Abstract
Starting from benzofuran-2-methanal, 6-substituted benzothiazole-2-amines and malonic esters, sixteen title compounds were designed and synthesized seeking to introduce anti-TMV activity. The structures of the newly synthesized compounds were confirmed by 1H-NMR, 13C-NMR, IR spectra, and MS (HREI) analysis. The bioassays identified some of these new compounds as having moderate to good anti-TMV activity. The compounds 5i and 5m have good antiviral activity against TMV with a curative rate of 52.23% and 54.41%, respectively, at a concentration of 0.5 mg/mL.
1. Introduction
Benzothiazoles have varied biological activities [1,2,3]. They are widely found in bioorganic and medicinal chemistry with applications in drug discovery and are still of great scientific interest nowadays [4]. Benzothiazole moieties are part of compounds showing numerous biological activities such as anti-bacterial, anti-microbial, anthelmintic, antitumor, anti-inflammatory properties [5,6,7,8]. Compounds containing benzofuran moieties are also widespread in Nature, with a broad spectrum of physiological bioactivities, used as pesticidal, anti-bacterial, insecticide, anti-tumor, anti-inflammatory [9,10,11], and so on.
β-Amino ester derivatives are key intermediate in the synthesis of β-lactam antibiotics, which are important components of many natural products and therapeutic agents [12,13,14]. Therefore, the asymmetric synthesis of β-amino ester derivatives has become a field of increasing interest in organic synthetic chemistry over the past few years [15,16,17,18,19,20].
As an extension study of our group’s research [19,21], we have now synthesized a series of novel β-amino ester derivatives containing benzofuran and benzothiazole units. The structures of these newly synthesized compounds were confirmed by 1H-NMR, 13C-NMR, IR spectra, and MS (HREI) analysis. Bioassays identified these new compounds as possessing weak to good antiviral activities.
2. Results and Discussion
2.1. Experimental Condition Optimization
Our strategy is outlined in Scheme 1. Benzofuran-2-methanal (1) reacted with 6-substituted benzothiazoles 2a–d under reflux conditions in toluene, with some acetic acid as catalyst affording the imines 3a–d. After recrystallization by ethanol, the final compounds were isolated in good yields. All products 3a–d were characterized by spectroscopic methods.
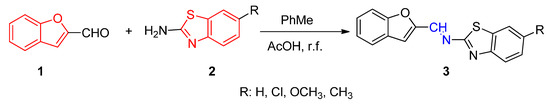
Scheme 1.
Synthesis of imines.
According to the Scheme 2, we tested the influence of the reaction temperature, solvent, and reaction times. The reaction temperature had a pronounced effect on the yield, solvents such as THF, acetone, and toluene showed lower yields compared to DCM (Table 1). According to an optimized procedure, the best result was achieved at 35 °C in DCM.
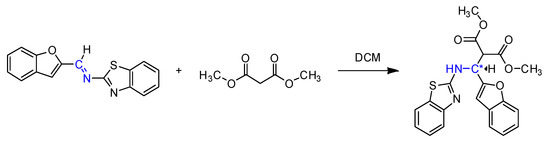
Scheme 2.
Screening of the reaction conditions.

Table 1.
The optimization of reaction conditions.
| Entry | Solvent | Temp. (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | THF | r.t. | 24 | 34 |
| 2 | THF | r.f. | 12 | 38 |
| 3 | PhMe | r.t. | 24 | 46 |
| 4 | PhMe | r.f. | 12 | 72 |
| 5 | DCM | r.t. | 24 | 62 |
| 6 | DCM | 35 | 10 | 78 |
Having established the ideal reaction conditions, the synthetic scope of the reaction was evaluated with different imines and malonic esters (Scheme 3). The results of our studies are summarized in Table 2. It can be seen that the reactions afforded good yields.
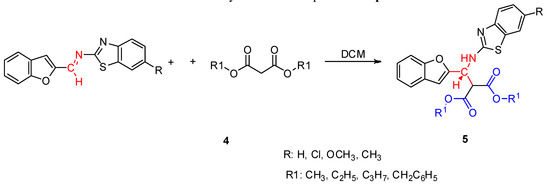
Scheme 3.
Synthesis of compounds 5a−p.

Table 2.
The yields of the Mannich reactions of imines and malonic esters.
| Entry | 5 | R | R1 | Time(h) | Yield(% a) |
|---|---|---|---|---|---|
| 1 | 5a | 6-H | -CH3 | 12 | 82 |
| 2 | 5b | 6-H | -C2H5 | 12 | 80 |
| 3 | 5c | 6-H | -C3H7 | 12 | 78 |
| 4 | 5d | 6-H | -CH2C6H5 | 24 | 62 |
| 5 | 5e | 6-Cl | -CH3 | 12 | 85 |
| 6 | 5f | 6-Cl | -C2H5 | 12 | 86 |
| 7 | 5g | 6-Cl | -C3H7 | 12 | 82 |
| 8 | 5h | 6-Cl | -CH2C6H5 | 24 | 67 |
| 9 | 5i | 6-OCH3 | -CH3 | 12 | 81 |
| 10 | 5j | 6-OCH3 | -C2H5 | 12 | 80 |
| 11 | 5k | 6-OCH3 | -C3H7 | 12 | 79 |
| 12 | 5l | 6-OCH3 | -CH2C6H5 | 24 | 60 |
| 13 | 5m | 6-CH3 | -CH3 | 12 | 81 |
| 14 | 5n | 6-CH3 | -C2H5 | 12 | 76 |
| 15 | 5o | 6-CH3 | -C3H7 | 12 | 76 |
| 16 | 5p | 6-CH3 | -CH2C6H5 | 24 | 64 |
a Isolated yield after chromatographic purification.
2.2. Anti-TMV Activity
The antiviral activity of compound 5 against TMV was assayed by the reported method [9]. As it can be seen from the results presented in Table 3, some compounds possess good anti-TMV activity, such as the curative rates against TMV of compounds 5i and 5m which were 52.23% and 54.41%. These values are close to that of the the commercial control ningnanmycin (curative rate 55.27%).

Table 3.
The in vivo antiviral activity towards TMV of the new compounds at 0.5 (mg/mL).
| Entry | Compound | Protection Effect % | Curative Effect % | Inhibition Effect % |
|---|---|---|---|---|
| 1 | 5a | 57.95 | 48.27 | 39.72 |
| 2 | 5b | 63.08 | 47.18 | 43.42 |
| 3 | 5c | 62.11 | 43.83 | 33.29 |
| 4 | 5d | 48.58 | 49.17 | 28.45 |
| 5 | 5e | 58.67 | 46.28 | 33.50 |
| 6 | 5f | 68.32 | 48.34 | 43.98 |
| 7 | 5g | 56.76 | 48.27 | 46.77 |
| 8 | 5h | 49.77 | 44.20 | 44.21 |
| 9 | 5i | 59.79 | 52.23 | 40.98 |
| 10 | 5j | 58.76 | 48.03 | 39.27 |
| 11 | 5k | 40.20 | 46.73 | 41.04 |
| 12 | 5l | 52.34 | 35.53 | 29.08 |
| 13 | 5m | 68.75 | 54.41 | 46.88 |
| 14 | 5n | 66.04 | 48.09 | 39.70 |
| 15 | 5o | 58.97 | 47.93 | 42.33 |
| 16 | 5p | 46.36 | 38.46 | 45.96 |
| 17 | Ningnanmycin | 82.03 | 55.27 | 52.16 |
3. Experimental
3.1. Instruments and Chemicals
Melting points were determined on a XT-4 binocular microscope (Beijing Tech Instrument Co., Beijing, China) and were not corrected. IR spectra were recorded on a Bruker VECTOR 22 spectrometer in KBr disks. 1H- and 13C-NMR spectral analyses (solvent CDCl3 or DMSO-d6) were performed on a JEOL-ECX 500 NMR spectrometer at room temperature using TMS as an internal standard. Elemental analyses were performed on an Elementar Vario-III CHN analyzer. MS spectra were recorded with a VG Autospec-3000 spectrometer. Analytical TLC was performed on silica gel GF254. Column chromatographic purification was carried out using silica gel GF254. Commercial reagents were used as received, unless otherwise indicated. Reactions were performed under a positive pressure of dry argon in oven-dried or flame-dried glassware equipped with a magnetic stir bar. Standard inert atmosphere techniques were used in handling all air and moisture sensitive reagents. All reagents were of analytical reagent grade or chemically pure. All solvents were dried, deoxygenated and redistilled before use.
3.2. Synthesis
3.2.1. General Synthetic Methods for 3a–d
To a magnetically stirred solution of 6-substituted benzothiazole (6.80 mmol) in toluene (5 mL) benzofuran-2-methanal (6.80 mmol) dissolved in toluene (5 mL) was added dropwise at room temperature. After attaching a Dean Stark trap, the reaction was allowed to reflux after adding acetic acid (0.5 mL). Complete consumption of starting materials was observed after 24 h. After recrystallization from ethanol the final compounds 3a–d were isolated in good yields.
3.2.2. Characterization of 3a–d
N-(Benzofuran-2-ylmethylene)benzo[d]thiazol-2-amine (3a): yellow solid; mp:173–174 °C; yield: 77%; 1H-NMR (CDCl3) δ (ppm): 8.11 (d, 1H, J = 5 Hz, 11-CH), 7.98 (d, 1H, J = 5 Hz, 9-CH), 7.89 (d, 1H, J = 5 Hz, 6-CH), 7.77 (d, 1H, J = 10 Hz, 17-CH), 7.61–7.54 (m, 2H, 7-CH, 8-CH), 7.52 (s, 1H, 20-CH), 7.47–7.46 (m, 2H, 18-CH, 19-CH), 7.43-7.39 (m, 1H, 14-CH); 13C-NMR (DMSO-d6) δ (ppm): 171.5 (2-C), 156.4 (16-C), 154.8 (4-C), 151.8 (11-C), 135.1 (12-C), 129.8 (5-C), 129.4 (15-C), 128.0 (8-C), 127.4 (7-C), 125.9 (19-C), 124.7 (18-C), 123.9 (6-C), 123.3 (9-C), 120.2 (17-C), 113.0 (20-C), 112.6 (14-C); IR (KBr, cm−1) ν: 3049, 608, 1556, 1546, 1417, 1309, 1116, 954, 813, 750, 729; MS (ESI): m/z = 279 ([M+H]+), 301 ([M+Na]+).
N-(Benzofuran-2-ylmethylene)-6-chlorobenzo[d]thiazol-2-amine (3b): yellow solid; mp: 209–212 °C; yield: 76%; 1H-NMR (CDCl3) δ (ppm): 9.17 (s, 1H, 6-CH), 8.23 (s, 1H, 11-CH), 7.96 (s, 1H, 17-CH), 7.91 (d, 1H, J = 5 Hz, 9-CH), 7.84 (d, 1H, J = 5 Hz, 8-CH), 7.72 (s, 1H, 20-CH), 7.54–7.54 (m, 2H, 18-CH, 19-CH), 7.36 (s, 1H, 14-CH); 13C-NMR (DMSO-d6) δ (ppm): 172.5 (2-C), 156.5 (16-C), 155.3 (4-C), 152.2 (11-C), 150.6 (6-C), 136.5 (12-C), 130.2 (5-C), 129.5 (15-C), 129.2 (8-C), 127.8 (7-C), 124.7 (19-C), 124.4 (18-C), 123.9 (9-C), 122.7 (17-C), 120.6 (20-C), 112.7 (14-C); IR (KBr, cm−1) ν: 3086, 1598, 1539, 1425, 1330, 1294, 1166, 1122, 950, 812, 802, 738, 704, 605; MS (ESI): m/z = 313 ([M+H]+), 335 ([M+Na]+).
N-(Benzofuran-2-ylmethylene)-6-methoxybenzo[d]thiazol-2-amine (3c): yellow solid; mp: 147–150 °C; yield: 64%; 1H-NMR (CDCl3) δ (ppm): 9.09–9.07 (m, 1H, 11-CH), 7.88 (d, 1H, J = 5 Hz, 17-CH ), 7.81 (t, 2H, J = 15 Hz, 6-CH, 8-CH), 7.70 (t, 1H, J = 15 Hz, 9-CH), 7.62 (d, 1H, J = 5 Hz, 20-CH), 7.51 (d, 1H, J = 5 Hz, 19-CH), 7.34 (s, 1H, 14-CH), 7.09-7.07 (m, 1H, 18-CH), 3.81 (s, 3H, 22-C OCH3); 13C-NMR (DMSO-d6) δ (ppm): 168.9 (2-C), 157.9 (16-C), 156.3 (4-C), 153.5 (11-C), 152.4 (6-C), 146.1 (12-C), 136.5 (5-C), 129.1 (15-C), 128.0 (8-C), 124.6 (7-C), 124.0 (19-C), 123.7 (18-C), 119.4 (9-C), 116.6 (17-C), 112.5 (20-C), 105.6 (14-C), 56.2 (22-C); IR (KBr, cm−1) ν: 3093, 1598, 1556, 1541, 1485, 1452, 1429, 1263, 1226, 1120, 1056, 1024, 954, 908, 833, 817, 756, 609; MS (ESI): m/z = 309 ([M+H]+), 331 ([M+Na]+).
N-(Benzofuran-2-ylmethylene)-6-methylbenzo[d]thiazol-2-amine (3d): yellow solid; mp: 185–190 °C; yelid: 78%; 1H-NMR (CDCl3) δ (ppm): 8.37-8.36 (m, 2H, 11-CH, 6-CH), 8.05–8.00 (m, 2H, 9-CH, 17-CH), 7.92–7.90 (m, 2H, 18-CH, 19-CH), 7.51–7.47 (m, 2H, 14-CH, 20-CH), 7.46-7.40 (m, 1H, 8-CH), 2.43 (s, 3H, 21-CH3); 13C-NMR (DMSO-d6) δ (ppm): 181.4 (2-C), 170.5 (16-C), 166.2 (4-C), 154.3 (11-C), 129.8 (6-C), 129.3 (12-C), 128.8 (5-C), 126.9 (15-C), 124.7 (8-C), 123.8 (7-C), 122.9 (19-C), 122.5 (18-C), 121.3 (9-C), 120.0 (17-C), 117.9 (20-C), 112.9 (14-C), 21.7 (22-C); IR (KBr, cm−1) ν: 3028, 1602, 1579, 1562, 1473, 1433, 1361, 1305, 1213, 1186, 1120, 956, 916, 858, 825, 750, 740, 686, 609; MS (ESI): m/z = 293 ([M+H]+), 315 ([M+Na]+).
3.2.3. General Synthetic Methods for 5a–p
To a magnetically stirred solution of imines (0.50 mmol) in DCM (5 mL) malonic ester (0.7 mmol) was added dropwise at room temperature. The reaction was allowed to reach 35 °C, and complete consumption of starting materials was observed after 12–24 h. After removing the solvent by reduced pressure distillation, the mixture was subjected to column chromatography on silica gel (EA/PE = 1:7) to afford compounds 5a–p.
Dimethyl 2-((Benzo[d]thiazol-2-ylamino)(benzofuran-2-yl)methyl) malonate (5a): white solid; mp: 70–72 °C; yield 82%; 1H-NMR (CDCl3) δ (ppm): 7.61–7.58 (m, 2H, 24-CH, 27-CH), 7.52–7.47 (m, 1H, 14-CH), 7.45–7.41 (m, 1H, 17-CH), 7.33–7.26 (m, 2H, 25-CH, 26-CH), 7.24–7.18 (m, 1H, 16-CH), 7.15-7.09 (m, 1H, 15-CH), 6.71 (s, 1H, NH), 6.75 (d, 1H, J = 15 Hz, 11-CH), 6.07 (d, 1H, J = 10 Hz, 8-CH), 4.37–4.33 (m, 1H, 2-CH), 3.76 (s, 6H, 28-CH3, 29-CH3); 13C-NMR (CDCl3) δ (ppm): 168.4 (20-C), 167.1 (1-C), 165.8 (3-C), 155.0 (10-C), 154.1 (13-C), 152.1 (22-C), 131.0 (23-C), 128.1 (12-C), 126.1 (15-C), 124.5 (16-C), 123.1 (25-C), 122.3 (26-C), 121.3 (24-C), 121.0 (27-C), 119.7 (14-C), 111.3 (17-C), 104.7 (11-C), 53.9 (8-C), 53.8 (2-C), 53.1 (28-C), 52.8 (29-C); MS (ESI): m/z = 411 ([M+H]+), 433 ([M+Na]+); MS (HREI): C21H18N2O5S Na for +, calculated 410.0940, found 410.0940; IR (KBr, cm−1) ν 3385, 2951, 1745, 1732, 1595, 1539, 1452, 1435, 1355, 1259, 1207, 1172, 1014, 966, 750, 725.
Diethyl 2-((Benzo[d]thiazol-2-ylamino)(benzofuran-2-yl)methyl) malonate (5b): white solid; mp: 110–112 °C; yield 80%; 1H-NMR (CDCl3) δ (ppm): 7.59 (d, 2H, J = 5 Hz, 24-CH, 27-CH), 7.50 (s, 1H, 14-CH), 7.43 (s, 1H, 17-CH), 7.31–7.26 (m, 3H, 25-CH, 26-CH, 16-CH), 7.20 (s, 1H, 15-CH), 7.12 (s, 1H, NH), 6.75 (s, 1H, 11-CH), 6.08 (s, 1H, 8-CH), 4.33 (d, 1H, J = 5 Hz, 2-CH), 4.25–4.17 (m, 4H, 28-CH2, 30-CH2 ), 1.21 (s, 6H, 29-CH3, 31-CH3); 13C-NMR (CDCl3) δ (ppm): 168.1 (20-C), 166.7 (1-C), 165.9 (3-C), 155.0 (10-C), 154.3 (13-C), 152.1 (22-C), 131.0 (23-C), 128.1 (12-C), 126.0 (15-C), 124.5 (16-C), 123.1 (25-C), 122.2 (26-C), 121.3 (24-C), 120.9 (27-C), 119.6 (14-C), 111.2 (17-C), 104.7 (11-C), 62.5 (28-C), 62.1 (30-C), 54.1 (8-C), 52.8 (2-C), 14.1 (29-C), 14.0 (31-C); MS (ESI): m/z = 439 ([M+H]+), 461 ([M+Na]+); MS (HREI): C23H22N2O5S Na for +, calculated 438.1249, found 438.1253; IR (KBr, cm−1) ν 3369, 1739, 1718, 1537, 1485, 1454, 1286, 1242, 1201, 1184, 1176, 1018, 947, 802, 758.
Dipropyl 2-((Benzo[d]thiazol-2-ylamino)(benzofuran-2-yl)methyl) malonate (5c): white solid; mp: 127–129 °C; yield 78%; 1H-NMR (CDCl3) δ (ppm): 7.59–7.55 (m, 2H, 24-CH, 27-CH), 7.48 (d, 1H, J = 5 Hz, 14-CH), 7.42 (d, 1H, J = 5 Hz, 17-CH), 7.30–7.23 (m, 2H, 25-CH, 26-CH), 7.19 (t, 1H, J = 5 Hz, 16-CH), 7.09 (t, 1H, J = 5 Hz, 15-CH), 6.73 (d, 1H, J = 5 Hz, 11-CH), 6.07 (s, 1H, NH), 4.35–4.32 (m, 1H, 8-CH), 4.13-4.09 (m, 4H, 28-CH2, 29- CH2), 3.40 (d, 1H, J = 5 Hz, 2-CH), 1.61–1.56 (m, 4H, 30-CH2, 31-CH2), 0.87-0.82 (m, 6H, 32-CH3, 33-CH3); 13C-NMR (CDCl3) δ (ppm): 168.3 (20-C), 166.8 (1-C), 165.7 (3-C), 155.0 (10-C), 154.4 (13-C), 152.2 (22-C), 131.0 (23-C), 128.1 (12-C), 126.0, (15-C) 124.4 (16-C), 123.1 (25-C), 122.2 (26-C), 121.3 (24-C), 120.9 (27-C), 119.7 (14-C), 111.2 (17-C), 104.6 (11-C), 68.0 (28-C), 67.7 (29-C), 54.0 (8-C), 52.8 (2-C), 21.9 (30-C), 21.8 (31-C), 10.3 (32-C), 10.2 (33-C); MS (ESI): m/z = 467 ([M+H]+), 489 ([M+Na]+); MS (HREI): C25H26N2O5S Na for +, calculated 438.1249, found 466.1550; IR (KBr, cm−1) ν 3356, 2962, 1751, 1724, 1600, 1564, 1548, 1454, 1442, 1386, 1313, 1271, 1176, 1136, 1053, 925, 815, 759, 754.
Dibenzyl 2-((Benzo[d]thiazol-2-ylamino)(benzofuran-2-yl)methyl) malonate (5d): white solid; mp: 110–112 °C; yield 62%; 1H-NMR (CDCl3) δ (ppm): 7.57 (d, 1H, J = 10 Hz, 24-CH), 7.54 (d, 1H, J = 10 Hz, 27-CH), 7.44 (d, 1H, J = 10 Hz, 14-CH), 7.34–7.32 (m, 10H, 31-CH, 32-CH, 33-CH, 34-CH, 35-CH, 37-CH, 38-CH, 39-CH, 40-CH, 41-CH), 7.25 (s, 1H, NH), 7.19–7.18 (m, 1H, 17-CH), 7.16–7.15 (m, 2H, 25-CH, 26-CH), 7.13–7.11 (m, 2H, 15-CH, 16-CH), 6.65 (s, 1H, 11-CH), 5.17 (s, 4H, 28-CH2, 29-CH2), 4.44 (d, 1H, J = 5 Hz, 8-CH), 3.48 (s, 1H, 2-CH); 13C-NMR (CDCl3) δ (ppm): 167.9 (20-C), 166.4 (1-C), 166.3 (3-C), 165.5 (10-C), 155.0 (13-C), 154.1 (22-C), 152.1 (30-C), 135.3 (36-C), 134.8 (23-C), 134.7 (32-C), 131.1 (34-C), 128.7 (38-C), 128.6 (40-C), 128.5 (12-C), 128.5 (31-C), 128.4 (35-C), 128.3 (37-C), 128.2 (41-C), 128.1 (39-C), 126.0 (33-C), 124.5 (15-C), 123.1 (26-C), 122.2 (25-C), 121.3 (16-C), 120.9 (24-C), 119.7 (17-C), 111.3 (27-C), 104.7 (14-C), 67.4 (11-C), 54.1 (28-C), 54.0 (29-C), 52.6 (8-C), 41.7 (2-C); MS (ESI): m/z = 563 ([M+H]+), 585 ([M+Na]+); MS (HREI): C33H26N2O5S Na for +, calculated 562.1562, found 562.1569; IR (KBr, cm−1) ν 3352, 2922, 1747, 1716, 1537, 1454, 1444, 1348, 1249, 1172, 954, 750, 727.
Dimethyl 2-(Benzofuran-2-yl((6-chlorobenzo[d]thiazol-2-yl)amino)methyl) malonate (5e): white solid; mp: 115–117 °C; yield 85%; 1H-NMR (CDCl3) δ (ppm): 7.53 (s, 1H, 24-CH), 7.49 (d, 1H, J = 5 Hz, 27-CH), 7.46 (d, 1H, J = 5 Hz, 14-CH), 7.42 (d, 1H, J = 5 Hz, 17-CH), 7.26 (d, 1H, J = 5 Hz, 25-CH), 7.22–7.17 (m, 2H, 26-CH, 15-CH), 6.73 (d, 1H, J = 5 Hz, 11-CH), 6.03 (s, 1H, NH), 5.29 (d, 1H, J = 5 Hz, 8-CH), 4.32-4.31 (m, 1H, 2-CH), 3.73 (s, 6H, 29-CH3, 30-CH3); 13C-NMR (CDCl3) δ (ppm): 168.4 (20-C), 167.0 (1-C), 165.9 (3-C), 155.0 (10-C), 153.8 (13-C), 150.8 (22-C), 132.2 (23-C), 128.0 (12-C), 127.4 (15-C), 126.5 (16-C), 124.6 (25-C), 123.2 (26-C), 121.4 (24-C), 120.6 (27-C), 120.3 (14-C), 111.3 (17-C), 104.7 (11-C), 53.7 (8-C), 53.4 (2-C), 53.1 (29-C), 52.7 (30-C); MS (ESI): m/z = 445 ([M+H]+), 467 ([M+Na]+); MS (HREI): C21H17ClN2O5S Na for +, calculated 444.0547, found 444.0547; IR (KBr, cm−1) ν 3340, 2954, 1745, 1593, 1537, 1483, 1436, 1359, 1226, 1161, 1138, 1037, 974, 954, 875, 812, 759.
Diethyl 2-(Benzofuran-2-yl((6-chlorobenzo[d]thiazol-2-yl)amino)methyl) malonate (5f): white solid; mp: 92–94 °C; yield 86%; 1H-NMR (CDCl3) δ (ppm): 7.53 (s, 1H, 24-CH), 7.49 (d, 1H, J = 5 Hz, 27-CH), 7.45 (d, 1H, J = 5 Hz, 14-CH), 7.42 (d, 1H, J = 5 Hz, 17-CH), 7.26 (d, 1H, J = 5 Hz, 26-CH), 7.22–7.17 (m, 2H, 15-CH, 16-CH), 6.94 (s, 1H, 11-CH), 6.72 (s, 1H, 8-CH), 6.05 (s, 1H, NH), 4.28 (d, 1H,J = 5 Hz, 2-CH), 4.24–4.13 (m, 4H, 28-CH2, 30-CH2), 1.21–1.16 (m, 6H, 29-CH3, 31-CH3); 13C-NMR (CDCl3) δ (ppm): 168.1 (20-C), 166.6 (1-C), 165.9 (3-C), 155.0 (10-C), 154.1 (13-C), 150.8 (22-C), 132.2 (23-C), 128.0 (12-C), 127.3 (15-C), 126.5 (16-C), 124.5 (25-C), 123.1 (26-C), 121.3 (24-C), 120.6 (27-C), 120.3 (14-C), 111.3 (17-C), 104.7 (11-C), 62.5 (28-C), 62.2 (30-C), 54.0 (8-C), 52.7 (2-C), 14.1 (29-C), 14.0 (31-C); MS (ESI): m/z = 473 ([M+H]+), 495 ([M+Na]+); MS (HREI): C23H21ClN2O5S Na for +, calculated 472.0860, found 472.0850; IR (KBr, cm−1) ν 3346, 2974, 1741, 1718, 1537, 1483, 1396, 1367, 1301, 1242, 1172, 1029, 974, 812, 763.
Dipropyl 2-(Benzofuran-2-yl((6-chlorobenzo[d]thiazol-2-yl)amino)methyl) malonate (5g): white solid; mp: 88–90 °C; yield 82%; 1H-NMR (CDCl3) δ (ppm): 7.54 (s, 1H, 24-CH), 7.49 (d, 1H, J = 5 Hz, 27-CH), 7.45 (d, 1H,J = 5 Hz, 14-CH), 7.42 (d, 1H, J = 5 Hz, 17-CH), 7.27–7.22 (m, 2H, 25-CH, 26-CH), 7.20-7.17 (m, 1H, 15-CH), 6.93 (s, 1H, 11-CH), 6.72 (s, 1H, 8-CH), 6.05 (s, 1H, NH), 4.31 (d, 1H, J = 5 Hz, 2-CH), 4.14-4.06 (m, 4H, 29-CH2, 30-CH2), 1.61–1.57 (m, 4H, 31-CH2, 32-CH2), 0.87–0.83 (m, 6H, 33-CH3, 34-CH3); 13C-NMR (CDCl3) δ (ppm): 168.3 (20-C), 166.7 (1-C), 165.8 (3-C), 155.0 (10-C), 154.1 (13-C), 150.8 (22-C), 132.2 (23-C), 128.1 (12-C), 127.3 (15-C), 126.5 (16-C), 124.5 (25-C), 123.1 (26-C), 121.3 (24-C), 120.6 (27-C), 120.3 (14-C), 111.3 (17-C), 104.7 (11-C), 68.0 (29-C), 67.7 (30-C), 54.0 (8-C), 52.7 (2-C), 21.9 (31-C), 21.8 (32-C), 10.4 (33-C), 10.3 (34-C); MS (ESI): m/z = 501 ([M+H]+), 523 ([M+Na]+); MS (HREI): C25H25ClN2O5S Na for +, calculated 500.1173, found 500.1171; IR (KBr, cm−1) ν 3357, 2966, 1747, 1720, 1593, 1533, 1483, 1446, 1392, 1354, 1290, 1238, 1197, 1172, 1053, 945, 812, 759.
Dibenzyl 2-(Benzofuran-2-yl((6-chlorobenzo[d]thiazol-2-yl)amino)methyl) malonate (5h): white solid; mp: 116–118 °C; yield 67%; 1H-NMR (CDCl3) δ (ppm): 7.53 (s, 1H, 24-CH), 7.45 (d, 1H, J = 10 Hz, 27-CH), 7.42 (d, 1H, J = 5 Hz, 14-CH), 7.36–7.66 (m, 7H, 31-CH, 32-CH, 33-CH, 34-CH, 35-CH, 37-CH, 38-CH), 7.26–7.24 (m, 2H, 39-CH, 40-CH), 7.19–7.18 (m, 2H, 17-CH, 41-CH), 7.17–7.15 (m, 2H, 15-CH, 26-CH), 7.13–7.11 (m, 2H, 11-CH, 26-CH), 6.81 (s, 1H, NH), 6.11 (s, 1H, 8-CH), 5.17–5.14 (m, 4H, 28-CH2, 29-CH2), 3.47 (s, 1H, 2-CH); 13C-NMR (CDCl3) δ (ppm): 168.0 (20-C), 166.4 (1-C), 165.6 (3-C), 155.0 (10-C), 153.8 (13-C), 150.8 (22-C), 135.3 (30-C), 134.7 (36-C), 134.6 (23-C), 132.3 (32-C), 128.7 (34-C), 128.6 (38-C), 128.6 (40-C), 128.5 (12-C), 128.5 (31-C), 128.5 (35-C), 128.4 (37-C), 128.4 (41-C), 128.2 (39-C), 128.0 (33-C), 127.4 (15-C), 126.5 (26-C), 124.6 (25-C), 123.2 (16-C), 121.4 (24-C), 120.5 (17-C), 120.4 (27-C), 111.3 (14-C), 104.7 (11-C), 68.2 (28-C), 67.8 (29-C), 54.0 (8-C), 41.9 (2-C); MS (ESI): m/z = 597 ([M+H]+), 619 ([M+Na]+); MS (HREI): C33H25ClN2O5S Na for +, calculated 596.1173, found 596.1189; IR (KBr, cm−1) ν 3388, 2966, 1745, 1730, 1599, 1543, 1529, 1487, 1454, 1381, 1259, 1220, 1147, 1004, 817, 748, 694.
Dimethyl 2-(Benzofuran-2-yl((6-methoxybenzo[d]thiazol-2-yl)amino)methyl) malonate (5i): white solid; mp: 85–87 °C; yield 81%; 1H-NMR (CDCl3) δ (ppm): 7.49–7.45 (m, 2H, 24-CH, 27-CH), 7.42 (d, 1H, J = 5 Hz, 14-CH), 7.27–7.23 (m, 1H, 17-CH), 7.20–7.17 (m, 1H, 25-CH), 7.11 (s, 1H, 26-CH), 6.89 (d, 1H, J = 5 Hz, 15-CH), 6.73 (d, 1H, J = 5 Hz, 11-CH ), 6.64 (s, 1H, NH), 6.01 (s, 1H, 8-CH), 4.34–4.32 (m, 1H, 2-CH), 3.80 (s, 3H, 29-C-OCH3), 3.73 (s, 6H, 30-CH3, 31-CH3); 13C-NMR (CDCl3) δ (ppm): 168.4 (20-C), 167.1 (1-C), 164.1 (3-C), 155.6 (10-C), 154.9 (13-C), 154.2 (22-C), 146.3 (23-C), 132.0 (12-C), 128.1 (15-C), 124.5 (16-C), 123.1 (25-C), 121.3 (26-C), 120.0 (24-C), 113.7 (27-C), 111.3 (14-C), 105.3 (17-C), 104.7 (11-C), 56.1 (8-C), 53.9 (2-C), 53.3 (29-C), 53.1 (30-C), 52.8 (31-C); MS (ESI): m/z = 441 ([M+H]+), 463 ([M+Na]+); MS (HREI): C22H20N2O6S Na for +, calculated 440.1042, found 440.1047; IR (KBr, cm−1) ν 3377, 2953, 1745, 1724, 1604, 1544, 1483, 1471, 1454, 1436, 1359, 1247, 1170, 1058, 1029, 968, 815, 759.
Diethyl 2-(benzofuran-2-yl((6-methoxybenzo[d]thiazol-2-yl)amino)methyl) malonate (5j): white solid; mp: 85–88 °C; yield 80%; 1H-NMR (CDCl3): δ (ppm) 7.50–7.45 (m, 2H, 24-CH, 27-CH), 7.42 (d, 1H, J = 5 Hz, 14-CH), 7.26–7.23 (m, 1H, 17-CH), 7.20–7.17 (m, 1H, 26-CH), 7.11 (s, 1H, 15-CH), 6.90–6.87 (m, 1H, 16-CH), 6.72 (s, 1H, 11-CH), 6.70 (s, 1H, NH), 6.02 (s, 1H, 8-CH), 4.29 (d, 1H, J = 5 Hz, 2-CH), 4.24–4.14 (m, 4H, 28-CH2, 30-CH2), 3.80 (s, 3H, 25-C-OCH3), 1.21–1.17 (m, 6H, 29-CH3, 31-CH3); 13C-NMR (CDCl3) δ (ppm): 168.1 (20-C), 166.7 (1-C), 164.1 (3-C), 155.5 (10-C), 154.9 (13-C), 154.5 (22-C), 146.3 (23-C), 131.9 (12-C), 128.1 (15-C), 124.4 (16-C), 123.1 (25-C), 121.3 (26-C), 120.0 (24-C), 113.7 (27-C), 111.2 (14-C), 105.3 (17-C), 104.6 (11-C), 62.4 (28-C), 62.1 (30-C), 55.9 (8-C), 54.1 (25-C), 52.7 (2-C), 14.1 (29-C), 14.0 (31-C); MS (ESI): m/z = 469 ([M+H]+), 491 ([M+Na]+); MS (HREI): C24H24N2O6S Na for +, calculated 468.1355, found 468.1336; IR (KBr, cm−1) ν 3377, 2974, 1745, 1712, 1602, 1543, 1490, 1469, 1436, 1280, 1188, 1033, 981, 855, 825, 756.
Dipropyl 2-(Benzofuran-2-yl((6-methoxybenzo[d]thiazol-2-yl)amino)methyl) malonate (5k): white solid; mp: 67–70 °C; yield 79%; 1H-NMR (CDCl3) δ (ppm): 7.49–7.45 (m, 3H, 24-CH, 27-CH, 14-CH), 7.25–7.06 (m, 3H, 17-CH, 25-CH, 26-CH), 6.89-6.81 (m, 1H, 15-CH), 6.72 (s, 1H, 11-CH), 6.62 (s, 1H, NH), 5.98 (s, 1H, 8-CH), 4.31–4.26 (m, 1H, 2-CH), 4.08–4.01 (m, 4H, 29-CH2, 30-CH2), 3.80 (s, 3H, 28-C-OCH3), 1.60-1.52 (m, 4H, 31-CH2, 32-CH2), 0.87–0.77 (m, 6H, 33-CH3, 34-CH3); 13C-NMR (CDCl3) δ (ppm): 168.2 (20-C), 166.8 (1-C), 164.0 (3-C), 155.5 (10-C), 155.0 (13-C), 154.5 (22-C), 146.3 (23-C), 132.0 (12-C), 128.2 (15-C), 124.4 (16-C), 123.0 (25-C), 121.2 (26-C), 120.0 (24-C), 113.6 (27-C), 111.2 (14-C), 105.3 (17-C), 104.6 (11-C), 67.9 (29-C), 67.6 (30-C), 56.0 (8-C), 54.1 (2-C), 52.7 (28-C), 21.8 (31-C), 21.7 (32-C), 10.3 (33-C), 10.2 (34-C); MS (ESI): m/z = 497 ([M+H]+), 519 ([M+Na]+); MS (HREI): C26H28N2O6S Na for +, calculated 496.1668, found 496.1669; IR (KBr, cm−1) ν 3348, 2951, 1741, 1716, 1604, 1543, 1483, 1357, 1288, 1172, 1064, 947, 850, 806, 759.
Dibenzyl 2-(Benzofuran-2-yl((6-methoxybenzo[d]thiazol-2-yl)amino)methyl) malonate (5l): white solid; mp: 110–112 °C; yield 60%; 1H-NMR (CDCl3): δ (ppm) 7.42 (d, 2H, J = 5 Hz, 27-CH, 24-CH), 7.35 (d, 1H, J = 5 Hz, 14-CH), 7.27–7.23 (m, 14H, 31-CH, 32-CH, 33-CH, 34-CH, 35-CH, 37-CH, 38-CH, 39-CH, 40-CH, 17-CH, 41-CH, 15-CH, 26-CH, 11-CH), 6.90–6.88 (m, 1H, 26-CH), 6.64 (s, 1H, NH), 6.01 (s, 1H, 8-CH), 5.19-5.06 (m, 4H, 28-CH2, 29-CH2), 4.43 (d, 1H, J = 5 Hz, 2-CH), 3.80 (s, 3H, 25-C-OCH3); 13C-NMR (CDCl3) δ (ppm): 167.9 (20-C), 166.5 (1-C), 163.8 (3-C), 155.6 (10-C), 155.0 (13-C), 154.2 (22-C), 146.3 (30-C), 134.8 (36-C), 134.7 (23-C), 132.1 (32-C), 128.7 (34-C), 128.6 (38-C), 128.5 (40-C), 128.5 (12-C), 128.5 (31-C), 128.4 (35-C), 128.4 (37-C), 128.3 (41-C), 128.2 (39-C), 128.1 (33-C), 124.4 (15-C), 123.1 (26-C), 121.3 (25-C), 120.1 (16-C), 113.7 (24-C), 111.3 (17-C), 105.3 (27-C), 104.7 (14-C), 68.1 (11-C), 67.8, 67.8 (28-C, 29-C), 56.0 (25-C), 54.2 (8-C), 52.6 (2-C); MS (ESI): m/z = 593 ([M+H]+), 615 ([M+Na]+); MS (HREI): C34H28N2O6S Na for +, calculated 592.1668, found 592.1687; IR (KBr, cm−1) ν 3350, 2954, 1747, 1716, 1602, 1543, 1469, 1454, 1348, 1222, 1172, 1028, 954, 823, 750, 696.
Dimethyl 2-(Benzofuran-2-yl((6-methylbenzo[d]thiazol-2-yl)amino)methyl) malonate (5m): white solid; mp: 108–109 °C; yield 81%; 1H-NMR (CDCl3) δ (ppm): 7.48 (d, 1H, J = 5 Hz, 24-CH), 7.45 (d, 1H, J = 5 Hz, 27-CH), 7.42 (d, 1H, J = 5 Hz, 14-CH), 7.38 (s, 1H, 17-CH), 7.25 (t, 1H, J = 15 Hz, 25-CH), 7.29 (t, 1H, J = 15 Hz, 26-CH), 7.10 (d, 1H, J = 5 Hz, 15-CH), 6.72 (s, 1H, 11-CH), 6.71 (s, 1H, NH ), 6.01 (s, 1H, 8-CH), 4.43 (d, 1H, J = 5 Hz, 2-CH), 3.73 (s, 6H, 29-CH3, 30-CH3), 2.38 (s, 3H, 28-CH3); 13C-NMR (CDCl3) δ (ppm): 168.4 (20-C), 167.0 (1-C), 165.1 (3-C), 155.0 (10-C), 154.2 (13-C), 150.0 (22-C), 132.1 (23-C), 131.0 (12-C), 128.1 (15-C), 127.2 (16-C), 124.5 (25-C), 123.1 (26-C), 121.3 (24-C), 121.0 (27-C), 119.3 (14-C), 111.3 (17-C), 104.7 (11-C), 53.8 (8-C), 53.3 (2-C), 53.0 (29-C), 52.8 (30-C), 21.3 (28-C); MS (ESI): m/z = 425 ([M+H]+), 447 ([M+Na]+); MS (HREI): C22H20N2O5S Na for +, calculated 424.1093, found 424.1097; IR (KBr, cm−1) ν 3361, 2954, 1743, 1724, 1539, 1487, 1454, 1435, 1357, 1224, 1172, 1147, 1033, 972, 817, 759.
Diethyl 2-(Benzofuran-2-yl((6-methylbenzo[d]thiazol-2-yl)amino)methyl) malonate (5n): white solid; mp: 105–107 °C; yield 76%; 1H-NMR (CDCl3) δ (ppm): 7.48-7.47 (d, 1H, J = 5 Hz, 24-CH), 7.45 (d, 1H, J = 5 Hz, 27-CH), 7.42 (d, 1H, J = 5 Hz, 14-CH), 7.38 (s, 1H, 17-CH), 7.25 (t, 1H, J = 15 Hz, 26-CH), 7.20–7.17 (m, 1H, 15-CH), 7.10 (d, 1H, J = 5 Hz, 16-CH), 6.74 (s, 1H, NH), 6.72 (s, 1H, 11-CH), 6.03 (s, 1H, 8-CH), 4.29 (m, 1H, 2-CH), 4.24–4.14 (m, 4H, 28-CH2, 30-CH2), 2.38 (s, 3H, 25-CH3), 1.21–1.16 (m, 6H, 29-CH3, 31-CH3); 13C-NMR (CDCl3) δ (ppm): 168.1 (20-C), 165.1 (1-C), 155.0 (3-C), 154.5 (10-C), 150.0 (13-C), 133.7 (22-C), 132.0 (23-C), 131.0 (12-C), 128.1 (15-C), 127.2 (16-C), 124.4 (25-C), 123.0 (26-C), 121.3 (24-C), 121.0 (27-C), 119.2 (14-C), 111.2 (17-C), 104.6 (11-C), 62.4 (28-C), 62.1 (30-C), 54.1 (8-C), 52.8 (2-C), 21.3 (25-C), 14.1 (29-C), 14.0 (31-C); MS (ESI): m/z = 453 ([M+H]+), 475 ([M+Na]+); MS (HREI): C24H24N2O5S Na for +, calculated 452.1406, found 452.1407; IR (KBr, cm−1) ν 3352, 2976, 1743, 1720, 1600, 1560, 1541, 1487, 1456, 1336, 1301, 1238, 1172, 1029, 939, 858, 810, 763.
Dipropyl 2-(Benzofuran-2-yl((6-methylbenzo[d]thiazol-2-yl)amino)methyl) malonate (5o): white solid; mp: 76–78 °C; yield 73%; 1H-NMR (CDCl3) δ (ppm): 7.48–7.43 (m, 3H, 24-CH, 27-CH, 14-CH), 7.41 (d, 1H, J = 10 Hz, 17-CH), 7.38 (s, 1H, 25-CH), 7.25–7.23 (m, 1H, 26-CH), 7.10–7.08 (m, 1H, 15-CH), 6.80 (s, 1H, 11-CH), 6.71 (s, 1H, NH), 6.04 (s, 1H, 8-CH), 4.33-4.32 (m, 1H, 2-CH), 4.13–4.06 (m, 4H, 29-CH2, 30-CH2), 2.38 (s, 3H, 28-CH3), 1.61-1.56 (m, 4H, 31-CH2, 32-CH2), 0.87–0.82 (m, 6H, 33-CH3, 34-CH3); 13C-NMR (CDCl3) δ (ppm): 168.2 (20-C), 166.8 (1-C), 165.1 (3-C), 155.0 (10-C), 154.5 (13-C), 150.0 (22-C), 132.0 (23-C), 131.0 (12-C), 128.1 (15-C), 127.2 (16-C), 124.4 (25-C), 123.0 (26-C), 121.3 (24-C), 121.0 (27-C), 119.2 (14-C), 111.2 (17-C), 104.6 (11-C), 68.0 (29-C), 67.6 (30-C), 54.1 (8-C), 52.8 (2-C), 21.9 (28-C), 21.8 (31-C), 21.3 (32-C), 10.3 (33-C), 10.2 (34-C); MS (ESI): m/z = 481([M+H]+), 503 ([M+Na]+); MS (HREI): C26H28N2O5S Na for +, calculated 480.1719, found 480.1711; IR (KBr, cm−1) ν 3365, 2966, 1747, 1722, 1535, 1483, 1456, 1354, 1290, 1172, 1055, 954, 808, 761.
Dibenzyl 2-(Benzofuran-2-yl((6-methylbenzo[d]thiazol-2-yl)amino)methyl) malonate (5p): white solid; mp: 126–128 °C; yield 64%; 1H-NMR (CDCl3) δ (ppm): 7.43–7.41 (m, 2H, 27-CH, 24-CH), 7.35–7.31 (m, 3H, 14-CH, 31-CH, 32-CH), 7.23–7.14 (m, 10H, 33-CH, 34-CH, 35-CH, 37-CH, 38-CH, 39-CH, 40-CH, 17-CH, , 41-CH, 15-CH), 7.12–7.08 (m, 3H, 26-CH, 11-CH, 26-CH), 6.64–6.62 (m, 1H, NH), 6.10 (s, 1H, 8-CH), 5.17–5.04 (m, 4H, 28-CH2, 29-CH2), 4.43-4.41 (m, 1H, 2-CH), 2.39–2.37 (s, 3H, 25-CH3); 13C-NMR (CDCl3) δ (ppm): 167.9 (20-C), 166.5 (1-C), 165.0 (3-C), 155.0 (10-C), 154.2 (13-C), 150.0 (22-C), 134.8 (30-C), 134.7 (36-C), 132.0 (23-C), 131.1 (32-C), 128.7 (34-C), 128.7 (38-C), 128.6 (40-C), 128.6 (12-C), 128.5 (31-C), 128.5 (35-C), 128.2 (37-C), 128.2 (41-C), 128.1 (39-C), 128.1 (33-C), 127.2 (15-C), 127.2 (26-C), 124.7 (25-C), 123.1 (16-C), 121.4 (24-C), 121.0 (17-C), 119.3 (27-C), 111.3 (14-C), 104.7 (11-C), 68.1 (28-C), 67.8 (29-C), 54.1 (8-C), 52.7 (2-C), 21.4 (25-C); MS (ESI): m/z = 577 ([M+H]+), 599 ([M+Na]+); MS (HREI): C34H28N2O5S Na for +, calculated 576.1719, found 576.1702; IR (KBr, cm−1) ν 3348, 2954, 1743, 1724, 1537, 1487, 1452, 1382, 1238, 1170, 1012, 910, 808, 731, 702.
3.3. Anti-TMV Activity Section
3.3.1. Purification of Tobacco Mosaic Virus
Using Gooding’s method [21], the upper leaves of Nicotiana tabacum inoculated with TMV were selected and ground in phosphate buffer and then filtered through double-layer pledget. The filtrate was centrifuged at 10,000 g, treated with PEG twice, and centrifuged again. The whole experiment was processed at 4 °C. Absorbance value was estimated at 260 nm by ultraviolet spectrophotometer:
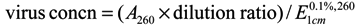
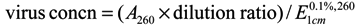
3.3.2. Inhibition Effect of Compound on TMV in Vivo [22]
The virus was inhibited by mingling with the compound solution at the same volume for 30 min. The mixture was then inoculated on the left side of the leaves of N. tabacum L., whereas the right side of the leaves was inoculated with the mixture of solvent and the virus for control. The local lesion numbers were recorded 3–4 days after inoculation [10]. Three repetitions were conducted for each compound.
3.3.3. Curative Effect of Compounds on TMV in Vivo [22]
The leaves of N. tabacum L. growing at the same ages were selected. TMV at a concentration of 6 × 10−3 mg/mL was dipped and inoculated on the whole leaves. Then the leaves were washed with water and dried. The compound solution was smeared on the left side, and the solvent was smeared on the right side for control. The local lesion numbers were then recorded 3–4 days after inoculation. For each compound, three repetitions were conducted to ensure the reliability of the results.
3.3.4. Protective Effect of Compounds on TMV in Vivo [22]
The leaves of N. tabacum L. growing at the same ages were selected. The compound solution was smeared on the left side for 12 h and the solvent was smeared on the right side for control. The TMV at a concentration of 6 × 10−3 mg/mL was inoculated on the whole leaves. The local lesion numbers were then recorded 3–4 days after inoculation. For each compound, three repetitions were conducted to ensure the reliability of the results:


4. Conclusions
Sixteen novel β-amino ester derivatives containing benzofuran and benzothiazole units were designed and synthesized seeking anti-TMV activity. The bioassays identified these new compounds as possessing weak to good antiviral activities. The bioassay test results showed that compounds 5i and 5m have good antiviral activity against TMV, with curative rates of 52.23% and 54.41% at a concentration of 0.5 mg/mL.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/11/13623/s1.
Acknowledgments
We are grateful for the Key project of the National Natural Science Foundation of China (No.2132003) and the National Natural Science Foundation of China (No.20872021) for the financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sawada, Y.; Yanai, T.; Nakagawa, H. Synthesis and insecticidal activity of benzoheterocycil analofues of N`-benzoheterocyclic analogues of N`-benzoyl-N-(tert-butyl)benzohydrazide: Patr 1. Design of benzoheterocyclic analogues. Pest Manag. Sci. 2003, 59, 25–35. [Google Scholar] [CrossRef]
- Mahran, M.A.; El-nassry, S.M.F.; Allam, S.R. Synthesis of some new benzothiazole derivatives as potential antimicrobial and antiparasitic agents. Pharmazie 2003, 58, 527–530. [Google Scholar]
- Khanitha, P.; Kazuki, K.; Hiroki, Te. Synthesis of three classes of rhodacyanine dyes and ebalitation of their in vitro and in vivo antimalarial activity. Bioorg. Med. Chem. 2006, 14, 8550–8563. [Google Scholar] [CrossRef]
- Victor, F.; Raisa, R.; Claudia, R.B.G.; Thatyana, R.A.V. Chemistry and Biological Activities of 1,3-Benzothiazoles. Mini Rev. Org. Chem. 2012, 9, 44–53. [Google Scholar] [CrossRef]
- Charris, J.; Monasterios, M.; Domingguez, J. Synthesis of some 5-nitro-2-furfurylidene derivatives and their antibacterial and antifungal activities. Heterocycl. Commun. 2002, 8, 275–280. [Google Scholar]
- Helal, M.H.M.; Salem, M.A.; El-Gaby, M.S.A.; Aljahdali, M. Synthesis and biological evaluation of some novel thiazole compounds as potential anti-inflammatory agents. Eur. J. Med. Chem. 2013, 65, 517–526. [Google Scholar] [CrossRef]
- Sidoova, E.; Loos, D.; Bujdakova, H. New Anticandidous 2-Alkylthio-6-aminobenzothiazoles. Molecules 1997, 2, 36–42. [Google Scholar]
- Hassan, M.; Chohan, Z.H.; Supuran, C.T. Antibacterial Co (II) and Ni (II) complexes of benzothiazole-dereved Schiff bases. Synth. Reactiv. Inorg. Metal Org. Chem. 2002, 32, 1445–1461. [Google Scholar] [CrossRef]
- Albert, W.B.; Leonard, R.W. Coumarone. Organic Synth. 1973, 5, 251. [Google Scholar]
- Muthu, K.K.; Amol, B.S.; Aparna, S.C.; Prashik, B.D.; Rahul, P.W.; Maheshwar, S.M.; Sandeep, G. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar]
- Benaim, G.; Paniz, M.; Alberto, E. The emerging role of amiodarone and dronedarone in Chagas disease. Nat. Rev. Cardiol. 2012, 9, 605–609. [Google Scholar] [CrossRef]
- Giannis, A. Peptidomimetics for receptor ligands-discovery, development, and medical perspectives. Angew. Chem. Int. Ed. 1993, 32, 1244–1267. [Google Scholar] [CrossRef]
- Goody, R.S.; Alexandrov, K.; Engelhard, M. Combining chemical and biological techniques to produce modified proteins. ChemBioChem 2002, 3, 399–403. [Google Scholar] [CrossRef]
- Wang, L.; Schultz, P.G. Expanding the genetic code. Chem. Commun. 2002, 2002, 1–11. [Google Scholar] [CrossRef]
- Simon, S.; Markus, W.; Thomas, I.; Irina, S.; Harald, G. Kinetic investigation of a solvent-free, chemoenzymatic reaction sequence towards enantioselective synthesis of a β-amino acid ester. Biotechnol. Bioeng. 2012, 109, 1479–1489. [Google Scholar] [CrossRef]
- Eusebio, J.; Delia, Q.; Jaime, E. Enantioselective Synthesis of beta-Amino Acids. Aldrichimica Acta 1994, 27, 3–11. [Google Scholar]
- Jerry, D.C.; Gerald, A.W.; Keithm, A.; Pierre, J.C. Pilot Plant Preparation of an α v β 3 Integrin Antagonist. Part 1. Process Research and Development of a (S)-β-Amino Acid Ester Intermediate: Synthesis via a Scalable, Diastereoselective Imino-Reformatsky Reaction. Org. Process Res. Dev. 2004, 8, 51–61. [Google Scholar] [CrossRef]
- Bai, S.; Liang, X.P.; Bhadury, P.; Hu, D.H.; Yang, S. Asymmetric Mannich reactions catalyzed by cinchona alkaloid thiourea: Enantioselective one-pot synthesis of novel β-amino ester derivatives. Tetrahedron 2011, 22, 518–523. [Google Scholar]
- Dockichev, T.V.; Latypova, D.R.; Shakirov, R.R.; Biglova, R.Z.; Talipov, R.F. Catalyzed synthesis of β-amino acids esters. Russ. J. Organ. Chem. 2010, 46, 755–757. [Google Scholar] [CrossRef]
- Zhang, K.K.; Liang, X.P.; He, M.; Wu, J.; Zhang, Y.P.; Xue, W.; Jin, L.H.; Yang, S.; Hu, D.Y. One-Pot Synthesis of Novel Chiral β-Amino Acid Derivatives by Enantioselective Mannich Reactions Catalyzed by SquaramideCinchona Alkaloids. Molecules 2013, 18, 6142–6152. [Google Scholar] [CrossRef]
- Gooding, G.V.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1290. [Google Scholar]
- Li, S.Z.; Wang, D.M.; Jiao, S.M. Pesticide Experiment Methods-Fungicide Sector; Agriculture Press of China: Beijing, China, 1991; pp. 93–94. [Google Scholar]
- Sample Availability: Samples of the compounds 5a–5p are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).