Development of Bioorthogonal Reactions and Their Applications in Bioconjugation
Abstract
:1. Introduction
2. Bioorthogonal Reactions and Recent Development
2.1. Staudinger Ligation

2.2. “Click” Reactions
2.2.1. Copper-Catalyzed Click Reaction

2.2.2. Strain-Promoted Click Reaction


2.3. Tetrazine Ligation
2.4. Photo-Click Reaction

3. Applications of Bioorthogonal Chemistry for Biomolecule Bioconjugations
3.1. Applications of Bioorthogonal Chemistry for Protein Bioconjugations

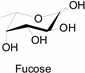
3.2. Application of Bioorthogonal Chemistry for Glycan Bioconjugations
| Natural Monosaccharide | Chemical Report Bearing Monosaccharide |
|---|---|
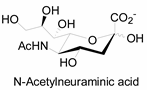 | 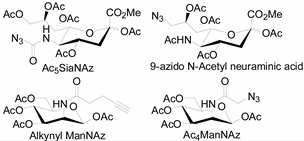 |
 | 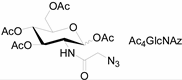 |
 | 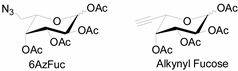 |
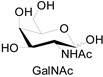 | 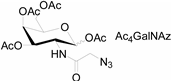 |

3.3. Application of Bioorthogonal Chemistry for Nucleic Acids Bioconjugations


3.4. Application of Bioorthogonal Chemistry for Lipids Bioconjugations
4. Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kalia, J.; Raines, R.T. Advances in bioconjugation. Curr. Org. Chem. 2010, 14, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Patterson, D.M.; Nazarova, L.A.; Prescher, J.A. Finding the right (bioorthogonal) chemistry. ACS Chem. Biol. 2014, 9, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Ramil, C.P.; Lin, Q. Bioorthogonal chemistry: Strategies and recent developments. Chem. Commun. 2013, 49, 11007–11022. [Google Scholar] [CrossRef]
- Shieh, P.; Bertozzi, C.R. Design strategies for bioorthogonal smart probes. Org. Biomol. Chem. 2014, 12, 9307–9320. [Google Scholar] [CrossRef] [PubMed]
- Debets, M.F.; van Hest, J.C.; Rutjes, F.P. Bioorthogonal labelling of biomolecules: New functional handles and ligation methods. Org. Biomol. Chem. 2013, 11, 6439–6455. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, H.; Meyer, J. Uber neue organische phosphorverbindeugen iii. Phosphinmethylenederivate und phosphinimine. Helv. Chim. Acta 1919, 2, 635–646. [Google Scholar] [CrossRef]
- Saxon, E.; Bertozzi, C.R. Cell surface engineering by a modified staudinger reaction. Science 2000, 287, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
- Kiick, K.L.; Saxon, E.; Tirrell, D.A.; Bertozzi, C.R. Incorporation of azides into recombinant proteins for chemoselective modification by the staudinger ligation. Proc. Natl. Acad. Sci. USA 2002, 99, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Prescher, J.A.; Dube, D.H.; Bertozzi, C.R. Chemical remodelling of cell surfaces in living animals. Nature 2004, 430, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Michael, A. Ueber die einwirkung von diazobenzolimid auf acetylendicarbonsauremethylester. J. Prakt. Chem. 1893, 48, 94–95. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-dipolar cycloadditions past and future. Angew. Chem. Int. Ed. 1963, 2, 565–632. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligationˮ of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.M.; Bertozzi, C.R. From mechanism to mouse: A tale of two bioorthogonal reactions. Acc. Chem. Res. 2011, 44, 666–676. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.S.; Finn, M.G. Click chemistry in complex mixtures: Bioorthogonal bioconjugation. Chem. Biol. 2014, 21, 1075–1101. [Google Scholar] [CrossRef] [PubMed]
- Hong, V.; Steinmetz, N.F.; Manchester, M.; Finn, M.G. Labeling live cells by copper-catalyzed alkyne—Azide click chemistry. Bioconjugate Chem. 2010, 21, 1912–1916. [Google Scholar] [CrossRef]
- Soriano Del Amo, D.; Wang, W.; Jiang, H.; Besanceney, C.; Yan, A.C.; Levy, M.; Liu, Y.; Marlow, F.L.; Wu, P. Biocompatible copper(I) catalysts for in vivo imaging of glycans. J. Am. Chem. Soc. 2010, 132, 16893–16899. [Google Scholar] [CrossRef] [PubMed]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A strain-promoted [3+2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Prescher, J.A.; Laughlin, S.T.; Agard, N.J.; Chang, P.V.; Miller, I.A.; Lo, A.; Codelli, J.A.; Bertozzi, C.R. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 16793–16797. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Guo, J.; Wolfert, M.A.; Boons, G.J. Visualizing metabolically labeled glycoconjugates of living cells by copper-free and fast huisgen cycloadditions. Angew. Chem. Int. Ed. 2008, 47, 2253–2255. [Google Scholar] [CrossRef]
- Codelli, J.A.; Baskin, J.M.; Agard, N.J.; Bertozzi, C.R. Second-generation difluorinated cyclooctynes for copper-free click chemistry. J. Am. Chem. Soc. 2008, 130, 11486–11493. [Google Scholar] [CrossRef] [PubMed]
- Debets, M.F.; van Berkel, S.S.; Schoffelen, S.; Rutjes, F.P.; van Hest, J.C.; van Delft, F.L. Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3+2) cycloaddition. Chem. Commun. 2010, 46, 97–99. [Google Scholar] [CrossRef]
- Dommerholt, J.; Schmidt, S.; Temming, R.; Hendriks, L.J.; Rujtes, F.P.; van Hest, J.C.; Lefeber, D.J.; Friedl, P.; van Delft, F.L. Readily accessbile bicyclononynes for bioorthogonal labeling and three-dimensional imaging of living cells. Angew. Chem. Int. Ed. 2010, 49, 9422–9425. [Google Scholar] [CrossRef]
- Balcar, J.; Chrisam, G.; Huber, F.X.; Sauer, J. Reaktivität von stickstoff-heterocyclen genenüber cyclooctin als dienophil. Tetrahedron Lett. 1983, 24, 1481–1484. [Google Scholar] [CrossRef]
- Thalhammer, F.; Wallfahrer, U.; Sauer, J. Reaktivität einfacher offenkettiger und cyclischer dienophile bei diels-alder-reaktionen mit inversem elektronenbedarf. Tetrahedron Lett. 1990, 31, 6851–6854. [Google Scholar] [CrossRef]
- Sauer, J.; Heldmann, D.K.; Hetzenegger, J.; Krauthan, J.; Sichert, H.; Schuster, J. 1,2,4,5-tetrazine: Synthesis and reactivity in [4+2] cycloadditions. Eur. J. Org. Chem. 1998, 1998, 2885–2896. [Google Scholar] [CrossRef]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine ligation: Fast bioconjugation based on inverse-electron-demand diels-alder reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, N.K.; Weissleder, R.; Hilderbrand, S.A. Tetrazine-based cycloadditions: Application to pretargeted live cell imaging. Bioconjugate Chem. 2008, 19, 2297–2299. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Hilderbrand, S.; Upadhyay, R.; Mazitschek, R.; Weissleder, R. Bioorthogonal turn-on probes for imaging small molecules inside living cells. Angew. Chem. Int. Ed. 2010, 49, 2869–2872. [Google Scholar] [CrossRef]
- Patterson, D.M.; Nazarova, L.A.; Xie, B.; Kamber, D.N.; Prescher, J.A. Functionalized cyclopropenes as bioorthogonal chemical reporters. J. Am. Chem. Soc. 2012, 134, 18638–18643. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Seckute, J.; Cole, C.M.; Devaraj, N.K. Live-cell imaging of cyclopropene tags with fluorogenic tetrazine cycloadditions. Angew. Chem. Int. Ed. 2012, 51, 7476–7479. [Google Scholar] [CrossRef]
- Lang, K.; Davis, L.; Torres-Kolbus, J.; Chou, C.; Deiters, A.; Chin, J.W. Genetically encoded norbornene directs site-specific cellular protein labelling via a rapid bioorthogonal reaction. Nat. Chem. 2012, 4, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Clovis, J.S.; Eckell, A.; Huisgen, R.; Sustmann, R. 1.3-dipolare cycloadditionen, xxv. Der nachweis des freien diphenylnitrilimins als zwischenstufe bei cycloadditionen. Chem. Ber. 1967, 100, 60–70. [Google Scholar]
- Wang, Y.; Vera, C.I.; Lin, Q. Convenient synthesis of highly functionalized pyrazolines via mild, photoactivated 1,3-dipolar cycloaddition. Org. Lett. 2007, 9, 4155–4158. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, Y.; Qu, J.; Lin, Q. Selective functionalization of a genetically encoded alkene-containing protein via “photoclick chemistryˮ in bacterial cells. J. Am. Chem. Soc. 2008, 130, 9654–9655. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, Y.; Qu, J.; Madden, M.M.; Lin, Q. A photoinducible 1,3-dipolar cycloaddition reaction for rapid, selective modification of tetrazole-containing proteins. Angew. Chem. Int. Ed. 2008, 47, 2832–2835. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Song, W.; Wang, Y.; Yu, Z.; Li, J.; Wu, M.; Wang, L.; Zang, J.; Lin, Q. A biosynthetic route to photoclick chemistry on proteins. J. Am. Chem. Soc. 2010, 132, 14812–14818. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pan, Y.; Wang, Z.; Wang, J.; Lin, Q. Genetically encoded cyclopropene directs rapid, photoclick-chemistry-mediated protein labeling in mammalian cells. Angew. Chem. Int. Ed. 2012, 51, 10600–10604. [Google Scholar] [CrossRef]
- An, P.; Yu, Z.; Lin, Q. Design of oligothiophene-based tetrazoles for laser-triggered photoclick chemistry in living cells. Chem. Commun. 2013, 49, 9920–9922. [Google Scholar] [CrossRef]
- Yu, Z.; Ohulchanskyy, T.Y.; An, P.; Prasad, P.N.; Lin, Q. Fluorogenic, two-photon-triggered photoclick chemistry in live mammalian cells. J. Am. Chem. Soc. 2013, 135, 16766–16769. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hao, P.; Li, L.; Tan, C.Y.; Cheng, X.; Chen, G.Y.; Sze, S.K.; Shen, H.M.; Yao, S.Q. Design and synthesis of minimalist terminal alkyne-containing diazirine photo-crosslinkers and their incorporation into kinase inhibitors for cell- and tissue-based proteome profiling. Angew. Chem. Int. Ed. 2013, 52, 8551–8556. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Li, L.; Pan, S.; Na, Z.; Tan, C.Y.; Yao, S.Q. “Minimalistˮ cyclopropene-containing photo-cross-linkers suitable for live-cell imaging and affinity-based protein labeling. J. Am. Chem. Soc. 2014, 136, 9990–9998. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ho, L.Y.; Lin, Q. Rapid, photoactivatable turn-on fluorescent probes based on an intramolecular photoclick reaction. J. Am. Chem. Soc. 2011, 133, 11912–11915. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, W.; Hu, W.J.; Lin, Q. Fast alkene functionalization in vivo by photoclick chemistry: Homo lifting of nitrile imine dipoles. Angew. Chem. Int. Ed. 2009, 48, 5330–5333. [Google Scholar] [CrossRef]
- Seitchik, J.L.; Peeler, J.C.; Taylor, M.T.; Blackman, M.L.; Rhoads, T.W.; Cooley, R.B.; Refakis, C.; Fox, J.M.; Mehl, R.A. Genetically encoded tetrazine amino acid directs rapid site-specific in vivo bioorthogonal ligation with trans-cyclooctenes. J. Am. Chem. Soc. 2012, 134, 2898–2901. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, Z.; Xu, H.; Li, L.; Chen, S.; Li, J.; Hao, Z.; Chen, P.R. Site-specific incorporation of photo-cross-linker and bioorthogonal amino acids into enteric bacterial pathogens. J. Am. Chem. Soc. 2011, 133, 20581–20587. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yan, H.; Li, L.; Yang, M.; Peng, B.; Chen, S.; Li, W.; Chen, P.R. Site-specific engineering of chemical functionalities on the surface of live hepatitis D virus. Angew. Chem. Int. Ed. 2013, 52, 13970–13974. [Google Scholar] [CrossRef]
- Yang, M.; Jalloh, A.S.; Wei, W.; Zhao, J.; Wu, P.; Chen, P.R. Biocompatible click chemistry enabled compartment-specific ph measurement inside E. coli. Nat. Commun. 2014, 5, 4981. [Google Scholar] [CrossRef]
- Li, J.; Jia, S.; Chen, P.R. Diels-alder reaction-triggered bioorthogonal protein decaging in living cells. Nat. Chem. Biol. 2014, 10, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Hong, S.; Chen, X. Cell-selective metabolic labeling of biomolecules with bioorthogonal functionalities. Curr. Opin. Chem. Biol. 2013, 17, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Haltiwanger, R.S.; Lowe, J.B. Role of glycosylation in development. Annu. Rev. Biochem. 2004, 73, 491–537. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, S.T.; Bertozzi, C.R. Imaging the glycome. Proc. Natl. Acad. Sci. USA 2009, 106, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, S.T.; Baskin, J.M.; Amacher, S.L.; Bertozzi, C.R. In vivo imaging of membrane-associated glycans in developing zebrafish. Science 2008, 320, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Dube, D.H.; Sletten, E.M.; Bertozzi, C.R. A strategy for the selective imaging of glycans using caged metabolic precursors. J. Am. Chem. Soc. 2010, 132, 9516–9518. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Hong, S.; Feng, L.; Rong, J.; Chen, X. Cell-selective metabolic glycan labeling based on ligand-targeted liposomes. J. Am. Chem. Soc. 2012, 134, 9914–9917. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Han, J.; Dong, L.; Tan, Y.; Yang, H.; Feng, L.; Wang, Q.W.; Meng, R.; Zhao, J.; Wang, S.Q.; et al. Glycan imaging in intact rat hearts and glycoproteomic analysis reveal the upregulation of sialylation during cardiac hypertrophy. J. Am. Chem. Soc. 2014, 136, 17468–17476. [Google Scholar]
- Qu, D.; Wang, G.; Wang, Z.; Zhou, L.; Chi, W.; Cong, S.; Ren, X.; Liang, P.; Zhang, B. 5-ethynyl-2'-deoxycytidine as a new agent for DNA labeling: Detection of proliferating cells. Anal. Biochem. 2011, 417, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.H.; Woods, P.S.; Hughes, W.L. The organization and duplication of chromosomes as revealed by autoradiographic studies using tritium-labeled thymidinee. Proc. Natl. Acad. Sci. USA 1957, 43, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Gratzner, H.G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science 1982, 218, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Salic, A.; Mitchison, T.J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Cinquin, O.; Crittenden, S.L.; Morgan, D.E.; Kimble, J. Progression from a stem cell-like state to early differentiation in the C. elegans germ line. Proc. Natl. Acad. Sci. USA 2010, 107, 2048–2053. [Google Scholar] [CrossRef]
- Neef, A.B.; Luedtke, N.W. Dynamic metabolic labeling of DNA in vivo with arabinosyl nucleosides. Proc. Natl. Acad. Sci. USA 2011, 108, 20404–20409. [Google Scholar] [CrossRef] [PubMed]
- Rieder, U.; Luedtke, N.W. Alkene-tetrazine ligation for imaging cellular DNA. Angew. Chem. Int. Ed. 2014, 53, 9168–9172. [Google Scholar] [CrossRef]
- Arndt, S.; Wagenknecht, H.A. “Photoclick” postsynthetic modification of DNA. Angew. Chem. Int. Ed. 2014, 53, 14580–14582. [Google Scholar] [CrossRef]
- Song, C.X.; Szulwach, K.E.; Fu, Y.; Dai, Q.; Yi, C.; Li, X.; Li, Y.; Chen, C.H.; Zhang, W.; Jian, X.; et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat. Biotechnol. 2011, 29, 68–72. [Google Scholar]
- Maier, O.; Oberle, V.; Hoekstra, D. Fluorescent lipid probes: Some properties and applications (a review). Chem. Phys. Lipids 2002, 116, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Jao, C.Y.; Roth, M.; Welti, R.; Salic, A. Metabolic labeling and direct imaging of choline phospholipids in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 15332–15337. [Google Scholar] [CrossRef] [PubMed]
- Neef, A.B.; Schultz, C. Selective fluorescence labeling of lipids in living cells. Angew. Chem. Int. Ed. 2009, 48, 1498–1500. [Google Scholar] [CrossRef]
- Gubbens, J.; Ruijter, E.; de Fays, L.E.; Damen, J.M.; de Kruijff, B.; Slijper, M.; Rijkers, D.T.; Liskamp, R.M.; de Kroon, A.I. Photocrosslinking and click chemistry enable the specific detection of proteins interacting with phospholipids at the membrane interface. Chem. Biol. 2009, 16, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Tully, S.E.; Cravatt, B.F. Activity-based probes that target functional subclasses of phospholipases in proteomes. J. Am. Chem. Soc. 2010, 132, 3264–3265. [Google Scholar] [CrossRef] [PubMed]
- Hang, H.C.; Geutjes, E.J.; Grotenbreg, G.; Pollington, A.M.; Bijlmakers, M.J.; Ploegh, H.L. Chemical probes for the rapid detection of fatty-acylated proteins in mammalian cells. J. Am. Chem. Soc. 2007, 129, 2744–2745. [Google Scholar] [CrossRef] [PubMed]
- Charron, G.; Zhang, M.M.; Yount, J.S.; Wilson, J.; Raghavan, A.S.; Shamir, E.; Hang, H.C. Robust fluorescent detection of protein fatty-acylation with chemical reporters. J. Am. Chem. Soc. 2009, 131, 4967–4975. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Ascano, J.M.; Hang, H.C. Bioorthogonal chemical reporters for monitoring protein acetylation. J. Am. Chem. Soc. 2010, 132, 3640–3641. [Google Scholar] [CrossRef] [PubMed]
- Rangan, K.J.; Yang, Y.Y.; Charron, G.; Hang, H.C. Rapid visualization and large-scale profiling of bacterial lipoproteins with chemical reporters. J. Am. Chem. Soc. 2010, 132, 10628–10629. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, S.; Wang, J.; Jia, S.; Yang, M.; Hao, Z.; Zhang, X.; Chen, P.R. Ligand-free palladium-mediated site-specific protein labeling inside gram-negative bacterial pathogens. J. Am. Chem. Soc. 2013, 135, 7330–7338. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lim, R.K.; Edwardraja, S.; Lin, Q. Copper-free sonogashira cross-coupling for functionalization of alkyne-encoded proteins in aqueous medium and in bacterial cells. J. Am. Chem. Soc. 2011, 133, 15316–15319. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dong, T.; Liu, X.; Lei, X. A bioorthogonal ligation enabled by click cycloaddition of o-quinolinone quinone methide and vinyl thioether. J. Am. Chem. Soc. 2013, 135, 4996–4999. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dong, T.; Li, Q.; Lei, X. Probing the anticancer mechanism of (−)-ainsliatrimer a through diverted total synthesis and bioorthogonal ligation. Angew. Chem. Int. Ed. 2014, 53, 12111–12115. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. A bioorthogonal quadricyclane ligation. J. Am. Chem. Soc. 2011, 133, 17570–17573. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Temming, R.P.; Dommerholt, J.; Guo, J.; Ania, D.B.; Debets, M.F.; Wolfert, M.A.; Boons, G.J.; van Delft, F.L. Protein modification by strain-promoted alkyne-nitrone cycloaddition. Angew. Chem. Int. Ed. 2010, 49, 3065–3068. [Google Scholar] [CrossRef]
- Tang, W.; Becker, M.L. “Click” reactions: A versatile toolbox for the synthesis of peptide-conjugates. Chem. Soc. Rev. 2014, 43, 7013–7039. [Google Scholar] [CrossRef] [PubMed]
- Kolodych, S.; Rasolofonjatovo, E.; Chaumontet, M.; Nevers, M.C.; Creminon, C.; Taran, F. Discovery of chemoselective and biocompatible reactions using a high-throughput immunoassay screening. Angew. Chem. Int. Ed. 2013, 52, 12056–12060. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Zheng, L.; Zhang, P.; Li, J.; Zhang, Y. Development of Bioorthogonal Reactions and Their Applications in Bioconjugation. Molecules 2015, 20, 3190-3205. https://doi.org/10.3390/molecules20023190
Zheng M, Zheng L, Zhang P, Li J, Zhang Y. Development of Bioorthogonal Reactions and Their Applications in Bioconjugation. Molecules. 2015; 20(2):3190-3205. https://doi.org/10.3390/molecules20023190
Chicago/Turabian StyleZheng, Mengmeng, Li Zheng, Peiyuan Zhang, Jinbo Li, and Yan Zhang. 2015. "Development of Bioorthogonal Reactions and Their Applications in Bioconjugation" Molecules 20, no. 2: 3190-3205. https://doi.org/10.3390/molecules20023190
APA StyleZheng, M., Zheng, L., Zhang, P., Li, J., & Zhang, Y. (2015). Development of Bioorthogonal Reactions and Their Applications in Bioconjugation. Molecules, 20(2), 3190-3205. https://doi.org/10.3390/molecules20023190





