Abstract
A palladium(II)-catalyzed ortho-benzoxylation of 2-arylpyridines with aryl acylperoxides was developed. With pyridyl as directing group, the benzoxylation reaction exhibits remarkable regioselectivity and excellent functional group tolerance, providing the products in up to 87% yield.
1. Introduction
Site selective direct functionalization of C–H bonds is attracting considerable current attention for developing sustainable chemical synthesis. Without relying on prefunctionalized substrates, the direct C–H functionalization approach would streamline the synthetic routes and improve atom economy. With transition metal catalysis, innovative transformations based on regioselective aryl C–H bond cleavage with the formation of C–C and C–X (X = halogen) bonds have been achieved [1,2,3]. Recently, significant advances have been made in the analogous transformations with C–O bond formation on arenes. For instance, the research groups of Sanford, Crabtree and Wang achieved acetoxylation of aryl C–H bonds with oxidants such as Oxone®, PhI(OAc)2, and K2S2O8 [4,5,6]. The analogous acetoxylation of selected sp3 C–H bonds has also been achieved independently by Sanford, Yu and Corey [7,8,9]. Notably, Yu and co-workers accomplished Pd-catalyzed arene hydroxylation with dioxygen as oxidant [10]. Apart from Pd, Cu(OAc)2 has also been shown to exhibit promising reactivities toward catalytic acetoxylation of aryl C–H bonds [11].
Compared to acetoxylation, the analogous benzoxylation is less developed [12]. Aryl benzoates are known to form structures of some natural products such as the gilvocarcins (Figure 1) [13,14]. In 2009, Cheng and co-workers reported the Rh(I)-catalyzed ortho-benzoxylation of 2-arylpyridines with benzoic acids [15]. Later, the same group also reported the analogous Cu(OAc)2-catalyzed benzoxylations with benzoic anhydride and derivatives [16,17]. With a continuing interest in developing catalytic C–H bond cross coupling reactions, we have been investigating the regioselective C–C and C–N bond cross coupling reactions based on the coupling reactions of arylpalladium and –rhodium complexes with carboradicals [18,19,20,21], carbenes [22] and nitrenoids [23,24,25]. Previously, we described the successful development of the Pd-catalyzed decarboxylative arylation of 2-arylpyridines with aryl acylperoxides [19]. Prompted by this work, here we disclose the Pd-catalyzed regioselective benzoxylation of 2-arylpyridines with aryl acylperoxides.

Figure 1.
Examples of gilvocarins.
Figure 1.
Examples of gilvocarins.
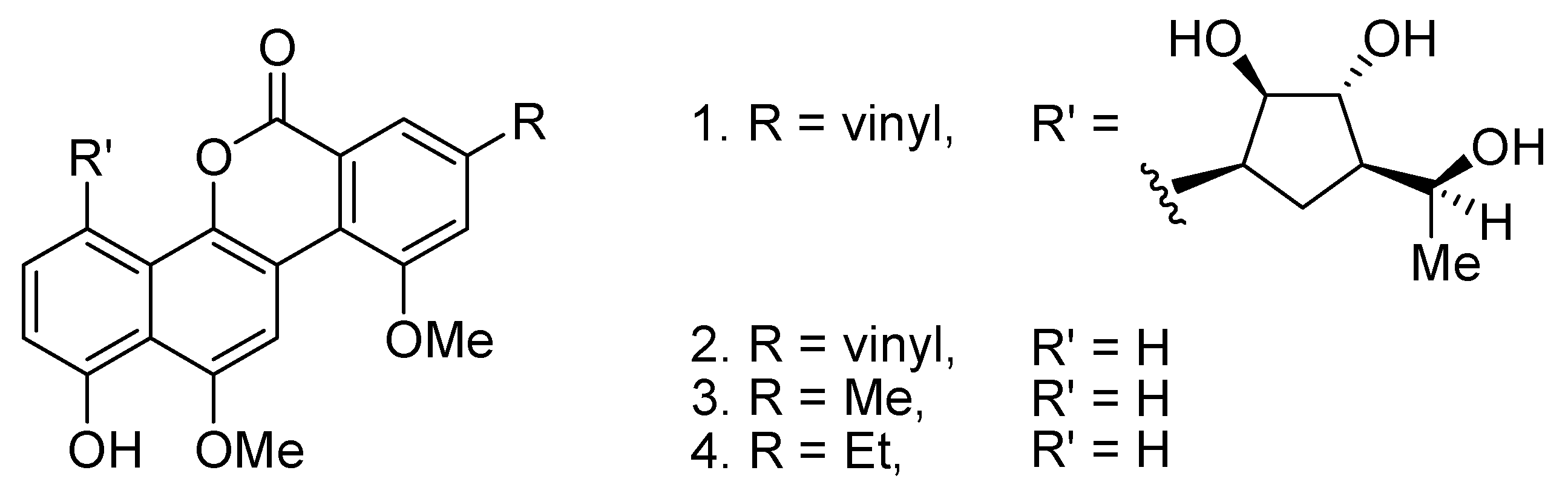
2. Results and Discussion
In our earlier study on decarboxylative arylation, we found that treating 2-phenylpyridine (1a, 0.25 mmol) with Pd(OAc)2 (5 mol%) with a methoxy-substituted aryl acylperoxide (4 × 0.5 equiv./0.5 h) at 100 °C for 2 h in CH3CN (Scheme 1) furnished 2k in 90% isolated yield. However, when simple benzoyl peroxide was employed as reagent, the analogous reaction of 1a produced 2a in only 7% yield. To develop a general benzoxylation reaction, we first examined the solvent effect (Table 1). With 1a as substrate and benzoyl peroxide as reagent (4 × 0.5 equiv./0.5 h), the best result (2a: 49%) was obtained when xylene was employed as solvent (entry 8). As expected, toluene gave comparable results; the use of other donor solvents (e.g., dioxane, THF, DMF and DCE) produced far inferior outcomes.
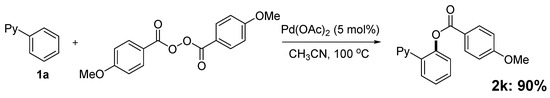
Scheme 1.
Initial studies of othro-C-H-arylcarboxylation by aryl acylperoxide.
Scheme 1.
Initial studies of othro-C-H-arylcarboxylation by aryl acylperoxide.
We next turned to optimize other experimental parameters, and the results are summarized in Table 2. We found that when raising the reaction temperature to 120 °C led to slightly lower yield of 41% for the benzoxylation of 1a (entry 2). Notably, further increasing the temperature to 130 °C afforded 2a in 59% yield (entry 3). Either prolonged reaction time or higher temperature (150 °C) did not give better results (entries 4–5). After several trials, we found that employing 3.5 equiv. of benzoyl peroxide added in a batchwise fashion (7 × 0.5 equiv./0.5 h) improved the benzoxylation yield to 64% (entry 6). Interestingly, when dried benzoyl peroxide was employed, 2a was obtained in 70% yield. Up to 76% yield of 2a was obtained when 10 mol% of Pd(OAc)2 was employed for the benzoxylation reaction (entry 9).

Table 1.
Solvent effect. 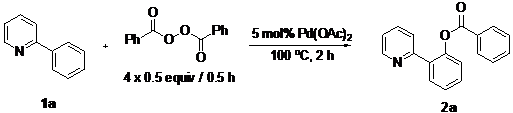
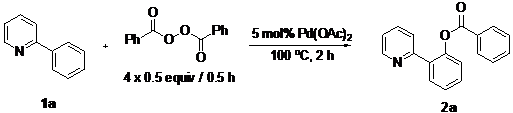
| Entry | Solvent (2 mL) | GC Yield (%) |
|---|---|---|
| 1 | CH3CN | 7 |
| 2 | dioxane | 10 |
| 3 | THF | 29 |
| 4 | DMF | 33 |
| 5 | DMA | 3 |
| 6 | DCE | 11 |
| 7 | toluene | 47 |
| 8 | xylene | 49 |

Table 2.
Effect of other experimental parameters. 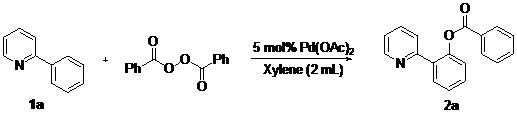
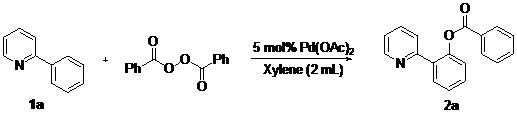
| Entry | Addition method | Temp. (°C) | Time (h) | GC Yield (%) |
|---|---|---|---|---|
| 1 | 4 × 0.5 equiv./0.5 h | 100 | 2 | 49 |
| 2 | 4 × 0.5 equiv./0.5 h | 120 | 2 | 41 |
| 3 | 4 × 0.5 equiv./0.5 h | 130 | 2 | 59 |
| 4 | 4 × 0.5 equiv./1 h | 130 | 4 | 53 |
| 5 | 4 × 0.5 equiv./0.5 h | 150 | 2 | 52 |
| 6 | 7 × 0.5 equiv./0.5 h | 130 | 3.5 | 64 |
| 7 | 8 × 0.5 equiv./0.5 h | 130 | 4 | 60 |
| 8 * | 7 × 0.5 equiv./0.5 h | 130 | 3.5 | 70 |
| 9 *,# | 7 × 0.5 equiv./0.5 h | 130 | 3.5 | 76 |
* Peroxide was washed with diethyl ether and air dry; # 10 mol% of Pd(OAc)2 was used.
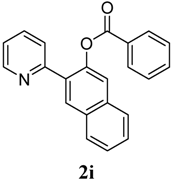
Table 3 depicts the substrate scope study, and the Pd-catalyzed benzoxylation is broadly applicable to a variety of 2-arylpyridines. In all cases, benzoxylation occurs at the ortho-C-H bond to the pyridyl group. For meta-substituted arenes (e.g., 1d and 1e), benzoxylation occurred regioselectively at the less hindered ortho-C-H bond (entries 4–7). Apparently, those aryl groups bearing electron-donating substituent such as Me, MeO produced better yields of 2d and 2e (83%–87%), whereas the analogous reactions of substrates with electron-withdrawing CF3 and F groups resulted in 54%–62% yields. Higher benzoxylation yield was also observed with 1h, which contains a Me group on the pyridyl function. Presumably, stronger coordination of the pyridyl group would facilitate cyclopalladation, thereby speeding up the substrate conversion. Consistent with this notion is that benzoxylation of benzoquinoline 1j was equally facile with 2j being obtained in 85% yield. The rigid scaffold of 1j should lower the entropic cost for the cyclopalladation (entry 10). The molecular structure of 2i has been established by X-ray crystallography. CCDC 933423 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk), see Supporting Information.

Table 3.
Pd-catalyzed benzoxylation of arylpyridines a.
| Entry | Substrate | Product | Isolated Yield (%) | ||
|---|---|---|---|---|---|
| 1 |  1a 1a | 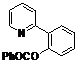 2a 2a | 71 | ||
| 2 | 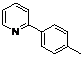 1b 1b | 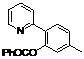 2b 2b | 70 | ||
| 3 | 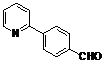 1c 1c | 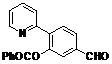 2c 2c | 44 | ||
| 4 | 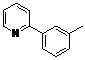 1d 1d | 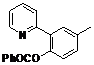 2d 2d | 87 | ||
| 5 | 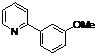 1e 1e | 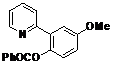 2e 2e | 83 | ||
| 6 | 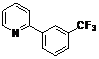 1f 1f | 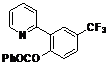 2f 2f | 54 | ||
| 7 | 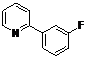 1g 1g | 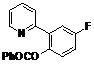 2g 2g | 62 | ||
| 8 | 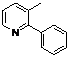 1h 1h | 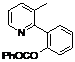 2h 2h | 87 | ||
| 9 | 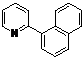 1i 1i | 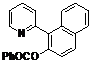 2i 2i | 57 | ||
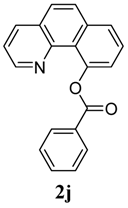
| 10 | 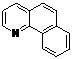 1j 1j | 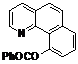 2j 2j | 85 | ||
a Reaction conditions: substrate (0.5 mmol), Pd(OAc)2 (5 mol%), peroxide (7 × 0.5 equiv./0.5 h), at 130 °C for 3.5 h in xylene (2 mL).
Table 4 depicts the scope of the aryl acylperoxides; the Pd-catalyzed benzoxylations of 1a afforded the corresponding benzoates in 27%–81% yields. Peroxides with electron-donating (e.g., Me, MeO, EtO) and -withdrawing (e.g., CF3) are effective reagents for the benzoxylation. Peroxides with bulky substituents such as tBu and naphthalene were also tolerated with benzoates being formed in 44%–77% yields.

Table 4.
Benzoxylation with various aryl acylperoxides a. 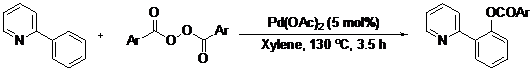
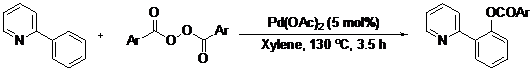
| Entry | Ar | Isolated Yield (%) |
|---|---|---|
| 1 | 4-OMe-C6H5 | 54 |
| 2 | 3-OMe-C6H5 | 69 |
| 3 | 2-Cl-C6H5 | 43 |
| 4 | 3-Cl-C6H5 | 73 |
| 5 | 2-Me-C6H5 | 66 |
| 6 | 4-Me-C6H5 | 64 |
| 7 | 4-F-C6H5 | 55 |
| 8 | 4-Br-C6H5 | 30 |
| 9 | 4-CF3-C6H5 | 81 |
| 10 | 4-OEt-C6H5 | 27 |
| 11 | 4-tBu-C6H5 | 77 |
| 12 | 2,6-F2-C6H4 | 46 |
| 13 | 2-naphthalene | 44 |
a Reaction conditions: substrate (0.5 mmol), Pd(OAc)2 (5 mol% ), peroxide (7 × 0.5 equiv./0.5 h), at 130 °C for 3.5 h in xylene (2 mL).
A plausible mechanism is shown in Scheme 2. The benzoxylation is probably initiated by the pyridyl-assisted cyclopalladation of the ortho-C-H of the arenes. Our earlier study showed that the regioselectivity of the cyclopalladation is determined by steric factor [19]. Substrates with a meta-substituted group would be palladated at the least hindered site. Regarding to the nature of the palladation step, our previous studies established a linear free energy relationship (ρ+ = −0.74) for the Pd-catalyzed oxidative acylation of pivilanilides, consistent with an electrophilic mechanism [21]. We conjectured that homolytic O–O cleavage of the aryl acylperoxide should afford arylcarboxy radicals [26], which would react with the palladacyclic intermediate leading to the C–O bond formation.
3. Experimental
3.1. General
2-Arylpyridines were prepared by reacting the corresponding arylboronic acids with 2-bromopyridines using reported procedures [27]. Aryl acylperoxides were prepared by reacting acid chlorides with hydrogen peroxide (35 wt. % in H2O) and sodium hydroxide by the reported procedures [28]. Benzoyl peroxide (Luperox® A75) was obtained commercially and washed with diethyl ether and air dry. Thin layer chromatography was performed on silica gel plates. Silica gel (Merck, 230–400 mesh) and aluminum oxide (Merck, 50–200 mesh) were used for flash column chromatography. 1H 400 MHz) and 13C-NMR spectra (100 MHz) were recorded on a Brüker DPX 400 NMR spectrometer, chemical shift (δ) valued are given in ppm and are referenced to the residual solvent peaks. Coupling constants (J) were reported in Hertz (Hz). Mass spectra and high resolution mass spectra (HRMS) were obtained on a VG Micromass Fison VG platform, a Finnigan Model Mat 95 ST instrument, or a Brüker APEX 47e FT-ICR mass spectrometer. Infrared analysis were measured on a Nicolet Magna 750 FTIR spectrometer. Melting points were measured on a Büchi B-545 melting point apparatus. The X-ray crystal structure was obtained on a Brüker CCD area detector diffractometer.
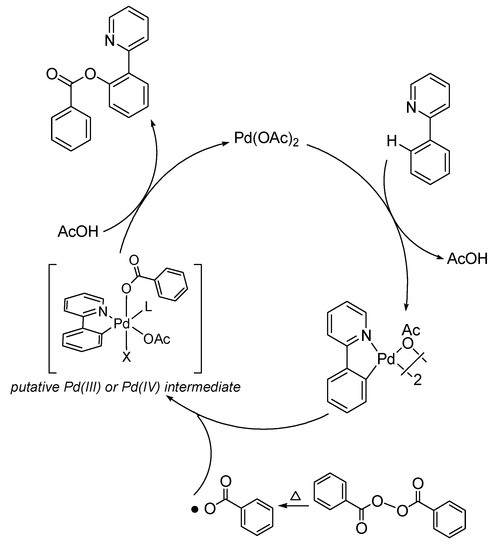
Scheme 2.
Plausible mechanism of othro-C-H-benzoxylation by aryl acylperoxides.
Scheme 2.
Plausible mechanism of othro-C-H-benzoxylation by aryl acylperoxides.
3.2. General Procedure for the Pd-Catalyzed Benzoxylation
A mixture of substrate (0.5 mmol), Pd(OAc)2 (0.025 mmol, 5 mol%), aryl acylperoxide [1.75 mmol; addition interval: 7 × 0.5 equiv./0.5 h] in xylene (2 mL) was sealed in a 8-mL vial with a Teflon-lined cap. The mixture was heated at 130 °C (oil bath temperature) for 3.5 h. After cooling down to room temperature, the reaction mixture was filtered through a plug of silica gel, and the filtrate was concentrated under vacuum to afford an oily substance. The crude product was dissolved in dichloromethane and treated with saturated aqueous NaHCO3 (3 × 10 mL) solution and extracted with dichloromethane (4 × 10 mL). The combined organic extracts were dried over Na2SO4 and evaporated to dryness by a rotary evaporator. The residue was loaded to a silica gel column for purification by flash chromatography using 60% n-hexane/40% diethyl ether as eluent.
3.3. Characterization of Products
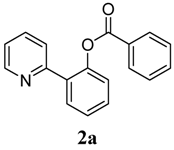
2-(Pyridin-2-yl)phenyl benzoate (2a). Yellow oil (71% yield). 1H-NMR (CDCl3): δH 8.59 (d, J = 4.7 Hz, 1H), 8.09 (d, J = 7.8 Hz, 2H), 7.78 (d, J = 7.6 Hz, 1H), 7.55–7.63 (m, 3H), 7.38–7.52 (m, 4H), 7.31 (d, J = 8.0 Hz, 1H), 7.13–7.16 (m, 1H). 13C-NMR (CDCl3): δC 165.2 (C=O), 154.7(C), 150.2 (C-O), 149.4 (C-H), 136.7 (C-H), 134.0 (C-H), 131.5 (C-H), 130.7 (C-H), 130.3 (C), 129.1 (C), 128.9 (C-H), 128.4 (C-H), 127.0 (C-H), 124.2 (C-H), 123.9 (C-H), 122.7 (C-H). MS (EI): 275 (M+, 20), 105 (100), 77 (40). HRMS (ESI): calcd. for C14H13NO2H+: 276.1025, found: 276.1023. IR (KBr, cm−1): 1739.

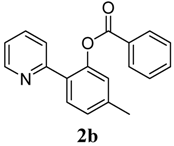
5-Methyl-2-(pyridin-2-yl)phenyl benzoate (2b). Yellow oil (70% yield). 1H-NMR (CDCl3): δH 8.57 (d, J = 4.4 Hz, 1H), 8.10 (d, J = 7.5 Hz, 2H), 7.69 (d, J = 7.9 Hz, 1H), 7.50–7.61 (m, 3H), 7.45 (t, J = 7.7 Hz, 2H), 7.21 (d, J = 8.3 Hz, 1H), 6.93–6.97 (m, 1H), 2.43 (s, 3H). 13C-NMR (CDCl3): δC 165.9 (C=O), 156.2 (C), 150.1 (C-O), 149.4 (C-H), 140.8 (C), 136.7 (C-H), 134.0 (C), 131.3 (C-H), 131.0 (C-H), 130.6 (C-H), 130.2 (C), 129.1 (C-H), 128.4 (C-H), 124.2 (C), 122.5 (C-H), 122.1 (C-H), 21.9 (CH3). MS (EI): 289 (M+, 40), 105 (100), 77 (40). HRMS (ESI): calcd. for C19H15NO2H+: 290.1181, found: 290.1188. IR (KBr, cm−1): 1736.

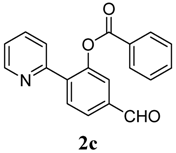
5-Formyl-2-(pyridin-2-yl)phenyl benzoate (2c). Yellow oil (44% yield based on conversion). 1H-NMR (CDCl3): δH 10.07 (s, 1H), 8.62 (d, J = 4.6 Hz, 1H), 8.08 (d, J = 7.3 Hz, 2H), 7.95–8.00 (m, 2H), 7.91 (dd, J = 7.9, 1.2 Hz, 1H), 7.83 (d, J = 1.2 Hz, 1H), 7.60–7.67 (m, 2H), 7.48 (d, J = 8.2 Hz, 1H), 7.21–7.23 (m, 1H). 13C-NMR (CDCl3): δC 191.5 (C=O), 165.5 (C=O), 154.9 (C), 150.5 (C-O), 146.7 (C-H), 139.6 (C-H), 137.0 (C-H), 134.5 (C), 132.5 (C-H), 130.9 (C), 129.6 (C-H), 127.4 (C-H), 126.6 (C-H), 125.2 (C-H), 123.6 (C-H), 120.7 (C-H). MS (EI): 303 (M+, 10), 105 (100), 77 (30). HRMS (ESI): calcd. for C19H13NO3H+: 304.0974, found: 304.0981. IR (KBr, cm−1): 1738, 1697.

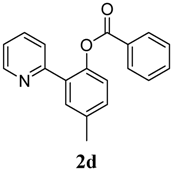
4-Methyl-2-(pyridin-2-yl)phenyl benzoate (2d). Yellow oil (87% yield). 1H-NMR (CDCl3): δH 8.61 (d, J = 4.7 Hz, 1H), 8.09 (d, J = 7.4 Hz, 2H), 7.55–7.61 (m, 4H), 7.44 (t, J = 7.7 Hz, 2H), 7.28 (dd, J = 9.0, 2.1 Hz, 1H), 7.19 (d, J = 8.2 Hz, 1H), 7.12–7.15 (m, 1H), 2.44(s, 3H). 13C-NMR (CDCl3): δC 165.9 (C=O), 156.2 (C), 150.2 (C-H), 146.6 (C), 136.7 (C-H), 134.0 (C), 133.4 (C-H), 131.9 (C-H), 130.9 (C), 130.7 (C-H), 130.1 (C-H), 129.1 (C-H), 128.4 (C-H), 124.3 (C-H), 123.6 (C-H), 122.2 (C-H), 21.5 (CH3). MS (EI): 289 (M+, 30), 105 (100), 77 (30). HRMS (ESI): calcd. for C19H15NO2H+: 290.1181, found: 290.1184. IR (KBr, cm−1): 1738.

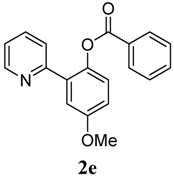
4-Methoxy-2-(pyridin-2-yl)phenyl benzoate (2e). Yellow oil (83% yield). 1H-NMR (CDCl3): δH 8.61 (d, J = 4.7 Hz, 1H), 8.09 (d, J = 7.4 Hz, 2H), 7.57–7.62 (m, 2H), 7.45 (t, J = 7.7 Hz, 2H), 7.34 (d, J = 3.0 Hz, 1H), 7.22 (d, J = 8.8 Hz, 1H), 7.13–7.17 (m, 2H), 7.02 (dd, J = 8.8, 3.0 Hz, 1H), 3.88 (s, 3H). 13C-NMR (CDCl3): δC 166.1 (C=O), 158.2 (C-O), 156.0 (C), 150.2 (C-H), 149.2 (C-O), 142.4 (C-H), 136.8 (C-H), 134.6 (C-H), 134.0 (C), 131.5 (C-H), 129.1 (C-H), 126.4 (C), 124.8 (C-H), 124.3 (C-H), 116.3 (C-H), 115.8 (C-H), 56.3 (CH3). MS (EI): 305 (M+, 40), 105 (100), 77 (30). HRMS (ESI): calcd. for C19H15NO3H+: 306.1130, found: 306.1133. IR (KBr, cm−1): 1737.

4-Methoxy-2-(pyridin-2-yl)phenyl benzoate (2f). Yellow oil (54% yield). 1H-NMR (CDCl3): δH 8.63 (d, J = 4.5 Hz, 1H), 8.03–8.11 (m, 2H), 7.74 (dd, J = 8.5, 1.6 Hz, 1H), 7.57–7.69 (m, 4H), 7.42–7.50 (m, 3H), 7.22 (d, J = 5.3 Hz, 1H). 13C-NMR (CDCl3): δC 165.3 (C=O), 154.7 (C), 151.5 (C-O), 150.5 (C-H), 137.1 (C-H), 134.6 (C-H), 134.3 (C-H), 130.9 (C-H), 130.7 (C), 129.5 (C-H), 129.3 (C), 129.1 (C-H), 127.3 (C-H), 124.8 (C-H), 124.4 (CF3), 123.7 (C-H), 123.5 (C-H). MS (EI): 343 (M+, 20), 105 (100), 77 (40). HRMS (ESI): calcd. for C19H12NO2F3H+: 344.0898 found: 344.0905. IR (KBr, cm−1): 1739.
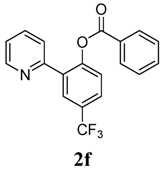

4-Fluoro-2-(pyridin-2-yl)phenyl benzoate (2g). Yellow oil (62% yield). 1H-NMR (CDCl3): δH 8.60 (d, J = 4.7 Hz, 1H), (d, J = 4.7 Hz, 1H), 8.08 (d, J = 7.4 Hz, 2H), 7.52–7.64 (m, 4H), 7.46 (t, J = 7.7 Hz, 2H), 7.25–7.28 (m, 1H), 7.13–7.18 (m, 2H). 13C-NMR (CDCl3): δC 165.8 (C=O), 159.8 (C-F), 154.9 (C), 150.4 (C-H), 144.7 (C-O), 136.9 (C-H), 135.4 (C-H), 134.2 (C-H), 130.8 (C-H), 129.8 (C), 129.2 (C-H), 125.5 (C), 124.2 (C-H), 123.2 (C-H), 117.9 (C-H), 117.1 (C-H). MS (EI): 293 (M+, 20), 105 (100), 77 (40). HRMS (ESI): calcd. for C18H12NO2FH+: 294.0930, found: 294.0927. IR (KBr, cm−1): 1741.
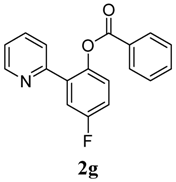

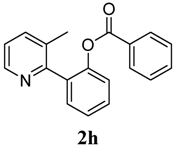
2-(3-Methylpyridin-2-yl)phenyl benzoate (2h). Yellow oil (87% yield). 1H-NMR (CDCl3): δH 8.42 (d, J = 4.5 Hz, 1H), 7.89 (d, J = 7.5 Hz, 1H), 7.26–7.54 (m, 8H), 7.04–7.07 (m, 1H), 2.21 (s, 3H). 13C-NMR (CDCl3): δC 165.0 (C=O), 155.9 (C), 148.8 (C-O), 147.1 (C-H), 138.3 (C-H), 134.0 (C-H), 133.8 (C-H), 132.6 (C), 130.9 (C-H), 130.6 (C), 130.1 (C-H), 129.7 (C), 128.8 (C-H), 126.4 (C-H), 123.3 (C-H), 123.0 (C-H), 19.5 (CH3). MS (EI): 289 (M+, 30), 105 (100), 77 (40). HRMS (ESI): calcd. for C19H15NO2H+: 290.1181, found: 290.1176. IR (KBr, cm−1): 1736.

1-(Pyridin-2-yl)naphthalen-2-yl benzoate (2i). Yellow oil (57% yield). 1H-NMR (CDCl3): δH 8.77 (d, J = 4.2 Hz, 1H), 7.93–8.01 (m, 4H), 7.71 (t, J = 7.6 Hz, 1H), 7.63 (d, J = 8.2 Hz, 1H), 7.55–7.57 (m, 1H), 7.44–7.54 (m, 4H), 7.40 (t, J = 7.6 Hz, 2H), 7.24–7.27 (m, 1H). 13C-NMR (CDCl3): δC 165.9 (C=O), 155.4 (C), 150.3 (C-O), 146.7 (C-H), 136.7 (C-H), 134.0 (C-H), 133.4 (C), 132.5 (C-H), 130.6 (C-H), 130.1 (C), 129.8 (C), 129.0 (C-H), 128.8 (C-H), 128.7 (C), 127.7 (C-H), 127.5 (C-H), 126.4 (C-H), 126.2 (C-H), 123.0 (C-H), 122.3 (C-H). MS (EI): 325 (M+, 50), 191 (10) 105 (100), 77 (40). HRMS (ESI): calcd. for C22H15NO2H+: 326.1130, found: 326.1129. IR (KBr, cm−1): 1735.

Benzo[h]quinolin-10-yl benzoate (2j). Yellow oil (85% yield). 1H-NMR (CDCl3): δH 8.39–8.42 (m, 3H), 8.08 (dd, J = 8.0, 1.8 Hz, 1H), 7.83–7.89 (m, 2H), 7.66–7.75 (m, 3H), 7.53–7.62 (m, 3H), 7.33 (q, J = 4.1 Hz, 1H). 13C-NMR (CDCl3): δC 167.5 (C=O), 149.7 (C-O), 148.5 (C-H), 146.1 (C), 136.6 (C-H), 136.1 (C), 133.3 (C-H), 132.0 (C), 131.1 (C-H), 129.0 (C-H), 128.7 (C), 128.5 (C-H), 127.6 (C-H), 127.3 (C-H), 127.0 (C-H), 124.0 (C), 123.0 (C-H), 122.1 (C-H). MS (EI): 299 (M+, 40), 105 (100), 77 (30). HRMS (ESI): calcd. for C20H13NO2H+: 300.1025, found: 300.1024. IR (KBr, cm−1): 1735.

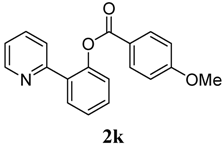
2-(Pyridin-2-yl)phenyl 4-methoxybenzoate (2k). Yellow oil (54% yield). 1H-NMR (CDCl3): δH 8.59 (d, J = 4.5 Hz, 1H), 8.03 (d, J = 8.8 Hz, 2H), 7.78 (dd, J = 7.6, 1.5 Hz, 1H), 7.54–7.63 (m, 2H), 7.45 (dt, J = 7.6, 1.5 Hz, 1H), 7.36 (dt, J = 7.5, 0.8 Hz, 1H), 7.29 (dd, J = 7.9, 0.7 Hz, 1H), 7.11–7.14 (m, 1H), 6.91 (d, J = 8.8 Hz, 2H), 3.82 (s, 3H). 13C-NMR (CDCl3): δC 165.4 (C=O), 164.3 (C), 156.1 (C), 150.1 (C), 148.9 (C-H), 136.6 (C-H), 133.9 (C-H), 132.8 (C-H), 131.4 (C-H), 130.2 (C), 126.8 (C-H), 123.9 (C-H), 122.6 (C), 122.2 (C-H), 119.2 (C-H), 114.3 (C-H), 56.0 (CH3). MS (EI): 305 (M+, 20), 135 (100). HRMS (ESI): calcd. for C19H15NO3H+: 306.1130, found: 306.1136. IR (KBr, cm−1): 1732.

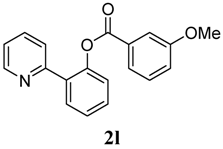
2-(Pyridin-2-yl)phenyl 3-methoxybenzoate (2l). Yellow oil (69% yield). 1H-NMR (CDCl3): δH 8.60 (d, J = 4.1 Hz, 1H), 7.78 (dd J = 7.7, 1.5 Hz, 1H), 7.69 (d, J = 6.6 Hz, 1H), 7.55–7.64 (m, 3H), 7.47 (dt J = 7.7, 1.6 Hz, 1H), 7.35–7.41 (m, 2H), 7.32 (dt J = 8.0, 1.0 Hz, 1H), 7.10–7.17 (m, 2H), 3.81 (s, 3H). 13C-NMR (CDCl3): δC 165.6 (C=O), 160.2 (C), 156.1 (C), 150.2 (C), 148.9 (C-H), 136.8 (C-H), 133.9 (C-H), 131.5 (C), 131.3 (C-H), 130.3 (C-H), 130.1 (C), 127.0 (C-H), 124.3 (C-H), 123.9 (C-H), 123.2 (C-H), 122.8 (C-H), 120.7 (C-H), 115.0 (C-H), 56.0 (CH3). MS (EI): 305 (M+, 20), 135 (100). HRMS (ESI): calcd. for C19H15NO3H+: 306.1130, found: 306.1134. IR (KBr, cm−1): 1736.

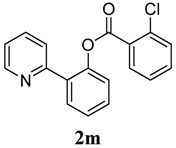
2-(Pyridin-2-yl)phenyl 2-chlorobenzoate (2m). Yellow oil (43% yield). 1H-NMR (CDCl3): δH 8.63 (d, J = 4.7 Hz, 1H), 7.88 (dd, J = 7.8, 1.2 Hz, 1H), 7.74 (dd, J = 7.7, 1.5 Hz, 1H), 7.68 (dt, J = 7.7, 1.7 Hz, 1H), 7.57 (d, J = 7.8 Hz, 1H), 7.37–7.52 (m, 4H), 7.31–7.34 (m, 2H), 7.18–7.21 (m, 1H). 13C-NMR (CDCl3): δC 165.2 (C=O), 156.4 (C), 150.2 (C-O), 148.7 (C-H), 137.0 (C-H), 135.0 (C-Cl), 134.0 (C-H), 133.6 (C-H), 132.5 (C-H), 131.8 (C-H), 131.5 (C), 130.5 (C-H), 130.0 (C), 127.2 (C-H), 124.4 (C-H), 124.0 (C-H), 122.9 (C-H), 122.1 (C-H). MS (EI): 309 (M+, 40), 139 (100), 111 (20). HRMS (ESI): calcd. for C18H12NO2ClH+: 310.0635, found: 310.0628. IR (KBr, cm−1): 1742.

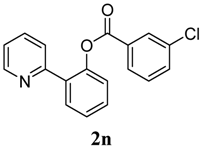
2-(Pyridin-2-yl)phenyl 3-chlorobenzoate (2n). Yellow oil (73% yield). 1H-NMR (CDCl3): δH 8.57 (d, J = 4.6 Hz, 1H), 8.06 (s, 1H), 7.95 (d, J = 7.8 Hz, 1H), 7.75 (dd, J = 7.7, 1.3 Hz, 1H), 7.65 (t, J = 7.8 Hz, 1H), 7.53–7.57 (m, 2H), 7.48 (t, J = 7.7 Hz, 1H), 7.37–7.42 (m, 2H), 7.30 (d, J = 4.5 Hz, 1H), 7.14–7.18 (m, 1H). 13C-NMR (CDCl3): δC 164.6 (C=O), 156.1 (C), 150.2 (C-O), 148.7 (C-H), 136.9 (C-H), 135.2 (C-Cl), 134.0 (C-H), 133.8 (C), 131.9 (C-H), 131.5 (C-H), 130.7 (C-H), 130.4 (C-H), 129.9 (C), 128.8 (C-H), 127.2 (C-H), 124.2 (C-H), 123.8 (C-H), 122.8 (C-H). MS (EI): 309 (M+, 20), 139 (100), 111 (40). HRMS (ESI): calcd. for C18H12NO2ClH+: 310.0635, found: 310.0640. IR (KBr, cm−1): 1740.

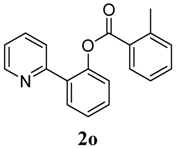
2-(Pyridin-2-yl)phenyl 2-methylbenzoate (2o). Yellow oil (66% yield). 1H-NMR (CDCl3): δH 8.61 (dd, J = 4.8, 0.8 Hz, 1H), 8.01 (dd, J = 7.1, 1.3 Hz, 1H), 7.76 (dd, J = 7.6, 1.6 Hz, 1H), 7.64 (dt, J = 7.7, 1.7 Hz, 1H), 7.56 (d, J = 7.9 Hz, 1H), 7.49 (dt, J = 7.7, 1.6 Hz, 1H), 7.38–7.45 (m, 2H), 7.24–7.30 (m, 4H), 7.15–7.19 (m, 1H), 2.54 (s, 3H). 13C-NMR (CDCl3): δC 166.3 (C=O), 156.5 (C), 150.2 (C-O), 150.0 (C-H), 141.8 (C), 136.8 (C-H), 134.2 (C-H), 133.1 (C-H), 132.7 (C-H), 132.4 (C-H), 131.5 (C-H), 130.4 (C), 129.2 (C), 128.5 (C-H), 126.9 (C-H), 126.4 (C-H), 124.1 (C-H), 122.8 (C-H), 22.2 (CH3). MS (EI): 289 (M+, 10), 119 (100), 91 (40). HRMS (ESI): calcd. for C19H15NO2H+: 290.1181, found: 290.1186. IR (KBr, cm−1): 1738.

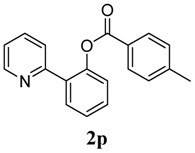
2-(Pyridin-2-yl)phenyl 4-methylbenzoate (2p). Yellow oil (64% yield). 1H-NMR (CDCl3): δH 8.60 (d, J = 4.7 Hz, 1H), 7.98 (d, J = 8.1 Hz, 2H), 7.79 (dd, J = 7.7, 1.5 Hz, 1H), 7.55–7.60 (m, 2H), 7.47 (dt, J = 7.7, 1.5 Hz, 1H), 7.39 (t, J = 7.5 Hz, 1H), 7.25 (d, J = 7.9 Hz, 2H), 7.14–7.16 (m, 1H), 7.04 (t, J = 4.7 Hz, 1H), 2.41 (s, 3H). 13C-NMR (CDCl3): δC 165.8 (C=O), 156.2 (C), 150.2 (C-O), 149.4 (C-H), 144.9 (C), 136.7 (C-H), 134.0 (C-H), 131.5 (C-H), 130.7 (C-H), 130.1 (C-H), 129.6 (C), 127.3 (C), 126.9 (C-H), 124.4 (C-H), 124.0 (C-H), 122.7 (C-H), 22.3 (CH3). MS (EI): 289 (M+, 10), 119 (100), 91 (40). HRMS (ESI): calcd. for C19H15NO2H+: 290.1181, found: 290.1173. IR (KBr, cm−1): 1737.

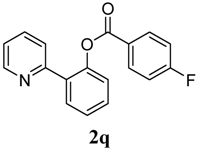
2-(Pyridin-2-yl)phenyl 4-fluorobenzoate (2q). Yellow oil (55% yield). 1H-NMR (CDCl3): δH 8.57 (d, J = 4.2 Hz, 1H), 8.08–8.11 (m, 2H), 7.76 (dd, J = 7.6, 1.5 Hz, 1H), 7.62 (dt, J = 7.7, 1.6 Hz, 1H), 7.53 (d, J = 7.9 Hz, 1H), 7.47 (dt, J = 7.7, 1.5 Hz, 1H), 7.39 (dt, J = 7.5, 0.9 Hz, 1H), 7.30 (dd, J = 7.9, 0.8 Hz, 1H), 7.09–7.16 (m, 3H). 13C-NMR (CDCl3): δC 167.9 (C-F), 164.8 (C=O), 156.1 (C), 150.1 (C-O), 148.8 (C-H), 136.8 (C-H), 133.9 (C-H), 133.4 (C-H), 131.5 (C-H), 130.3 (C), 127.1 (C-H), 126.3 (C), 123.0 (C-H), 122.8 (C-H), 116.4 (C-H), 116.2 (C-H). MS (EI): 293 (M+, 10), 123 (100), 95 (30). HRMS (ESI): calcd. for C18H12NO2FH+: 294.0930, found: 294.0931. IR (KBr, cm−1): 1738.

2-(Pyridin-2-yl)phenyl 4-bromobenzoate (2r). Yellow oil (30% yield). 1H-NMR (CDCl3): δH 8.56 (d, J = 4.3 Hz, 1H), 7.94 (d, J = 8.5 Hz, 2H), 7.76 (dd, J = 7.6, 1.4 Hz, 1H), 7.64 (dd, J = 7.8, 1.6 Hz, 1H), 7.46–7.53 (m, 3H), 7.40 (t, J = 7.0 Hz, 1H), 7.30 (d, J = 7.8 Hz, 1H), 7.14–7.17 (m, 1H). 13C-NMR (CDCl3): δC 165.1 (C=O), 156.2 (C), 150.2 (C-O), 148.8 (C-H), 136.9 (C-H), 133.8 (C-H), 132.5 (C-H), 132.3 (C-H), 131.9 (C-H), 130.4 (C-H), 129.3 (C), 129.1 (C), 127.2 (C-Br), 124.2 (C-H), 123.9 (C-H), 122.8 (C-H). MS (EI): 355 (M+, 20), 183 (100), 155 (20). HRMS (ESI): calcd. for C18H12NO2BrH+: 354.0130, found: 354.0133. IR (KBr, cm−1): 1737.
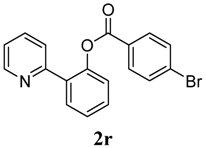

2-(Pyridin-2-yl)phenyl 4-(trifluoromethyl)benzoate (2s). Yellow oil (81% yield). 1H-NMR (CDCl3): δH 8.54 (d, J = 4.5 Hz, 1H), 8.19 (d, J = 8.4 Hz, 2H), 7.71–7.82 (m, 3H), 7.64 (dt, J = 7.8, 1.7 Hz, 1H), 7.47–7.54 (m, 2H), 7.41 (dt, J = 7.5, 1.0 Hz, 1H), 7.31 (d, J = 8.0 Hz, 1H), 7.14–7.17 (m, 1H). 13C-NMR (CDCl3): δC 164.7 (C=O), 156.2 (C), 150.1 (C-O), 148.7 (C-H), 137.0 (C), 135.6 (C-H), 135.2 (C-H), 133.8 (C-H), 133.4 (C), 131.5 (C-H), 131.2 (C-H), 130.5 (C), 127.3 (C-H), 125.6 (C-H), 124.1 (CF3), 123.9 (C-H), 122.9 (C-H). MS (EI): 343 (M+, 40), 173 (100), 145 (50). HRMS (ESI): calcd. for C19H12NO2F3H+: 344.0898, found: 344.0901. IR (KBr, cm−1): 1743.
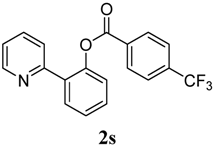

2-(Pyridin-2-yl)phenyl 4-ethoxybenzoate (2t). Yellow oil (27% yield). 1H-NMR (CDCl3): δH 8.61 (d, J = 4.6 Hz, 1H), 8.02 (d, J = 8.8 Hz, 2H), 7.78 (dd, J = 7.6, 1.3 Hz, 1H), 7.54–7.63 (m, 2H), 7.47 (dt, J = 7.6, 1.3 Hz, 1H), 7.38 (dt, J = 7.5, 0.9 Hz, 1H), 7.29 (d, J = 8.1 Hz, 1H), 7.13–7.17 (m, 1H), 6.91 (d, J = 8.8 Hz, 2H), 4.09 (q, J = 7.0 Hz, 2H), 1.44 (t, J = 7.0 Hz, 1H). 13C-NMR (CDCl3): δC 165.5 (C=O), 163.9 (C-O), 156.2 (C), 150.3 (C-O), 149.1 (C-H), 136.7 (C-H), 134.0 (C-H), 133.0 (C-H), 131.5 (C-H), 130.3 (C), 126.9 (C-H), 124.4 (C-H), 124.1 (C-H), 122.7 (C-H), 122.2 (C), 114.9 (C-H), 64.4 (CH2), 15.3 (CH3). MS (EI): 319 (M+, 10), 207 (20), 149 (100), 121 (40). HRMS (ESI): calcd. for C20H17NO3H+: 320.1287, found: 320.1285. IR (KBr, cm−1): 1731.
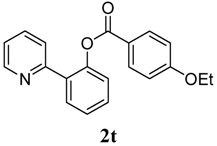

2-(Pyridin-2-yl)phenyl 4-tert-butylbenzoate (2u). Yellow oil (77% yield). 1H-NMR (CDCl3): δH 8.62 (d, J = 4.6 Hz, 1H), 8.04 (d, J = 8.3 Hz, 2H), 7.88 (d, J = 8.3 Hz, 2H), 7.45–7.54 (m, 2H), 7.36–7.42 (m, 2H), 7.11–7.14 (m, 1H), 1.33 (d, J = 13.6 Hz, 9H). 13C-NMR (CDCl3): δC 165.2 (C=O),157.7 (C), 156.1 (C-O), 149.9 (C), 148.9 (C-H), 136.7 (C-H), 133.9 (C-H), 131.5 (C-H), 130.5 (C-H), 130.2 (C), 126.8 (C), 125.9 (C-H), 124.3 (C-H), 123.9 (C-H), 122.6 (C-H), 121.2 (C-H), 35.6 (C), 31.6 (CH3). MS (EI): 331 (M+, 10), 161 (100), 146 (10). HRMS (ESI): calcd. for C22H21NO2H+: 332.1651, found: 332.1652. IR (KBr, cm−1): 1737cm−1.
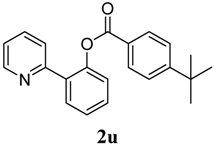

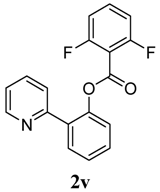
2-(Pyridin-2-yl)phenyl 2,6-difluorobenzoate (2v). Yellow oil (46% yield). 1H-NMR (CDCl3): δH 8.65 (d, J = 4.4 Hz, 1H), 7.76 (dd, J = 7.6,1.6 Hz, 1H), 7.70 (dt, J = 7.7, 1.7 Hz, 1H), 7.59 (d, J = 7.9 Hz, 1H), 7.49 (dt, J = 7.7, 1.6 Hz, 1H), 7.39–7.44 (m, 2H), 7.33 (dd, J = 8.0, 1.0 Hz, 1H), 7.20–7.23 (m, 1H), 6.95 (t, J = 8.3 Hz, 2H). 13C-NMR (CDCl3): δC 162.7 (C=O), 160.7 (C-F), 156.0 (C), 150.2 (C-O), 148.3 (C-H), 136.9 (C-H), 134.1 (C-H), 134.0 (C), 131.6 (C-H), 130.5 (C-H), 127.5 (C-H), 124.4 (C-H), 123.7 (C-H), 122.9 (C-H), 112.9 (C), 112.6 (C-H). MS (EI): 311 (M+, 40), 141 (100), 113 (10). HRMS (ESI): calcd. for C18H11NO2F2H+: 312.0836, found: 312.0841. IR (KBr, cm−1): 1751.

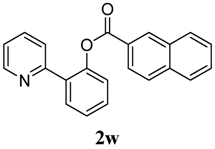
2-(Pyridin-2-yl)phenyl 2-naphthoate (2w). Yellow oil (44% yield). 1H-NMR (CDCl3): δH 8.69 (s, 1H), 8.59 (d, J = 4.8 Hz, 1H), 8.10 (dd, J = 8.6, 1.4 Hz, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.90 (d, J = 8.5 Hz, 2H), 7.82 (dd, J = 7.6, 1.5 Hz, 1H), 7.56–7.63 (m, 4H), 7.51 (dt, J = 7.8, 1.6 Hz, 1H), 7.41–7.45 (m, 1H), 7.38 (dd, J = 7.9, 0.7 Hz, 1H),7.11–7.15 (m, 1H). 13C-NMR (CDCl3): δC 166.0 (C=O), 156.3 (C), 150.3 (C-O), 149.1 (C-H), 136.8 (C-H), 136.4 (C), 134.0 (C-H), 133.1 (C), 132.6 (C-H), 131.6 (C-H), 130.4 (C-H), 130.1 (C-H), 129.2 (C), 128.9 (C-H), 128.4 (C), 127.4 (C-H), 127.3 (C-H), 127.1 (C-H), 126.1 (C-H), 124.3 (C-H), 124.0 (C-H), 122.8 (C-H). MS (EI): 325 (M+, 20), 155 (100), 127 (60). HRMS (ESI): calcd. for C22H15NO2H+: 326.1181, found: 326.1191. IR (KBr, cm−1): 1734 cm−1.

4. Conclusions
In summary, we developed a Pd-catalyzed regioselective C–H benzoxylation reaction with aryl acylperoxides as reagents. This catalytic protocol was convenient to operate, and the product benzoates were formed in good yield with high regioselectivity and functional group tolerance.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/18/4/4403/s1.
Acknowledgments
We thank the financial support from The Hong Kong Research Grants Council (PolyU 5031/09P, SEG_PolyU01). And also thank Jin-Quan Yu and Ramesh Giri for the invitation of the submission of this article on the special issue “Transition Metal Catalysis”.
References
- Liu, C.; Zhang, H.; Shi, W.; Lei, A. Bond formations between two nucleophiles: Transition metal catalyzed oxidative cross-coupling reactions. Chem. Rev. 2011, 111, 1780–1824. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef]
- Chen, X.; Engle, D.H.; Wang, D.H.; Yu, J.Q. Palladium(II)-catalyzed C–H activation/C–C cross coupling reactions: Versatility and practicality. Angew. Chem. Int. Ed. 2009, 48, 5094–5115. [Google Scholar] [CrossRef]
- Desai, L.V.; Malik, H.A.; Sanford, M.S. Oxone as an inexpensive, safe, and environmentally benign oxidant for C–H bond oxygenation. Org. Lett. 2006, 8, 1141–1144. [Google Scholar] [CrossRef]
- Yoneyama, T.; Crabtree, R.H. Pd(II) catalyzed acetoxylation of arenes with iodosyl acetate. J. Mol. Catal. A 1996, 108, 35–40. [Google Scholar] [CrossRef]
- Wang, G.W.; Yuan, T.T.; Wu, X.L. Direct ortho-acetoxylation of anilides via palladium-catalyzed sp2 C–H bond oxidative activation. J. Org. Chem. 2008, 73, 4717–4720. [Google Scholar] [CrossRef]
- Dick, A.R.; Hull, K.L.; Sanford, M.S. Palladium-catalyzed oxygenation of unactivated sp3 C–H bonds. J. Am. Chem. Soc. 2004, 126, 2300–2301. [Google Scholar] [CrossRef]
- Giri, R.; Liang, J.; Lei, J.G.; Li, J.J.; Wang, D.H.; Chen, X.; Naggar, I.C.; Guo, C.; Foxman, B.M.; Yu, J.Q. Pd-catalyzed stereoselective oxidation of methyl groups by inexpensive oxidants under mild conditions: A dual role for carboxylic anhydrides in catalytic C–H bond oxidation. Angew. Chem. Int. Ed. 2005, 44, 7420–7424. [Google Scholar] [CrossRef]
- Reddy, B.V.S.L.; Reddy, R.; Corey, E.J. Novel acetoxylation and C–C coupling reactions at unactivated positions in a-amino acid derivatives. Org. Lett. 2006, 8, 3391–3394. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Yu, J.Q. Pd(II)-catalyzed hydroxylation of arenes using 1 atm O2 or air. J. Am. Chem. Soc. 2009, 131, 14654–14655. [Google Scholar] [CrossRef]
- Chen, X.; Hao, X.S.; Goodhue, C.E.; Yu, J.Q. Cu(II)-catalyzed functionalizations of aryl C–H bonds using O2 as an oxidant. J. Am. Chem. Soc. 2006, 128, 6790–6791. [Google Scholar] [CrossRef]
- Luo, F.; Pan, C.; Cheng, J. Recent advances in transition-metal-catalyzed esterification. Synlett 2012, 23, 357–366. [Google Scholar] [CrossRef]
- James, C.A.; Snieckus, V. Combined directed metalation—cross coupling strategies. Total synthesis of the aglycones of gilvocarcin V, M and E. Tetrahedron Lett. 1997, 38, 8149–8152. [Google Scholar]
- Farr, R.N.; Kwok, D.I.; Daves, G.D., Jr. 8-Ethenyl-1-hydroxy-4-.beta.-D-ribofuranosyl-benzo[d]naphtho[1,2-b]pyran-6-one and 8-ethenyl-1-hydroxy-4-(2'-deoxy-.beta.-D-ribofurano-syl)benzo[d]naphtho[1,2-b]pyran-6-one. Synthetic C-glycosides related to the gilvocarcin, ravidomycin, and chrysomycin antibiotics. J. Org. Chem. 1992, 57, 2093–2100. [Google Scholar]
- Ye, Z.; Wang, W.; Luo, F.; Zhang, S.; Cheng, J. Rhodium-catalyzed ortho-benzoxylation of sp2 C–H bond. Org. Lett. 2009, 11, 3974–3977. [Google Scholar] [CrossRef]
- Wang, W.; Luo, F.; Zhang, S.; Cheng, J. Copper(II)-catalyzed ortho-acyloxylation of the 2-arylpyridines sp2 C–H bonds with anhydrides, using O2 as terminal oxidant. J. Org. Chem. 2010, 75, 2415–2418. [Google Scholar] [CrossRef]
- Wang, W.; Pan, C.; Chen, F.; Cheng, J. Copper(II)-catalyzed ortho functionalization of 2-arylpyridines with acyl chlorides. Chem. Commun. 2011, 47, 3978–3980. [Google Scholar] [CrossRef]
- Yu, W.Y.; Sit, W.N.; Lai, K.M.; Zhou, Z.; Chan, A.S.C. Palladium-catalyzed oxidative ethoxycarbonylation of aromatic C–H bond with diethyl azodicarboxylate. J. Am. Chem. Soc. 2008, 130, 3304–3306. [Google Scholar]
- Yu, W.Y.; Sit, W.N.; Zhou, Z.; Chan, A.S.C. Palladium-catalyzed decarboxylative arylation of C–H Bonds by aryl acylperoxides. Org. Lett. 2009, 11, 3174–3177. [Google Scholar] [CrossRef]
- Chan, C.W.; Zhou, Z.; Chan, A.S.C.; Yu, W.Y. Pd-catalyzed Ortho-C-H acylation/cross coupling of aryl ketone O-methyl oximes with aldehydes using tert-butyl hydroperoxide as oxidant. Org. Lett. 2010, 12, 3926–3929. [Google Scholar] [CrossRef]
- Chan, C.W.; Zhou, Z.; Yu, W.Y. Palladium(II)-catalyzed direct ortho-C-H acylation of anilides by oxidative cross-coupling with aldehydes using tert-butyl hydroperoxide as oxidant. Adv. Synth. Catal. 2011, 353, 2999–3006. [Google Scholar] [CrossRef]
- Chan, W.W.; Lo, S.F.; Zhou, Z.; Yu, W.Y. Rh-catalyzed intermolecular carbenoid functionalization of aromatic C–H bonds by α-diazomalonates. J. Am. Chem. Soc. 2012, 134, 13565–13568. [Google Scholar] [CrossRef]
- Ng, K.H.; Chan, A.S.C.; Yu, W.Y. Pd-catalyzed intermolecular ortho-C-H amidation of anilides by N-nosyloxycarbamate. J. Am. Chem. Soc. 2010, 132, 12862–12864. [Google Scholar] [CrossRef]
- Ng, K.H.; Zhou, Z.; Yu, W.Y. Rhodium(III)-catalyzed intermolecular direct amination of aromatic C–H bonds with N-chloroamines. Org. Lett. 2012, 14, 272–275. [Google Scholar] [CrossRef]
- Ng, K.H.; Ng, F.N.; Yu, W.Y. A convenient synthesis of anthranilic acids by Pd-catalyzed direct intermolecular ortho-C-H amidation of benzoic acids. Chem. Commun. 2012, 48, 11680–11682. [Google Scholar] [CrossRef]
- Moad, G.; Solomon, D.H. The Chemistry of Free Radical Polymerization; Elsevier: Boston, MA, USA, 2006. [Google Scholar]
- Navarro, O.; Kaur, H.; Mahjoor, P.; Nolan, S.P. Cross-coupling and dehalogenation reactions catalyzed by (N-heterocyclic carbene)Pd(allyl)Cl complexes. J. Org. Chem. 2004, 69, 3173–3180. [Google Scholar] [CrossRef]
- Flowers, G.C.; Leffler, J.E. Decomposition of bis(p-methoxybenzoyl) peroxide and the carboxy-inversion product on silica.xide and the carboxy-inversion product on silica. J. Org. Chem. 1985, 50, 4406–4408. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
