Mild Conditions for Deuteration of Primary and Secondary Arylamines for the Synthesis of Deuterated Optoelectronic Organic Molecules
Abstract
:1. Introduction


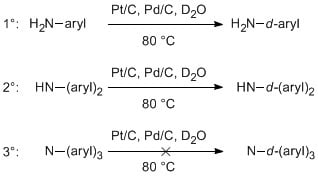
2. Results and Discussion


3. Experimental Section
3.1. Materials
3.2. Instrumentation
3.3. General Method
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yeh, S.J.; Tsai, C.Y.; Huang, C.-Y.; Liou, G.-S.; Cheng, S.-H. Electrochemical characterization of small organic hole-transport molecules based on the triphenylamine unit. Electrochem. Commun. 2003, 5, 373–377. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Hsiao, S.-H.; Su, T.-H.; Liou, G.-S. Novel aromatic poly(amine-imide)s bearing a pendent triphenylamine group: Synthesis, thermal, photophysical, electrochemical, and electrochromic characteristics. Macromolecules 2004, 38, 307–316. [Google Scholar] [CrossRef]
- Su, T.-H.; Hsiao, S.-H.; Liou, G.-S. Novel family of triphenylamine-containing, hole-transporting, amorphous, aromatic polyamides with stable electrochromic properties. J. Polym. Sci. Part. A: Polym. Chem. 2005, 43, 2085–2098. [Google Scholar] [CrossRef]
- Chou, M.-Y.; Leung, M.-k.; Su, Y.O.; Chiang, C.L.; Lin, C.-C.; Liu, J.-H.; Kuo, C.-K.; Mou, C.-Y. Electropolymerization of starburst triarylamines and their application to electrochromism and electroluminescence. Chem. Mater. 2004, 16, 654–661. [Google Scholar] [CrossRef]
- Tanaka, H.; Tokito, S.; Taga, Y.; Okada, A. Novel hole-transporting materials based on triphenylamine for organic electroluminescent devices. Chem. Commun. 1996, 2175–2176. [Google Scholar]
- Shirota, Y. Organic materials for electronic and optoelectronic devices. J. Mater. Chem. 2000, 10, 1–25. [Google Scholar] [CrossRef]
- Choi, K.; Yoo, S.J.; Sung, Y.-E.; Zentel, R. High contrast ratio and rapid switching organic polymeric electrochromic thin films based on triarylamine derivatives from layer-by-layer assembly. Chem. Mater. 2006, 18, 5823–5825. [Google Scholar] [CrossRef]
- Lee, K.H.; Schwenn, P.E.; Smith, A.R.G.; Cavaye, H.; Shaw, P.E.; James, M.; Krueger, K.B.; Gentle, I.R.; Meredith, P.; Burn, P.L. Morphology of all-solution-processed “bilayer” organic solar cells. Adv. Mater. 2011, 23, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Lee, P.; Armstrong, N.R.; Graham, A.; Evmenenko, G.A.; Dutta, P.; Marks, T.J. High-performance hole-transport layers for polymer light-emitting diodes. Implementation of organosiloxane cross-linking chemistry in polymeric electroluminescent devices. J. Am. Chem. Soc. 2005, 127, 3172–3183. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Forsythe, E.W.; Morton, D.C. Method of Making Benzazoles. US20130172570 A1, 4 July 2013. [Google Scholar]
- Takizawa, S.; Montes, V.A.; Anzenbacher, P. Phenylbenzimidazole-based new bipolar host materials for efficient phosphorescent organic light-emitting diodes. Chem. Mater. 2009, 21, 2452–2458. [Google Scholar] [CrossRef]
- Huang, J. Syntheses and applications of conducting polymer polyaniline nanofibers. Pure Appl. Chem. 2006, 78, 15–27. [Google Scholar] [CrossRef]
- Fehse, K.; Schwartz, G.; Walzer, K.; Leo, K. Combination of a polyaniline anode and doped charge transport layers for high-efficiency organic light emitting diodes. J. Appl. Phys. 2007, 101, 124509. [Google Scholar] [CrossRef]
- McKeown, N.B.; Badriya, S.; Helliwell, M.; Shkunov, M. The synthesis of robust, polymeric hole-transport materials from oligoarylamine substituted styrenes. J. Mater. Chem. 2007, 17, 2088–2094. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Hukic-Markosian, G.; Wang, F.; Wojcik, L.; Li, X.-G.; Ehrenfreund, E.; Vardeny, Z.V. Isotope effect in spin response of [π]-conjugated polymer films and devices. Nat. Mater. 2010, 9, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Keum, J.; Chen, J.; He, Y.; Chen, W.; Browning, J.F.; Jakowski, J.; Sumpter, B.G.; Ivanov, I.N.; Ma, Y.-Z.; et al. The isotopic effects of deuteration on optoelectronic properties of conducting polymers. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Tong, C.C.; Hwang, K.C. Enhancement of OLED efficiencies and high-voltage stabilities of light-emitting materials by deuteration. J. Phys. Chem. C 2007, 111, 3490–3494. [Google Scholar] [CrossRef]
- Wang, P.; Wang, F.-F.; Chen, Y.; Niu, Q.; Lu, L.; Wang, H.-M.; Gao, X.-C.; Wei, B.; Wu, H.-W.; Cai, X.; et al. Synthesis of all-deuterated tris(2-phenylpyridine)iridium for highly stable electrophosphorescence: The “deuterium effect”. J. Mater. Chem. C 2013, 1, 4821–4825. [Google Scholar] [CrossRef]
- Giebink, N.C.; D’Andrade, B.W.; Weaver, M.S.; Brown, J.J.; Forrest, S.R. Direct evidence for degradation of polaron excited states in organic light emitting diodes. J. Appl. Phys. 2009, 105, 124514. [Google Scholar] [CrossRef]
- Sivasubramaniam, V.; Brodkorb, F.; Hanning, S.; Buttler, O.; Loebl, H.P.; van Elsbergen, V.; Boerner, H.; Scherf, U.; Kreyenschmidt, M. Degradation of htl layers during device operation in pholeds. Solid State Sci. 2009, 11, 1933–1940. [Google Scholar] [CrossRef]
- Chang, J.; Yu, Y.-J.; Na, J.H.; An, J.; Im, C.; Choi, D.-H.; Jin, J.-I.; Lee, S.H.; Kim, Y.K. Photodegradation-induced photoluminescence behaviors of π-conjugated polymers upon the doping of organometallic triplet emitters. J. Polym. Sci. Part. B: Polym. Phys. 2008, 46, 2395–2403. [Google Scholar] [CrossRef]
- Giebink, N.C.; D’Andrade, B.W.; Weaver, M.S.; Mackenzie, P.B.; Brown, J.J.; Thompson, M.E.; Forrest, S.R. Intrinsic luminance loss in phosphorescent small-molecule organic light emitting devices due to bimolecular annihilation reactions. J. Appl. Phys. 2008, 103, 044509. [Google Scholar] [CrossRef]
- So, F.; Kondakov, D. Degradation mechanisms in small-molecule and polymer organic light-emitting diodes. Adv. Mater. 2010, 22, 3762–3777. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.R.; Mitchell, W.J.; Burn, P.L.; Thomas, R.K.; Fragneto, G.; Markham, J.P.J.; Samuel, I.D.W. Neutron reflection study on soluble and insoluble poly[2-(2'-ethylhexyloxy)-5-methoxy-1,4-phenylenevinylene) films. J. Appl. Phys. 2002, 91, 9066–9071. [Google Scholar] [CrossRef]
- Mitchell, W.J.; Burn, P.L.; Thomas, R.K.; Fragneto, G. Probing the polymer-electrode interface using neutron reflection. Appl. Phys. Lett. 2003, 82, 2724–2726. [Google Scholar] [CrossRef]
- Mitchell, W.J.; Burn, P.L.; Thomas, R.K.; Fragneto, G.; Markham, J.P.J.; Samuel, I.D.W. Relating the physical structure and optical properties of conjugated polymers using neutron reflectivity in combination with photoluminescence spectroscopy. J. Appl. Phys. 2004, 95, 2391–2396. [Google Scholar] [CrossRef]
- Jukes, P.C.; Martin, S.J.; Higgins, A.M.; Geoghegan, M.; Jones, R.A.L.; Langridge, S.; Wehrum, A.; Kirchmeyer, S. Controlling the surface composition of poly(3,4-ethylene dioxythiophene)–poly(styrene sulfonate) blends by heat treatment. Adv. Mater. 2004, 16, 807–811. [Google Scholar] [CrossRef]
- Higgins, A.M.; Cadby, A.; Lidzey, D.G.; Dalgliesh, R.M.; Geoghegan, M.; Jones, R.A.L.; Martin, S.J.; Heriot, S.Y. The impact of interfacial mixing on förster transfer at conjugated polymer heterojunctions. Adv. Funct. Mater. 2009, 19, 157–163. [Google Scholar] [CrossRef]
- Vickers, S.V.; Barcena, H.; Knights, K.A.; Thomas, R.K.; Ribierre, J.-C.; Gambino, S.; Samuel, I.D.W.; Burn, P.L.; Fragneto, G. Light-emitting dendrimer film morphology: A neutron reflectivity study. Appl. Phys. Lett. 2010, 96, 263302. [Google Scholar] [CrossRef]
- Cavaye, H.; Smith, A.R.G.; James, M.; Nelson, A.; Burn, P.L.; Gentle, I.R.; Lo, S.C.; Meredith, P. Solid-state dendrimer sensors: Probing the diffusion of an explosive analogue using neutron reflectometry. Langmuir 2009, 25, 12800–12805. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.R.G.; Lee, K.H.; Nelson, A.; James, M.; Burn, P.L.; Gentle, I.R. Diffusion—the hidden menace in organic optoelectronic devices. Adv. Mater. 2012, 24, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.R.G.; Ruggles, J.L.; Cavaye, H.; Shaw, P.E.; Darwish, T.A.; James, M.; Gentle, I.R.; Burn, P.L. Investigating morphology and stability of fac-tris (2-phenylpyridyl)iridium(III) films for oleds. Adv. Funct. Mater. 2011, 21, 2225–2231. [Google Scholar] [CrossRef]
- Darwish, T.A.; Smith, A.R.G.; Gentle, I.R.; Burn, P.L.; Luks, E.; Moraes, G.; Gillon, M.; Holden, P.J.; James, M. Deuteration of molecules for neutron reflectometry on organic light-emitting diode thin films. Tetrahedron Lett. 2012, 53, 931–935. [Google Scholar] [CrossRef]
- Atkinson, J.G.; Luke, M.O.; Stuart, R.S. A simplified preparation of fully deuterated, high molecular weight hydrocarbons. Can. J. Chem. 1967, 45, 1511–1518. [Google Scholar] [CrossRef]
- Darwish, T.A.; Luks, E.; Moraes, G.; Yepuri, N.R.; Holden, P.J.; James, M. Synthesis of deuterated [d32]oleic acid and its phospholipid derivative [d64]dioleoyl-sn-glycero-3-phosphocholine. J. Label. Compd. Radiopharm. 2013, 56, 520–529. [Google Scholar] [CrossRef]
- Yepuri, N.R.; Holt, S.A.; Moraes, G.; Holden, P.J.; Darwish, T.A.; Hossain, K.R.; Valenzuela, S.M.; James, M. Stereoselective synthesis of perdeuterated phytanic acid, its phospholipid derivatives and their formation into lipid model membranes for neutron reflectivity studies. Chem. Phys. Lipids 2014, 183, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Yepuri, N.R.; Jamieson, S.A.; Darwish, T.A.; Rawal, A.; Hook, J.M.; Thordarson, P.; Holden, P.J.; James, M. Synthesis of per-deuterated alkyl amines for the preparation of deuterated organic pyromellitamide gelators. Tetrahedron Lett. 2013, 54, 2538–2541. [Google Scholar] [CrossRef]
- Mun, S.; Lee, S.; Lee, B.; Choi, D.; Kim, D.; Park, J.; Park, J.; Kim, K.; Ju, J.; Park, Y.; et al. Aromatic Compound as an Electroluminescent Material or Electric Material for Optoelectronic Devices. WO/2012/177006, 27 December 2012. [Google Scholar]
- Dumrath, A.; Lübbe, C.; Neumann, H.; Jackstell, R.; Beller, M. Recyclable catalysts for palladium-catalyzed aminations of aryl halides. Chem. Eur. J. 2011, 17, 9599–9604. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Gong, J.-F.; Wu, Y.-J. Amination of aryl chlorides in water catalyzed by cyclopalladated ferrocenylimine complexes with commercially available monophosphinobiaryl ligands. Tetrahedron Lett. 2007, 48, 1619–1623. [Google Scholar] [CrossRef]
- Kommi, D.N.; Jadhavar, P.S.; Kumar, D.; Chakraborti, A.K. “All-water” one-pot diverse synthesis of 1,2-disubstituted benzimidazoles: Hydrogen bond driven ‘synergistic electrophile-nucleophile dual activation’ by water. Green Chem. 2013, 15, 798–810. [Google Scholar] [CrossRef]
- Spencer, A. Ruthenium-catalyzed rearrangements of azobenzenes. IV. Rearrangement of deuterated azobenzenes to 1-phenylbenzimidazole and N-phenyl-1,2-phenylenediamine derivatives. J. Organomet. Chem. 1985, 295, 199–210. [Google Scholar] [CrossRef]
- Werstiuk, N.H.; Ju, C. Protium-deuterium exchange of substituted pyridines in neutral D2O at elevated temperatures. Can. J. Chem. 1989, 67, 5–10. [Google Scholar] [CrossRef]
- Boix, C.; Poliakoff, M. Efficient H-D exchange of aromatic compounds in near-critical D2O catalysed by a polymer-supported sulphonic acid. Tetrahedron Lett. 1999, 40, 4433–4436. [Google Scholar] [CrossRef]
- Doak, W.A.; Knessl, C.; Labaziewicz, H.; O'Connor, S.E.; Pup, L.; Putz, G.; Schranz, M.; Walter, R.I.; Yang, L.; Werstiuk, N.H. The preparation of specifically deuterated diphenylamines. Can. J. Chem. 1985, 63, 3371–3373. [Google Scholar] [CrossRef]
- Derdau, V.; Atzrodt, J.; Zimmermann, J.; Kroll, C.; Brückner, F. Hydrogen–deuterium exchange reactions of aromatic compounds and heterocycles by NABD4-activated rhodium, platinum and palladium catalysts. Chem. Eur. J. 2009, 15, 10397–10404. [Google Scholar] [CrossRef] [PubMed]
- Odani, R.; Hirano, K.; Satoh, T.; Miura, M. Copper-mediated dehydrogenative biaryl coupling of naphthylamines and 1,3-azoles. J. Org. Chem. 2013, 78, 11045–11052. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Esaki, H.; Maesawa, T.; Imamiya, E.; Maegawa, T.; Sajiki, H. Efficient and selective Pt/C-catalyzed H-D exchange reaction of aromatic rings. Bull. Chem. Soc. Jpn. 2008, 81, 278–286. [Google Scholar] [CrossRef]
- Sajiki, H.; Ito, N.; Esaki, H.; Maesawa, T.; Maegawa, T.; Hirota, K. Aromatic ring favorable and efficient H–D exchange reaction catalyzed by pt/c. Tetrahedron Lett. 2005, 46, 6995–6998. [Google Scholar] [CrossRef]
- Yamamoto, M.; Oshima, K.; Matsubara, S. Platinum catalyzed H-D exchange reaction of various aromatic compounds under hydrothermal condition. Heterocycles 2006, 67, 353–359. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nishiyama, M.; Koie, Y. Palladium-catalyzed synthesis of triarylamines from aryl halides and diarylamines. Tetrahedron Lett. 1998, 39, 2367–2370. [Google Scholar] [CrossRef]
- Kwak, J.; Lyu, Y.-Y.; Noh, S.; Lee, H.; Park, M.; Choi, B.; Char, K.; Lee, C. Hole transport materials with high glass transition temperatures for highly stable organic light-emitting diodes. Thin Solid Films 2012, 520, 7157–7163. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–4 are available from the authors upon request.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krause-Heuer, A.M.; Yepuri, N.R.; Darwish, T.A.; Holden, P.J. Mild Conditions for Deuteration of Primary and Secondary Arylamines for the Synthesis of Deuterated Optoelectronic Organic Molecules. Molecules 2014, 19, 18604-18617. https://doi.org/10.3390/molecules191118604
Krause-Heuer AM, Yepuri NR, Darwish TA, Holden PJ. Mild Conditions for Deuteration of Primary and Secondary Arylamines for the Synthesis of Deuterated Optoelectronic Organic Molecules. Molecules. 2014; 19(11):18604-18617. https://doi.org/10.3390/molecules191118604
Chicago/Turabian StyleKrause-Heuer, Anwen M., Nageshwar R. Yepuri, Tamim A. Darwish, and Peter J. Holden. 2014. "Mild Conditions for Deuteration of Primary and Secondary Arylamines for the Synthesis of Deuterated Optoelectronic Organic Molecules" Molecules 19, no. 11: 18604-18617. https://doi.org/10.3390/molecules191118604
APA StyleKrause-Heuer, A. M., Yepuri, N. R., Darwish, T. A., & Holden, P. J. (2014). Mild Conditions for Deuteration of Primary and Secondary Arylamines for the Synthesis of Deuterated Optoelectronic Organic Molecules. Molecules, 19(11), 18604-18617. https://doi.org/10.3390/molecules191118604