Comparison of Two Species of Notopterygium by GC-MS and HPLC
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Volatile Compounds by GC-MS
| Peak No. | tR/min | Name | Formula | CAS | Area/% in NI | Area/% in NF | |
|---|---|---|---|---|---|---|---|
| 1a | 7.612 | 1R-alpha-pinene | C10H16 | 7785-70-8 | 34.61 | 8.86 | |
| 2a | 9.331 | b-thujene | C10H16 | 28634-89-1 | 3.91 | 4.49 | |
| 3a | 9.576 | beta-pinene | C10H16 | 18172-67-3 | 25.79 | 19.89 | |
| 4a | 11.98 | d-limonene | C10H16 | 5989-27-5 | 9.59 | 4.28 | |
| 5a | 13.243 | 4-isopropyl-1-methyl-1,4-cyclohexadiene | C10H16 | 99-85-4 | 2.18 | 21.23 | |
| 6a | 17.949 | l-terpinen-4-ol | C10H18O | 20126-76-5 | 1.63 | 1.02 | |
| 7 | 5.187 | ethylbenzene | C8H10 | 100-41-4 | 0.15 | 0.19 | |
| 8 | 7.237 | 3-thujene | C10H16 | 2867-5-2 | 0.49 | 0.22 | |
| 9 | 8.283 | camphene | C10H16 | 79-92-5 | 0.61 | 0.4 | |
| 10 | 10.146 | beta-myrcene | C10H16 | 123-35-3 | 0.4 | 0.54 | |
| 11 | 10.933 | alpha-phellandrene | C10H16 | 99-83-2 | 25.79 | 0.9 | |
| 12 | 11.034 | 3-carene | C10H16 | 13466-78-9 | 10.56 | 0.48 | |
| 13 | 11.409 | 2-carene | C10H16 | 554-61-0 | 0.62 | 0.48 | |
| 14 | 11.828 | 1-methyl-2-(methylethyl)-benzene | C10H14 | 527-84-4 | 0.92 | 11.38 | |
| 15 | 12.041 | 4-methylene-1-(1-methylethyl)-bicyclo[3.1.0]hexane | C10H16 | 3387-41-5 | 0.18 | 1.11 | |
| 16 | 12.766 | 3,7-dimethyl-1,3,6-octatriene | C10H16 | 3338-55-4 | 0 | 4.52 | |
| 17 | 14.181 | 1-methyl-4-(1-methylethyl)-cyclohexene | C20H36 | 34363-01-4 | 0.1 | 0 | |
| 18 | 14.325 | 2,6-dimethyl-2,4,6-octatriene | C10H16 | 673-84-7 | 0 | 0.38 | |
| 19 | 14.333 | 1-methyl-4-(1- methylethylidene)-cyclohexene | C10H16 | 586-62-9 | 0.56 | 0 | |
| 20 | 15.155 | pentanoic acid, 2-methylbutyl ester | C10H20O2 | 55590-83-5 | 0 | 0.25 | |
| 21 | 18.295 | 2-butenoic acid, 2-methyl-, 3-methylbutyl ester | C10H18O2 | 66917-62-2 | 0 | 1.21 | |
| 22 | 19.478 | 1-isopropyl-2-methoxy-4-methylbenzene | C11H16O | 1076-56-8 | 0 | 2.01 | |
| 23 | 19.796 | 3-methyl-2-butenoic acid | C11H16O2 | - | 0 | 0.93 | |
| 24 | 21.241 | (1S,2R,4S)-bicyclo[2.2.1]heptan-2-ol,1,7,7-trimethyl-, 2-acetate | C12H20O2 | 5655-61-8 | 1.86 | 1.21 | |
| 25 | 25.281 | Z-3-decen-1-yl acetate | C12H22O2 | 81634-99-3 | 0 | 2.78 | |
| 26 | 29.173 | shyobunone | C15H24O | - | 0.34 | 0 | |
| 27 | 29.845 | epicedrol | C15H26O | 19903-73-2 | 1.07 | 0 | |
| 28 | 29.981 | d-cadinene | C15H24 | 483-76-1 | 1.13 | 1.46 | |
| 29 | 30.214 | octahydro-4,4,8,8-tetramethyl-4a,7-methano-4aH-naphth[1,8a-b]oxirene | C15H24O | 67999-56-8 | 0 | 0.56 | |
| 30 | 30.249 | (6R)-1,1,5,9-tetramethylspiro [5.5]undeca-1,8-diene | C15H24 | 19912-83-5 | 0.77 | 0 | |
| 31 | 31.974 | guaiol | C15H26O | 489-86-1 | 0.64 | 0 | |
| 32 | 32.162 | dehydroxy-isocalamendiol | C15H24O | - | 0.4 | 0 | |
| 33 | 32.208 | r-eudesmol | C15H26O | 473-16-5 | 0.87 | 0 | |
| 34 | 32.514 | apiol | C12H14O4 | 523-80-8 | 0 | 0.75 | |
| 35 | 32.717 | 1R,4S,7S,11R-2,2,4,8-tetramethyltricyclo [5.3.1.0(4,11)]undec-8-ene | C15H24 | - | 0.15 | 0.65 | |
| 36 | 33.026 | 9-aristolene | C15H24 | 6831-16-9 | 0 | 0.68 | |
| 37 | 33.163 | agarospirol | C15H26O | 1460-73-7 | 0 | 4.75 | |
| 38 | 33.36 | bulnesol | C15H26O | 22451-73-6 | 0.29 | 0 | |
| 39 | 33.741 | alpha-bisabolol | C15H26O | 515-69-5 | 0 | 1.94 | |



2.2. Analysis of Non-Volatile Compounds by HPLC
| No. | Samples | Location | Species | Content (mg/g) | Similarities | |||
|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | |||||
| 1 | Xining * | Xining city, Qinghai province | NF | 0.77 | 0.16 | 0.36 | 33.42 | 0.975 |
| 2 | Xining * | Xining city, Qinghai province | NF | 0.77 | 0.16 | 0.35 | 33.41 | 0.972 |
| 3 | Weiyuan | Weiyuan county, Gansu province | NF | 0.21 | 1.05 | 0.17 | 13.12 | 0.957 |
| 4 | Minxian | Min county, Gansu province | NF | 1.38 | 1.12 | 0.58 | 20.07 | 0.994 |
| 5 | Lintao | Lintao county, Gansu province | NF | 1.08 | 0.94 | 1.92 | 22.08 | 0.972 |
| 6 | Rangtang | Rangtang county, Sichuan province | NI | 1.23 | 0.18 | 12.83 | 5.36 | 0.955 |
| 7 | Xiaojin | Xiaojin county, Sichuan province | NI | 0.68 | 0.76 | 8.24 | 1.29 | 0.887 |
| 8 | Aba | Aba county, Sichuan province | NI | 1.13 | 0.01 | 7.89 | 1.89 | 0.819 |
| 9 | Jiuzhi | Jiuzhi county , Qinghai province | NI | 0.66 | 0.02 | 11.28 | 2.96 | 0.983 |
| 10 | Gande | Gande county , Qinghai province | NI | 0.61 | 0.11 | 9.37 | 3.94 | 0.977 |
| 11 | Dari | Dari county , Qinghai province | NI | 0.57 | ND | 8.14 | 3.03 | 0.988 |
| 12 | Banma | Banma county , Qinghai province | NI | 0.56 | 0.07 | 10.40 | 3.20 | 0.981 |
| 13 | Kangding | Kangding county, Sichuan province | NI | 1.97 | 0.28 | 22.79 | 4.72 | 0.970 |
| 14 | Dege | Dege county, Sichuan province | NI | 0.91 | 0.11 | 13.88 | 4.21 | 0.933 |
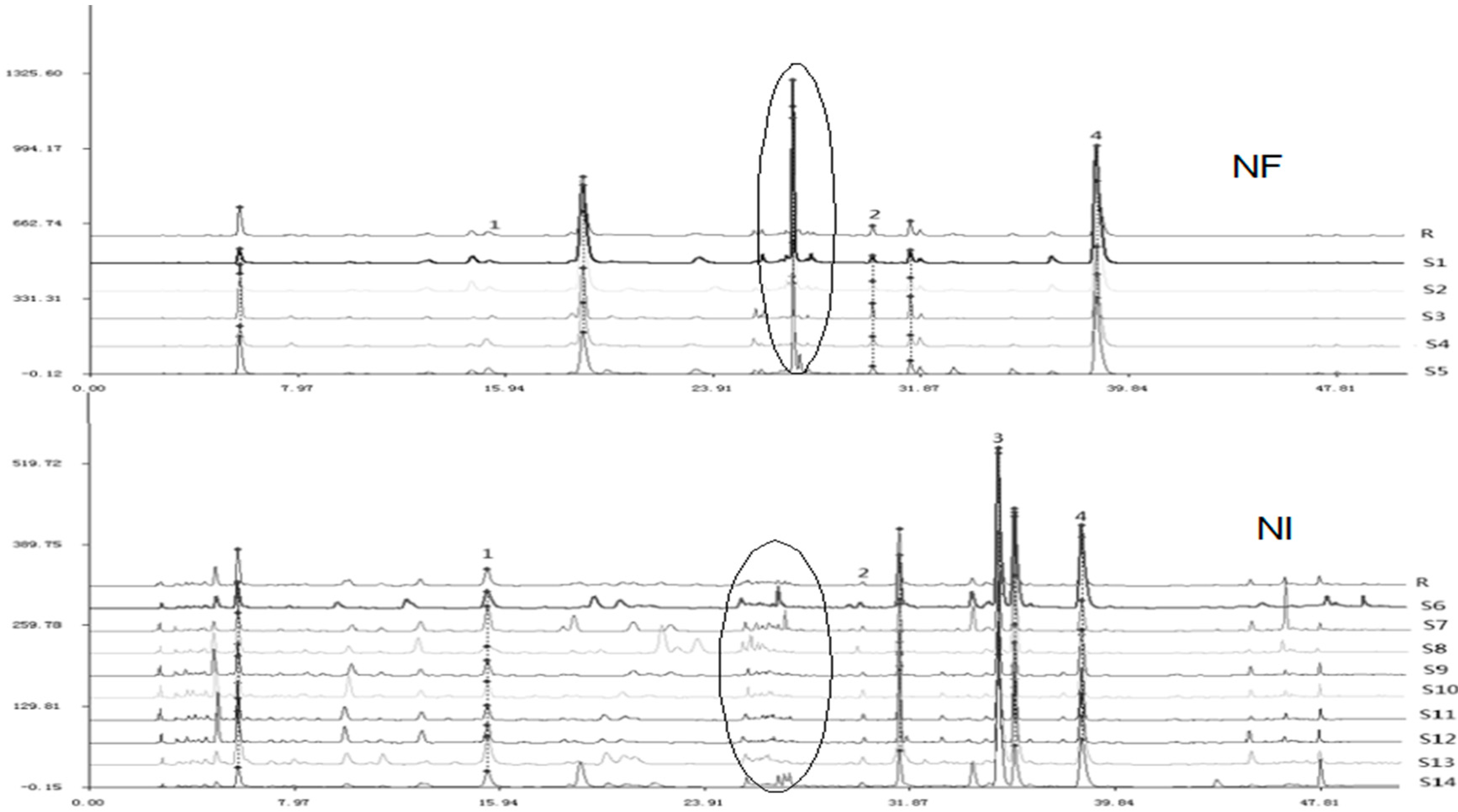
2.3. HPLC Fingerprint Analysis
3. Experimental Section
3.1. Plant Material
3.2. Chemicals and Reagents


3.3. Apparatus and Chromatographic Conditions
3.4. Sample Preparation
3.5. Data Processing and Multivariate Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; People’s Medical Publishing House: Beijing, China, 2010; Volume I, p. 170. [Google Scholar]
- Okuyama, E.; Nishimura, S.; Ohmori, S.; Ozaki, Y.; Satake, M.; Yamazaki, M. Analgesic component of Notopterygium incisum Ting. Chem. Pharm. Bull. 1992, 41, 926–929. [Google Scholar] [CrossRef]
- Guo, L.Q.; Taniguchi, M.; Chen, Q.Y.; Baba, K.; Yamazoe, Y. Inhibitory potential of herbal medicines on human cytochrome P450-mediated oxidation: Properties of umbelliferous or citrus crude drugs and their relative prescriptions. Jpn. J. Pharmacol. 2001, 85, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, S.Y.; Xu, K.J.; Sun, H.; Zhou, Y.; Xu, X.M.; Yi, J.H.; Gu, Y.C.; Ding, L.S. Quantitative analysis of chemical constituents in different commercial parts of Notopterygium incisum by HPLC-DAD-MS. J. Ethnopharmacol. 2009, 126, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Z.; Xu, W.; Chen, L.; Yuan, L.; Lee, D.Y.W. Novel coumarin glycoside and phenethyl vanillate from Notopterygium forbesii and their binding affinities for opioid and dopamine receptors. Bioorg. Med. Chem. 2008, 16, 3218–3223. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Q.; Baba, K.; Taniguchi, M.; Liu, X.H.; Sun, Y.F.; Kozawa, M. Coumapins from Notopterygium incisum Ting. Acta Pharmaceutica Sinica 1995, 4, 274–279. [Google Scholar]
- You, M.; Xiong, J.; Zhao, Y.; Cao, L.; Wu, S.B.; Xia, G.; Hu, J.F. Glycosides from the methanol extract of Notopterygium incisum. Planta Med. 2011, 77, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.Q.; Liang, Y.Z.; Xu, C.J.; Huang, L.F. Determination of the volatile chemical constituents of Notoptergium incium by gas chromatography-mass spectrometry and iterative or non-iterative chemometrics resolution methods. J. Chromatogr. A 2003, 1016, 99–110. [Google Scholar] [CrossRef] [PubMed]
- García-Argáez, A.N.; Ramírez Apan, T.O.; Parra Delgado, H.; Velázquez, G.; Martínez-Vázquez, M. Anti-inflammatory activity of coumarins from Decatropis bicolor on TPA ear mice model. Planta Med. 2000, 66, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Hosamani, K.M.; Shingalapur, R.V.; Hugar, M.H. Analgesic, anti-pyretic and DNA cleavage studies of novel pyrimidine derivatives of coumarin moiety. Eur. J. Med. Chem. 2010, 45, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.B.; Pang, F.; Wen, Y.; Zhang, H.F.; Zhao, Z; Hu, J.F. Antiproliferative and apoptotic activities of linear furocoumarins from Notopterygium incisum on cancer cell lines. Planta Med. 2010, 76, 82–85. [Google Scholar] [CrossRef]
- Xu, H.B.; Sun, X.B.; Zhao, Q.C. Pharmacological research on the volatile oil of Notopterygium incisum. Chin. Tradit. Herb. Drugs 1991, 22, 28–31. [Google Scholar]
- Huang, L.F.; Li, W.T.; Wang, Z.; Fu, J.; Chen, S.L. Correlative study between chemical constituents and ecological factors of Notopterygii Rhizoma Et Radix of endangered plateau plant. Acta Ecol. Sin. 2013, 33, 7667–7678. [Google Scholar]
- Tan, Y.; Zhang, H.; Sun, Y. Determination the content of nodakenin from Notopterygium incisum by TLC densitometry. Chin. J. Chin. Mater. Med. 1996, 21, 486–487. [Google Scholar]
- Qiu, Y.Q.; Lu, X.; Pang, T.; Zhu, S.K.; Kong, H.W.; Xu, G.W. Study of traditional Chinese medicine volatile oils from different geographical origins by comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry (GC× GC–TOFMS) in combination with multivariate analysis. J. Pharm. Biomed. Anal. 2007, 43, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Li, C.L.; Zhou, G.Y.; Che, G.D.; You, J.M.; Suo, Y.R. Determination of the carbohydrates from Notopterygium forbesii Boiss by HPLC with fluorescence detection. Carbohydr. Polym. 2013, 97, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; You, J.M.; Zhou, G.Y.; Li, C.L.; Suo, Y.R. Analysis of free fatty acids in Notopterygium forbesii Boiss by a novel HPLC method with fluorescence detection. Talanta 2012, 98, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Jiang, S.Y.; Zhou, Y.; Zhang, Y.X.; Xia, B.; Xu, X.M.; Zhou, Y.; Li, Y.F.; Wang, M.K.; Ding, L.S. Discrimination of the seeds of Notopterygium incisum and Notopterygium franchetii by validated HPLC-DAD-ESI-MS method and principal component analysis. J. Pharmaceut. Biomed. 2011, 56, 1089–1093. [Google Scholar] [CrossRef]
- Crowell, P.L.; Chang, R.R.; Ren, Z.B.; Elson, C.E.; Gould, M.N. Selective inhibition of isoprenylation of 21–26-kDa proteins by the anticarcinogen d-limonene and its metabolites. J. Biol. Chem. 1991, 266, 17679–17685. [Google Scholar] [PubMed]
- Chaudhary, S.C.; Siddiqui, M.S.; Athar, M.; Alam, M.S. d-Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Hum. Exp. Toxicol. 2012, 31, 798–811. [Google Scholar] [CrossRef]
- Gan, H.L.; Che Man, Y.B.; Tan, C.P.; NorAini, I.; Nazimah, S.A.H. Characterisation of vegetable oils by surface acoustic wave sensing electronic nose. Food Chem. 2005, 89, 507–518. [Google Scholar] [CrossRef]
- Yang, W.; Feng, C.; Kong, D.; Shi, X.; Cui, Y.; Liu, M.; Wang, Q.; Wang, Y.L.; Zhang, L.T. Simultaneous and sensitive determination of xanthotoxin, psoralen, isoimpinellin and bergapten in rat plasma by liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. B 2010, 878, 575–582. [Google Scholar] [CrossRef]
- Qian, G.S.; Wang, Q.; Jiang, Z.H.; Zhao, Z.Z.; Qin, Y.; Kelvin, S.Y.L. Quality assessment of Rhizoma et Radix Notopterygii by HPTLC and HPLC fingerprinting and HPLC quantitative analysis. J. Pharmaceut. Biomed. 2007, 44, 812–817. [Google Scholar] [CrossRef]
- Zhong, G. Studies on optimum extraction technology for volatile oil in Notopterygium by orthogonal test. Chin. J. Mod. Drug Appl. 2008, 11, 26–27. [Google Scholar]
- Lin, P.; Wang, Y.Z.; Zhu, H.B.; Chen, Q.M. Fingerprint profile of active components for Artemisia selengensis Turcz by HPLC-PAD combined with chemometrics. Food Chem. 2011, 125, 1064–1071. [Google Scholar] [CrossRef]
- Wei, H.; Sun, L.N.; Tai, Z.G.; Gao, S.H.; Xu, W.; Chen, W.S. A simple and sensitive HPLC method for the simultaneous determination of eight bioactive components and fingerprint analysis of Schisandra sphenanthera. Anal. Chim. Acta 2010, 662, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.D.; Wu, Y.S.; Zhou, J.S.; Lian, M.; Li, C.W.; Xu, Y.Q.; Liu, C.W.; Wang, C.; Meng, Q.X. The study of fingerprint characteristics of Dayi Pu-Erh tea using a fully automatic HS-SPME/GC-MS and combined chemometrics method. PLoS One 2014, 12, e116428. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds not are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Huang, L. Comparison of Two Species of Notopterygium by GC-MS and HPLC. Molecules 2015, 20, 5062-5073. https://doi.org/10.3390/molecules20035062
Wang Y, Huang L. Comparison of Two Species of Notopterygium by GC-MS and HPLC. Molecules. 2015; 20(3):5062-5073. https://doi.org/10.3390/molecules20035062
Chicago/Turabian StyleWang, Yaping, and Linfang Huang. 2015. "Comparison of Two Species of Notopterygium by GC-MS and HPLC" Molecules 20, no. 3: 5062-5073. https://doi.org/10.3390/molecules20035062
APA StyleWang, Y., & Huang, L. (2015). Comparison of Two Species of Notopterygium by GC-MS and HPLC. Molecules, 20(3), 5062-5073. https://doi.org/10.3390/molecules20035062






