro-2-methyl-2-pentene with 1,3-binucleophilic reagents. Bull. Korean Chem. Soc. 2002, 23, 1017–1020. [Google Scholar] [CrossRef]
- Ochiai, M.; Nishi, Y.; Hashimoto, S.; Tsuchimoto, Y.; Chen, D. Synthesis of 2,4-disubstituted thiazoles from (Z)-(2-acetoxyvinyl)phenyl-λ3-iodanes: Nucleophilic substitution of α-λ3-iodanyl ketones with thioureas and thioamides. J. Org. Chem. 2003, 68, 7887–7888. [Google Scholar] [CrossRef]
- Ochiai, M.; Tada, N. Domino Michael addition-carbene rearrangement-cyclization reaction of 1-alkynyl(aryl)-λ3-bromanes with 2-mercapto-1,3-benzazoles. Chem. Commun. 2005, 5083–5085. [Google Scholar] [CrossRef]
- El-Shaieb, K.M. Reaction of dimethyl acetylenedicarboxylate with 2-mercaptoperimidine and 2-mercaptobenzimidazole. Phosphorus, Sulfur Silicon Relat. Elem. 2006, 181, 675–681. [Google Scholar] [CrossRef]
- Thyagarajan, B.S.; Glowienka, J.A.; Nee, S. Novel synthesis of thiazolidines. Phosphorus Sulfur Silicon 1988, 39, 11–18. [Google Scholar]
- Gabillet, S.; Lecerclé, D.; Loreau, O.; Carboni, M.; Dézard, S.; Gomis, J.; Taran, F. Phosphine-catalyzed construction of sulfur heterocycles. Org. Lett. 2007, 9, 3925–3927. [Google Scholar]
- Sissouma, D.; Adjou, A.; Touré, S.A.; Zoakouma, S.; Gnon, B.; Téa, G.C. Reactivity of amidinium salts' access to benzo[4,5]imidazo[2,1-b]thiazoles. Phosphorus, Sulfur Silicon Relat. Elem. 2005, 180, 1375–1378. [Google Scholar] [CrossRef]
- Öehler, E.; Kang, H.; Zbiral, E. Regioselektive cyclisierungsreaktionen acylsubstituierter epoxyphosphosphonate mit 2-mercaptoazolen: synthesen von thiazolo[3,2-a]benzimidazol-, imidazo[2,1-b]thiazol- und thiazolo[3,2-b][1,2,4]triazol-derivaten. Chem. Ber. 1988, 121, 977–990. [Google Scholar] [CrossRef]
- Lee, K.J.; Jeong, J.U.; Choi, D.O.; Kim, S.H.; Kim, S.; Park, H. Synthesis of methoxythiazolidine fused heterocycles. Bull. Korean Chem. Soc. 1991, 12, 360. [Google Scholar]
- Korotkikh, N.I.; Raenko, G.F.; Aslanov, A.F.; Shvaika, O.P. Cyclization reactions. 31. Synthesis of 2-methyl-2-(halomethyl)benzimidazo[2,1-b]thiazolidinium salts and their transformations to N-(2-methyl-2,3-epithiopropyl)benzimidazol-2-ones. Khim Geterotsikl Soedin 1994, 706–710, Chem. Abstr.1995, 122, 265304w. [Google Scholar]
- Shvaika, O.P.; Korotkikh, N.I.; Aslanov, A.F. Recyclization reactions. New approach to synthesis of azocyclic derivatives of theitanes and selenetanes. Dokl. Akad. Nauk. Ukr. SSR 1991, 4, 112–115, Chem. Abstr. 1991, 115, 207769w. [Google Scholar]
- Khaliulin, F.A.; Katayev, V.A.; Zaks, A.S.; Terekhova, N.M.; Strokin, Y.V. Products of reaction of epithiochorohydrin with benzimidazoles: Pharmacological properties. Khim. Farm. Zh. 1993, 27, 25–26, Chem. Abstr. 1995, 122, 9937f. [Google Scholar]
- Kataev, V.A.; Spirkhin, L.V.; Khaliulin, A.N.; Gailyunas, I.A. Reaction of 2-chloro-5(6)-nitrobenzimidazole with cloromethylthiirane and isomeric composition of the products. Russ. J. Org. Chem. 2002, 38, 1507–1509. [Google Scholar]
- Roussel, C.; Andreoli, F.; Roman, M.; Hristova, M.; Vanthuyne, N. New route to 3-alkylthiazolo[3,2-a]benzimidazole derivatives. Molecules 2005, 10, 327–333. [Google Scholar] [CrossRef]
- Morel, G.; Marchand, E. Diisothiocyanates as heterodienophiles or dipolarophiles in the presence of α-thioxothioamides or mesoionic thiazoles. Heteroatom Chem. 2001, 12, 617–624. [Google Scholar] [CrossRef]
- Baird, C.L.; Griffitts, A.E.; Baffic, S.; Bryant, P.; Wolf, B.; Lutton, J.; Beradini, M.; Arvanitis, G.M. Synthesis, characterization and antitumor activity of platinum triamine complexes containing imidazothiazole ligands. Inorg. Chim. Acta 1997, 256, 253–262. [Google Scholar] [CrossRef]
- Hayashibe, S.; Kamikubo, T.; Tsukamoto, S.; Sakamoto, S. Regioselective synthesis of 6- and 7-substituted thiazol[3,2-a]benzimidazole derivatives using crystallization induced region-isomerization. Heterocycles 2004, 62, 815–819. [Google Scholar] [CrossRef]
- Slivka, N.Y.; Staninets, V.I.; Yu, I.; Gevaza, A.V. Ukrainskii Khimicheskii Zh. 2004, 70, 108, Chem. Abstr. 2004, 141, 295958x.
- Abdel-Aziz, H.A.; El-Zahabi, H.S.A.; Dawood, K.M. Regioselective synthesis and in-vitro anti-tumor activity of 1,3,4-triaryl-5-N-arylpyrazole-carboxamides. Eur. J.Med. Chem. 2010, 45, 2427–2432. [Google Scholar] [CrossRef]
- Abe, N.; Fujii, H.; Kakehi, A.; Shiro, M. Revised structures, 1-methylene-1H-[1,4]thiazino[4,3-a]-benzimidazole and 10-methylene-10H-imidazo[2,1-c][1,4]-benzothiazine derivatives, for the cycloadducts accompanying rearrangement from imidazo[2,1]benzothiazole and thiazolo[3,2-a]benzimidazole derivatives with propiolic esters. J. Chem. Res. 1999, (S), 322–323. [Google Scholar]
- Pattanaik, J.M.; Pattanaik, M.; Bhatta, D. Synthesis of some new Mannich bases and their fungicidal activities. Indian. J. Het. Chem. 1998, 8, 75–76. [Google Scholar]
- Abdel-Aziz, H.A.; Hamdy, N.A.; Farag, A.M.; Fakhr, I.M.I. Synthesis of some novel pyrazolo[1,5-a]pyrimidine, 1,2,4-triazolo[1,5-a]pyrimidine, pyrido[2,3-d]pyrimidine, pyrazolo[5,1-c]-1,2,4-triazine and 1,2,4-triazolo[5,1-c]-1,2,4-triazine derivatives incorporating a thiazolo[3,2-a]benzimidazole moiety. J. Het. Chem. 2008, 45, 1. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Saleh, T.S.; El-Zahabi, H.S.A. Facile synthesis and in-vitro anti-tumor activity of some pyrazolo[3,4-b]pyridines and pyrazolo[1,5-a]pyrimidines linked to thiazolo[3,2-a]benzimidazole moiety. Arch. Pharm. 2010, 343, 24–30. [Google Scholar]
- Farag, A.M.; Dawood, K.M.; Abdel-Aziz, H.A.; Hamdy, N.A.; Fakhr, I.M.I. Synthesis of some isoxazole, pyrazole, pyrimidine, pyran and benzo/naphto[b]furan derivatives incorporating thiazolo[3,2-a]benzimidazole nucleus. Het. Chem. 2010. submitted. [Google Scholar]
- Abdel-Aziz, H.A.; Hamdy, N.A.; Gamal-Eldeen, A.M.; Fakhr, I.M.I. Synthesis of new 2-substituted 6-bromo-3-methylthiazolo[3,2-a]benzimidazole derivatives and their biological activities. Z.Naturforsch. C 2010, in press. [Google Scholar]
- Hamdy, N.A.; Abdel-Aziz, H.A.; Farag, A.M.; Fakhr, I.M.I. Synthesis of some 1,3-thiazole, 1,3,4-thiadiazole, pyrazolo[5,1-c]-1,2,4-triazine, and 1,2,4-Triazolo[5,1-c]-1,2,4-triazine derivatives based on the thiazolo[3,2-a]benzimidazole moiety. Monatsh. Chem. 2007, 138, 1001–1010. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Hamdy, N.A.; Farag, A.M.; Fakhr, I.M.I. Synthesis and reactions of 3-methylthiazolo[3,2-a]benzimidazole-2-carboxylic acid hydrazide: Synthesis of some new pyrazole, 1,3-thiazoline, 1,2,4-triazole and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazine derivatives pendant to thiazolo[3,2-a]benzimidazole moiety. J. Chin. Chem. Soc. 2007, 54, 1573–1582. [Google Scholar]
- Abdel-Aziz, H.A.; Gamal-Eldeen, A.M.; Hamdy, N.A.; Fakhr, I.M.I. Immunomodulatory and anti-cancer activity of some novel 2-substituted-6-bromo-3-methylthiazolo[3,2-a]benzimidazole derivatives. Arch. Pharm. 2009, 342, 230–237. [Google Scholar] [CrossRef]
- Allanson, N.M.; Leslie, B.W.; Thomson, S. Preparation of benzimidazo[2,1-b]thiazolidin-2-ylmethylidine)arylbenzoates as antibacterials. Brit. U.K. Pat. Appl. GB 2,376,944, 2001. Chem. Abstr. 2003, 138, 55966t. [Google Scholar]
- Bercin, E.; Usal, M.; Noyanalpan, N. Synthesis of some new 2-(substituted benzylidene)-6-(or 7)- substituted thiazolo[3,2-a]benzimidazol-3-(2H)-one. J. Fac. Pharm. Gazi .Univ. 1993, 10, 143–151, Chem. Abstr. 1994, 121, 83176c. [Google Scholar]
- Augustin, M.; Doelling, W.; Elias, D. Reactions of thiazolo[3,2-a]benzimidazol-3-one with electrophiles. Z. Chem. 1989, 29, 206–207, Chem. Abstr. 1990, 112, 20939x. [Google Scholar]
- Mavrova, A.T.; Anichina, K.K.; Vuchev, D.I.; Tsenov, J.A.; Kondeva, M.S.; Micheva, M.K. Synthesis and antitrichinellosis activity of some 2-substituted-[1,3]thiazolo[3,2-a]benzimidazol-3(2H)-ones. Bioorg. Med. Chem. 2005, 13, 5550–5559. [Google Scholar] [CrossRef]
- Gašparová, R.; Lácová, M. Study of microwave irradiation effect on condensation of 6-R-3-formylchromones with active methylene compounds. Collec. Czechoslovak Chem. Comm. 1995, 60, 1178–1185. [Google Scholar] [CrossRef]
- Rábarová, E.; Koiš, P.; Lácová, M.; Krutošíková, A. Effect of microwave irradiation on reactions of 5-arylfuran-2-carboxaldehydes with some active methylene compounds. ARKIVOC 2004, (i), 110–122. [Google Scholar]
- Ketan, H.; Satyen, P.; Ashutosh, J.; Hansa, P. Synthesis and biological evaluation of some cyanopyridines and isoxazoles. IndianJ. Het. Chem. 2004, 13, 221–224. [Google Scholar]
- Pande, P.S.; Wadodkar, K.N. Microwave-assisted solvent-free synthesis of substituted 1,3a,4,5-tetrahydropyrazol[3,4-c]pyrazoles and benzo[4,5]imidazo-5H-thiazolo[5,4-c]2,3-dihydropyrazoles. Indian J. Het. Chem. 2007, 17, 19–22. [Google Scholar]
- Xiao-Fang, L.; Ya-Qing, F.; Da-Xin, S.; Hong-Liang, C. 4'-(2,4-Dichlorophenyl)-1'-methyl-2,3,2'',3''-tetrahydro-1H-indole-3-spiro-2'-pyrrolidine-3'-spiro-2''-(1,3-benzimidazo[2,1-b]thiazole)-2,3''-dione. Acta. Cryst. Sec. E 2003, E59, o1405–o1406. [Google Scholar]
- Mahmoud, A.M.; El-Ezbawy, S.R.; El-Sherief, H.A.H.; Sarhan, A.A.O. Synthesis of some new benzimidazoles bearing different heterocyclic moieties, part III. Rev. Roum. Chim. 1997, 42, 1155–1163. [Google Scholar]
- Sarhan, A.A.O.; Hozien, Z.A.; Mahmoud, A.M.; El-Sherief, H.A.H. Synthesis and reactions of new 4-chloro-2-methylpyrimidino[4′,5′:4,5]thiazolo[3,2-a]-benzimidazoles. Monatsh. Chem. 1997, 128, 1133–1141. [Google Scholar] [CrossRef]
- Sarhan, A.A.O. Synthesis, characterization and reactions of 4-hydrazino-2-methylpyrimidino[4',5':4,5]thiazolo[3,2-a]benzimidazole. J. Chin. Chem. Soc. 2000, 47, 1279–1286. [Google Scholar]
- Sarhan, A.A.O.; Al-Dhfyan, A.; Al-Mozaini, M.A.; Adra, C.N.; Aboul-Fadl, T. Cell cycle disruption and apoptotic activity of 3-aminothiazolo[3,2-a]benzimidazole-2-carbonitrile and its homologues. Eur. J. Med. Chem. 2010, 45, 2689–2694. [Google Scholar] [CrossRef]
- Davoodnia, A.; Roshani, M.; Nadim, E.S.; Bakavoli, M.; Hoseini, N.T. Microwave-assisted synthesis of new pyrimido[4',5':4,5]thiazolo[3,2-a]benzimidazol-4(3H)-one derivatives in solvent-free condition. Chin. Chem. Lett. 2007, 18, 1327–1330. [Google Scholar] [CrossRef]
- Park, Y.J.; Such, K.H.; Kang, E.C.; Yoon, H.S.; Kim, Y.H.; Kang, D.P.; Chang, M.S. Synthesis and biological activity of the new benzimidazole derivatives having a thiazolo[3,2-a]benzimidazole moiety. Korean J. Med. Chem. 1993, 3, 124–129, Chem. Abstr. 1994, 121, 9242e. [Google Scholar]
- Dianov, V.M. Synthesis and antioxidant properties of 3-methyl substituted thiazolo[3,2-a]benzimidazole. Pharm. Chem. J. 2007, 41, 308–309. [Google Scholar] [CrossRef]
- Oh, C.; Ham, Y.; Hong, S.; Cho, J. Synthesis and antibacterial activity of new 1β-methyl carbapenem having a thiazolo[3,2-a]benzimidazole moiety. Arch. Pharm. 1995, 328, 289–291. [Google Scholar] [CrossRef]
- Mavrova, A.Ts.; Anichina, K.K.; Vuchev, D.I.; Tsenov, J.A.; Denkova, P.S.; Kondeva, M.S.; Micheva, M.K. Antihelminthic activity of some newly synthesized 5(6)-(un)substituted-1H-benzimidazol-2-ylthioacetylpiperazine derivatives. Eur. J. Med. Chem. 2006, 41, 1412–1420. [Google Scholar] [CrossRef]
- Andrews, P.; Djakiew, D. Steroid homone and nonsteroidal anti-inflammatory drug (NSAID) combinations for inducing tumor cell apoptosis. PCT Int. Appl. WO 0,298,403, 2002. Chem. Abstr. 2003, 138, 11401a. [Google Scholar]
- Bakbardina, O.V.; Nurmagambetova, R.T.; Gazalieva, M.A.; Fazylov, S.D.; Temreshev, I.I. Synthesis and fungicidal activity of pseudo- thiohydatoins, their 5-arylidine derivatives, and 5-arylidine-3-β-aminothiazolid-2,4-one hhydrochlorides. Pharm. Chem. J. 2006, 40, 735–739. [Google Scholar]
- Bakbardina, O.V.; Nurmagambetova, R.T.; Gazalieva, M.A.; Fazylov, S.D.; Zhivotova, T.S. Synthesis of pseudothiohydantoins with a condensed imidazole ring and 5-arylidine derivatives and their hydrolysis. Izvestiya Natsional’noi Akademii Nauk Res-publiki Kazakhstan, Seriya Khimicheskaya 2005, 3, 86–89, Chem. Abstr. 2006, 144, 468068z. [Google Scholar]
- Chen, H.L.; Feng, Y.Q.; Li, X.F.; Wang, G. 4-(2-Chlorophenyl)-3-(2,6-dichlorophenyl)spiroisoxazoline-5,2'-benzo[4,5]imidazo[2,1-b]thiazol-3'-one dioxane hemisolvate. Acta. Crystalographica, Sec. E 2003, E59. o1128-o1129. [Google Scholar]
- Li, X.F.; Chen, H.L.; Feng, Y.Q.; Wang, G. 5'-(2-Chlorophenyl)-1'-methyl-2'',3''-dihydroindoline-3-spiro-3'-pyrrolidine-4'-spiro-2''-(1,3-benzimidazo[2,1-b]thiazole)-2,3''-dione dioxane hemisolvate. Acta. Crystalographica, Sec. E 2003, E59, o1307–o1308. [Google Scholar]
- Breemen, R.B.V.; Stogniew, M.; Fenselau, C. Characterization of acyl-linked glucuronides by electron impact and fast atom bombardment mass spectrometry. Biomed. Environ. Mass Spectro. 1988, 17, 97–103. [Google Scholar] [CrossRef]
- Perjessy, A.; Loos, D.; Perjesi, P.; Lacova, M. Investigation of transmission of substitutuent effects in 2-arylidene derivatives of cyclohexanone, tetralone, and 3-oxo-2.3-dihydro-1-thia-3a,8-diazocyclopent[a]indene by infrared spectroscopy and theoretical methods. Acta. Fac. Rerum. Nat. Univ. Comenianae Chem. 1993, 40, 33–53, Chem. Abstr. 1995, 123, 169137u. [Google Scholar]
- Venkatesan, A.M.; Mansour, T.S.; Abe, T.; Mihira, A.; Agarwal, A.; Ushirogochi, H.; Gu, Y.; Tamai, S.; Sum, F. Preparation of heterocyclic 6-alkylidene-penems as β-lactamase inhibitors for use against bacterial infections or diseases. PCT Int. Appl. WO 0,393,280, 2002. Chem. Abstr. 2003, 139, 381302u. [Google Scholar]
- Dianov, V.M.; Chikayeva, I.G.; Timirkhanova, G.A.; Strokin, Y.V.; Zarudiy, F.S.; Lifanov, V.A. Some aminomethyl derivatives of thiazoloazoles: Synthesis and pharmacological activity. Khim. Farm. Zh. 1994, 28, 21–23, Chem.Abstr. 1995, 123, 329538f. [Google Scholar]
- Yoshida, A.; Oda, K.; Tabata, K. Preparation of benzimidazole-2-thiol derivatives as k+-adenosine triphosphatase (ATPase) inhibitors. Jpn. Kokai Tokkyo Koho JP, 0,314,566, 1991. Chem. Abstr. 1991, 115, 71600z. [Google Scholar]
- Crossley, R. Preparation of 2,3-dihydrothiazolo- and thiazinobenzimidazoles as antiulcer and antihypersecretion agents. U.S. Patent 4,873,237,1989.
- Crossley, R.; Meade, P.J. Preparation and formulation of thiazolo- and thiazinobenzimidazoles as antiulcer and antihypersecretion agents. U.S. Patent 4,725,605,1988.
- Crossley, R.; Meade, P.J. Preparation of thiazolo- and thiazinobenzimidazoles as ulcer inhibitors. Brit. U.K. Pat. Appl. GB, 2,194,230, 1988. Chem.Abstr. 1988, 109, 931011m. [Google Scholar]
- Moormann, A.E.; Becker, D.P.; Flynn, D.L.; Li, H.; Vilamil, C.I. Preparation of benzimidazolylsulfinylmethyl-arylamines as (H+/K+) APTase inhibitors useful as antiviral agents. U.S. Patent 5,945,425,1999.
- Moormann, A.E.; Becker, D.P.; Flynn, D.L.; Li, H.; Vilamil, C.I. Preparation of sulphur-contaning heterocyclic (H+/K+) APTase inhibitors as antiviral agents. PCT Int. Appl. WO 9,529,897,1995. Chem. Abstr. 1996, 124, 202255b. [Google Scholar]
- Jaguelin, S.; Robert, A.; Gayral, P. Réaction des cyanoépoxydes avec le 2-mercaptobenzimidazole et la 2-aminothiazoline et évaluation biologique des produits formés. Eur. J. Med. Chem. 1991, 26, 51–57. [Google Scholar] [CrossRef]
- Whittaker, M.; Davidson, A.H.; Spavold, Z.M.; Bowles, S.A. Preparation of γ-butyrolactol ethers as platelet activating factor antagonists. PCT Int. Appl. WO 9,117,157, 1991. Chem. Abstr. 1992, 117, 26321q. [Google Scholar]
- Ao, E.; Tanaka, H.; Nakao, T.; Yamagami, K.; Fujii, A. Nicotinamide derivatives. Jpn. Kokai Tokkyo Koho JP 0,377,881, 1991. Chem Abstr. 1991, 115, 255995. [Google Scholar]
- Kovalev, G.V.; Spasov, A.A.; Bakumov, P.A.; Reshetov, M.E.; Anisimova, V.A.; Kuz''menko, T.A.; Strokin, Yu.V.; Dianov, V.M. Influence of condensed benzimidazole derivatives on gastric secretion. Pharm. Chem. J. 1990, 24, 123–127. [Google Scholar]
- Dijoseph, J.F.; Palumbo, G.J.; Crossley, R.; Santili, A.A.; Nielsen, S.T. Gastric antisecretory activity of an acid stable H+/K+ ATPase inhibitor, WY-26,769. Drug. Dev. Res. 1991, 23, 57–64. [Google Scholar] [CrossRef]
- Mavrova, A.T.; Anichina, K.K.; Vuchev, D.I. Synthesis, structure and antihelmintic activity of some novel derivatives of thiazolo[3,2-a]benzimidazole-3(2H)-one. J. Univ.Chem. Tech. Metall. 2003, 38, 251–256. [Google Scholar]
- Alcalde, E.; Perez-Garcia, L.; Dinares, I.; Frigola, J. Heterocyclic betaines. XXII. Azinium(Azolium) 4-nitrobenzimidazolate inner salts and their derivatives with several interannular spacers. Synthesis, characterization and antitrichomonal activity. Chem. Pham. Bull. 1995, 43, 493–498. [Google Scholar] [CrossRef]
- Mckee, T.D.; Suto, R.K. Pinl peptidyl prolyl isomerase-modulating compounds and methods of use in the treatment of cancer and other Pin1-associated conditions. PCT Int. Appl. WO 0,373999, 2002. Chem. Abstr. 2003, 139, 240337x. [Google Scholar]
- Itahana, H.; Okada, S.; Ohara, A.; Negoro, K.; Nozawa, S.; Kamikubo, T.; Sakamoto, S. Preparation of imidazothiazole derivatives as ligands for metabotropic glutamate receptor. Jpn. Kokai Tokkyo Koho JP 105,085, 2002. Chem.Abstr. 2002, 136, 294827p. [Google Scholar]
- Oku, T.; Kawai, Y.; Yatabe, T.; Sato, S.; Yamazaki, H.; Kayakiri, N.; Yoshihara, K. Preparation of benzimidazoles for the preventation and/or treatment of bone deseases. PCT Int. Appl. WO 9,710,219, 1997. Chem. Abstr. 1997, 126, 293352m. [Google Scholar]
- Rainsford, K.D. Effects of anti-inflammatory drugs on interleukinl-induced cartilage proteoglycan resorption in vitro: Inhibition by aurothiophosphines but no influence from perturbed eicosanoid metabolism. J. Pharm. Pharmacol. 1989, 41, 112–117. [Google Scholar] [CrossRef]
- Bercin, E.; Uysal, M. Synthesis and anthelmintic activities of some 2-benzylidenethiazolo[3,2-a]benzimidazol-3-(2H)-one derivatives I. J. Fac. Pharm. Gazi. Univ. 1993, 10, 47, Chem. Abstr. 1994, 120, 323375s. [Google Scholar]
- Liveridge, G.G.; Conzentino, P.; Cundy, K.C.; Sarpotdar, P.P. Surface-modified nonsteroidal anti-inflammatory drug (NSAID) nanoparticles. PCT Int. Appl. WO 9,325,190, 1993. Chem. Abstr. 1994, 120, 144167. [Google Scholar]
- Franson, N.M.; Snyder, D.R. Surface-modified nonsteroidal anti-inflammatory nanoparticles. PCT Int. Appl. WO 9,624,336, 1996. Chem. Abstr. 1996, 125, 230824. [Google Scholar]
- Eickhoff, W.M.; Engers, D.A.; Mueller, K.R. Nanoparticulate NSAID compositions. U.S. Patent 9,624,336,1996.
- Carlson, R.P.; Daniel, W.C.; Gregory, F.J.; Fenichel, L.R.; Gilman, S.C. Nonsteroidal Anti-Inlammatory Drugs, 2nd; Lewis, A.J., Furst, D.E., Eds.; Dekker: New York, N.Y.,USA, 1994; pp. 333–47. [Google Scholar]
- Calhoun, W.; Gilman, S.C.; Datko, L.J.; Copenhaver, T.W.; Carlson, R.P. Interaction studies of tilomisole, aspirin, and naproxen in acute and chronic inflammation with assessment of gastrointestinal irritancy in the rat. Agents Actions (Inflamm. Res.) 1992, 36, 99–106. [Google Scholar] [CrossRef]
- Dillman, R.O.; Ryan, K.P.; Dillman, J.B.; Shawler, D.L.; Maguire, R. WY 18,251 (Tilomisole), an analog of levamisole: tolerability, and immune modulating effects in cancer patients. Mol. Biother. 1992, 4, 10–14. [Google Scholar]
- Yocum, D.E. The use of immunomodulators in early rheumatoid arthritis. Semin. Arthritis Rheum. 1994, 23, 44–49. [Google Scholar] [CrossRef]
- Goodrich, K.H.; Alvarez, X.; Holcombe, R.F. Effect of levamisole on major histocompatibility complex class I expression in colorectal and breast carcinoma cell lines. Cancer 1993, 72, 225–230. [Google Scholar] [CrossRef]
- Kohara, A.; Toya, T.; Tamura, S.; Watabiki, T.; Nagakura, Y.; Shitaka, Y.; Hayashibe, S.; Kawabata, S.; Okada, M. Radioligand binding properties and pharmacological characterization of 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimidazole-2-carboxamide (YM-298198), a high-affinity, selective, and noncompetitive antagonist of metabotropic glutamate receptor type 1. J. Pharm. Exp. Ther. 2005, 315, 163–169. [Google Scholar] [CrossRef]
- Knöpfel, T. Two new non-competitive mGlu1 receptor antagonists are potent tools to unravel functions of this mGlu1 receptor subtype. Br. J. Pharmacol. 2007, 151, 723–724. [Google Scholar] [CrossRef]
- Fukunaga, I.; Yeo, C.; Batchelor, A.M. Potent and specific action of the mGlu1 antagonists YM-298198 and JNJ16259685 on synaptic transmission in rat cerebellar slices. Br. J. Pharmacol. 2007, 151, 870–876. [Google Scholar] [CrossRef]
- Hayashibe, S.; Itahana, H.; Okada, M.; Kohara, A.; Maeno, K.; Yahiro, K.; Shimada, I.; Tanabe, K.; Negoro, K.; Kamikubo, T.; Sakamoto, S. Preparation of imidazothiazole derivatives as ligands for metabotropic glutamate receptor. PCT Int. Appl. WO 059,913, 2000. Chem. Abstr. 2002, 136, 294827p. [Google Scholar]
- Cahusac, P.M.B.; Mavulati, S.C. Non-competitive mGlu1 receptor antagonists units in the rat sinus hair follicle. Neuroscience 2009, 163, 933–941. [Google Scholar] [CrossRef]
- Okada, M.; Nagakura, Y.; Kiso, T.; Toya, T.; Hayashibe, S. Remedies for neurogenic pains. PCT Int. Appl. WO 0,108,705, 2001. Chem. Abstr. 2001, 134, 141763y. [Google Scholar]
- Ohta, H.; Tanaka, T.; Sato, A.; Ohkubo, M.; Tsukamoto, N.; Mitsuya, M. Remedies for schizophrenia. PCT Int. Appl. WO 16,287, 2004. ChemAbstr. 2004, 140, 193088a. [Google Scholar]
- Itahana, H.; Fujiyasu, J.; Hayashibe, S.; Watanabe, T. Okada, M.; Toya, T. Preparation of aminomethyl-substituted thiazolobenzimidazole derivatives having metabotropic glutamate receptor activity. PCT Int. Appl. WO 0,378,441, 2002. Chem.Abstr. 2003, 139, 261301w. [Google Scholar]
- Itahana, H.; Fujiyasu, J.; Watanabe, T.; Okada, M.; Toya, T. Preparation of aminomethyl-substituted fluoro thiazolobenzimidazole derivatives with affinity for mGLuR1 receptors. PCT Int. Appl. WO 106,348, 2004. Chem. Abstr. 2005, 142, 38247z. [Google Scholar]
- Momose, S. Magnetic recording disk having thin film fluorine contaning lubricating coating. Jpn. Kokai Tokkyo Koho JP 09 115,126, 1997. Chem. Abstr. 1998, 127, 59685w. [Google Scholar]
- Ito, H. Silver halide photographic material containing hydrazine and thiazolothiazolium derivatives. Jpn. Kokai Tokkyo Koho JP, 06 258 75, 1994. Chem. Abstr. 1995, 122, 174217d. [Google Scholar]
- Harada, H.; Oka, S. Electrophotographic receptors using specific azo compound. Jpn. Kokai Tokkyo Koho JP, 05,150,523, 1993. Chem. Abstr. 1993, 119, 259520k. [Google Scholar]
- Kita, H.; Kaneko, Y.; Ikesu, S. Photographic cyan coupler with heat and moisture. Jpn. Kokai Tokkyo Koho JP, 04,260,035, 1992. Chem. Abstr. 1993, 118, 222791c. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2010 by the authors;
