Multidisciplinary Approach to Determine the Optimal Time and Period for Extracting the Essential Oil from Mentha suaveolens Ehrh †
Abstract
:1. Introduction
2. Results and Discussion
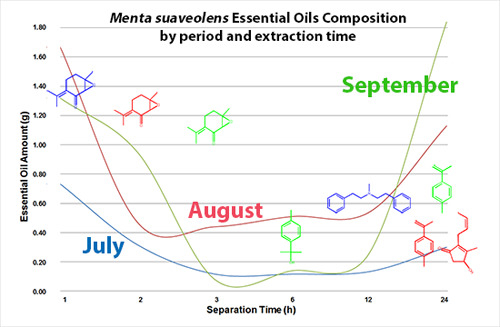
2.1. Essential Oil Extraction
| h 1 | July | August | September | |||
|---|---|---|---|---|---|---|
| EO (g) | Yield % | EO (g) | Yield % | EO (g) | Yield % | |
| 1 | 0.73 | 0.03 | 1.67 | 0.07 | 1.32 | 0.05 |
| 2 | 1.05 | 0.04 | 2.14 | 0.09 | 2.27 | 0.09 |
| 3 | 1.17 | 0.05 | 2.58 | 0.10 | 2.35 | 0.09 |
| 6 | 1.28 | 0.05 | 3.09 | 0.12 | 2.49 | 0.10 |
| 12 | 1.42 | 0.06 | 3.63 | 0.15 | 2.75 | 0.11 |
| 24 | 1.72 | 0.07 | 4.76 | 0.19 | 4.59 | 0.18 |
2.2. GC/MS EO Analysis
| # 1 | Name | Sample 2 | |||||
|---|---|---|---|---|---|---|---|
| J1h | J2h | J3h | J6h | J12h | J24h | ||
| 1 | (−)-spathulenol | 2.17 | 8.18 | 8.99 | 14.23 | 18.35 | |
| 4 | 3-octanol | 0.18 | |||||
| 5 | 3-octanol acetate | 0.11 | |||||
| 6 | α-cadinene | 0.24 | |||||
| 7 | α-cadinol | 0.50 | 1.75 | 9.09 | 10.69 | 7.62 | |
| 8 | α-cubebene | 0.30 | 0.31 | 0.09 | |||
| 13 | β-bourbonene | 0.17 | |||||
| 22 | borneol | 0.12 | |||||
| 23 | calamenene | 0.58 | 2.09 | 2.52 | 3.75 | 10.63 | 4.32 |
| 24 | carbitol | 0.55 | 2.36 | ||||
| 25 | β-caryophyllene oxide | 5.32 | 13.67 | 12.65 | 14.37 | 5.27 | 9.24 |
| 26 | cinerolone | 0.17 | 0.09 | 7.71 | |||
| 27 | citronellylacetate | 0.63 | 3.21 | 5.45 | |||
| 28 | copaene | 0.06 | 0.76 | ||||
| 29 | cubenol | 0.20 | 1.92 | 7.46 | 6.17 | 4.24 | |
| 31 | δ-cadinene | 0.12 | 4.89 | ||||
| 32 | demelverine | 0.59 | 0.51 | 2.18 | 9.52 | 43.46 | 20.28 |
| 34 | eucarvone | 0.08 | |||||
| 36 | gamma-cadinene | 0.86 | |||||
| 37 | p-cymen-8-ol | 0.12 | |||||
| 38 | p-menth-1-en-8-ol | 0.14 | |||||
| 40 | piperitenone oxide | 87.25 | 70.59 | 65.56 | 26.03 | 14.00 | |
| 41 | tau-cadinol | 0.72 | 1.39 | 1.37 | |||
| 42 | tau-muurolol | 0.18 | 1.23 | 2.14 | 3.29 | ||
| 46 | verbenone | 1.29 | 1.15 | 2.98 | 6.56 | 6.43 | |
| 47 | veridiflorol | 1.21 | 2.48 | 2.49 | 7.59 | 2.83 | |
| # 1 | Name | Sample 2 | |||||
|---|---|---|---|---|---|---|---|
| A1h | A2h | A3h | A6h | A12h | A24h | ||
| 1 | (−)-spathulenol | 0.79 | 1.48 | 2.47 | 2.12 | 0.32 | |
| 4 | 3-octanol | 0.73 | 0.11 | 0.07 | |||
| 5 | 3-octanol acetate | 2.08 | 1.02 | 0.90 | |||
| 7 | α-cadinol | 0.78 | 0.48 | 0.09 | |||
| 8 | α-cubebene | 5.07 | 4.36 | 10.08 | 3.64 | 0.45 | 0.14 |
| 10 | α-pharnesene | 5.15 | 8.01 | 16.54 | 9.09 | 2.56 | 0.37 |
| 11 | α-phellandrene | 0.11 | |||||
| 12 | α-pinene | 1.84 | |||||
| 14 | p-cymenene | 0.87 | 0.33 | 2.54 | 7.74 | 18.24 | 35.22 |
| 15 | β-elemene | 0.38 | 0.07 | ||||
| 16 | β-myrcene | 1.42 | |||||
| 17 | β-ocymene | 0.59 | |||||
| 18 | β-pharnesene | 0.73 | 1.03 | 2.29 | 2.24 | 0.89 | 0.18 |
| 19 | β-phellandrene | 0.42 | |||||
| 20 | β-pinene | 0.68 | |||||
| 21 | bicyclosesquiphellandrene | 0.88 | 0.98 | 2.37 | 1.89 | 0.41 | |
| 22 | borneol | 0.28 | 0.29 | 0.39 | 0.37 | 0.30 | 0.20 |
| 23 | calamenene | 0.44 | 1.07 | 1.56 | 1.33 | 0.65 | |
| 25 | β-caryophyllene oxide | 0.50 | 0.32 | 1.04 | 8.30 | 17.25 | 12.93 |
| 26 | cinerolone | 23.12 | 34.49 | 38.79 | |||
| 28 | copaene | 0.79 | 0.47 | ||||
| 29 | cubenol | 0.36 | 0.09 | 0.04 | |||
| 30 | d-limonene | 6.22 | |||||
| 31 | δ-cadinene | 0.09 | 0.27 | 1.07 | 2.32 | 1.54 | 0.44 |
| 32 | demelverine | 0.13 | 1.10 | 3.14 | 7.46 | 8.82 | 5.90 |
| 33 | eucalyptol | 4.21 | 0.47 | ||||
| 36 | δ-cadinene | 0.51 | 0.26 | 0.06 | |||
| 37 | p-cymen-8-ol | 0.21 | 0.54 | 0.66 | 0.94 | 0.97 | 0.55 |
| 39 | p-menthone | 0.39 | 0.46 | 2.63 | 5.70 | 5.61 | 2.25 |
| 40 | piperitenone oxide | 65.05 | 77.51 | 50.01 | 16.90 | 2.43 | |
| 44 | thymol | 0.39 | 0.32 | 0.24 | |||
| 46 | verbenone | 0.27 | 0.31 | 0.81 | 2.47 | 3.14 | 2.58 |
| 47 | veridiflorol | 0.39 | 0.17 | ||||
| 48 | ylangene | 0.85 | 0.43 | 1.50 | 0.64 | ||
| # 1 | Name | Sample 2 | |||||
|---|---|---|---|---|---|---|---|
| S1h | S2h | S3h | S6h | S12h | S24h | ||
| 1 | (−)-spathulenol | 3.67 | 8.95 | 2.54 | 3.62 | 1.69 | 0.99 |
| 2 | (z)-jasmone | 0.54 | |||||
| 3 | 2-caren-10-al | 0.32 | 0.76 | ||||
| 4 | 3-octanol | 3.13 | 4.74 | 0.82 | 0.61 | 1.19 | |
| 5 | 3-octanol acetate | 4.90 | 2.20 | 0.15 | 0.12 | 0.15 | |
| 7 | α-cadinol | 0.34 | 0.49 | 1.73 | 1.22 | 0.44 | |
| 8 | α-cubebene | 0.23 | 1.97 | 0.96 | 1.70 | ||
| 9 | α-muurolene | 0.09 | 0.13 | 0.51 | 0.58 | 0.15 | |
| 10 | α-pharnesene | 1.60 | 10.15 | 0.11 | 12.09 | 6.42 | 6.31 |
| 12 | α-pinene | 0.83 | |||||
| 14 | p-cymenene | 0.83 | 1.09 | 5.04 | 26.64 | ||
| 16 | β-myrcene | 0.35 | |||||
| 17 | β-ocymene | 0.25 | |||||
| 18 | β-pharnesene | 0.30 | 1.32 | 0.11 | 5.46 | 3.23 | 0.48 |
| 19 | β-phellandrene | 0.10 | |||||
| 21 | bicyclosesquiphellandrene | 0.19 | 2.33 | 1.16 | 0.77 | ||
| 22 | borneol | 0.23 | 0.34 | 0.14 | |||
| 23 | calamenene | 1.68 | 3.01 | 1.26 | 1.87 | 1.21 | 0.80 |
| 25 | β-caryophyllene oxide | 14.16 | 14.08 | 6.30 | 5.77 | 9.65 | 6.37 |
| 26 | cinerolone | 18.82 | 2.66 | 13.04 | 18.96 | ||
| 28 | copaene | 0.18 | 0.21 | 0.31 | 1.55 | 1.67 | 0.49 |
| 29 | cubenol | 0.40 | 1.77 | 0.70 | 1.44 | 0.84 | 0.18 |
| 30 | d-limonene | 0.69 | 4.84 | ||||
| 31 | delta-cadinene | 0.89 | 0.14 | 3.05 | 3.33 | 1.25 | |
| 32 | demelverine | 6.23 | 0.68 | 1.84 | 2.46 | 4.90 | 5.22 |
| 33 | eucalyptol | 1.16 | 0.43 | 2.24 | |||
| 35 | eugenol | 1.42 | 2.07 | 3.16 | 0.55 | ||
| 37 | p-cymen-8-ol | 1.67 | 9.22 | 0.37 | 26.77 | 24.68 | 4.52 |
| 38 | p-menth-1-en-8-ol | 0.55 | 0.20 | 0.88 | 0.72 | 0.22 | |
| 39 | p-menthone | 4.46 | 1.21 | 1.57 | 3.61 | ||
| 40 | piperitenone oxide | 38.69 | 35.64 | 69.52 | 13.20 | 5.53 | 5.61 |
| 43 | terpinen-4-ol | 0.16 | 0.30 | 0.16 | |||
| 44 | thymol | 1.34 | 0.25 | 1.04 | 0.15 | ||
| 45 | trans-2-caren-4-ol | 0.20 | |||||
| 46 | verbenone | 8.11 | 1.98 | 2.30 | 3.68 | 5.48 | 2.28 |
| 47 | veridiflorol | 0.42 | 1.74 | 0.99 | 1.80 | 0.96 | 0.28 |
| 48 | ylangene | 0.83 | 0.31 | 0.46 | |||
2.3. Antimicrobial Assay
| Sample 1 | MIC mg∙mL−1 | PO % |
|---|---|---|
| J1h | 0.10 | 87.25 |
| J2h | 0.10 | 70.59 |
| J3h | 0.10 | 65.56 |
| J6h | 6.25 | 26.03 |
| J12h | 6.25 | 14.78 |
| J24h | 12.50 | - |
| A1h | 0.10 | 65.05 |
| A2h | 0.02 | 77.51 |
| A3h | 0.10 | 50.01 |
| A6h | 0.78 | 16.90 |
| A12h | 3.12 | 2.43 |
| A24h | 6.25 | - |
| S1h | 0.20 | 38.69 |
| S2h | 0.20 | 35.64 |
| S3h | 0.10 | 69.52 |
| S6h | 6.25 | 13.20 |
| S12h | 6.25 | 5.53 |
| S24h | 0.20 | 5.61 |
| Miconazole | 0.016 |
2.4. Mutagenicity Test
| Sample | Compound Amount (μg∙plate−1) | Number of Revertant Colonies | |||||
|---|---|---|---|---|---|---|---|
| TA98 | TA100 | WP2uvrA | |||||
| −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | ||
| J1h | 50 | 44.0 ± 1.1 | 59.3 ± 1.8 | 153.3 ± 10.9 | 162.3 ± 2.6 | 48.2 ± 3.4 | 50.0 ± 2.5 |
| 100 | 45.2 ± 2.1 | 53.7 ± 3.5 | 162.8 ± 10.2 | 149.2 ± 7.1 | 42.0 ±6.8 | 67.0 ± 3.7 | |
| 250 | 43.7 ± 3.3 | 58.8 ± 3.7 | 146.7 ± 8.3 | 152.0 ± 8.3 | 38.3 ± 1.8 | 58.8 ± 11.9 | |
| 400 | 38.0 ± 3.0 | 62.7 ± 6.3 | 136.0 ± 8.3 | 149.3 ± 3.2 | 44.2 ± 2.8 | 63.7 ± 3.7 | |
| 550 | 29.7 ± 3.4 **,t | 56.5 ± 6.7 | 73.7 ± 11.2 **,t | 129.7 ± 5.4 | 32.5 ± 5.0 ~t | 57.2 ± 6.8 | |
| 850 | 15.0 ± 1.1 **,t | 30.3 ± 2.8 **,t | 40.0 ± 10.6 **,t | 97.0 ± 2.3 **,t | 19.3 ± 3.7 **,t | 34.7 ± 6.7 **,t | |
| A1h | 50 | 49.3 ± 3.8 | 68.0 ± 7.9 | 144.0 ± 14.0 | 133.3 ± 23.6 | 42.7 ± 3.5 | 77.3 ± 5.8 |
| 100 | 41.3 ± 3.5 | 60.7 ± 7.3 | 90.7 ± 14.3 **,t | 127.3 ± 12.6 | 43.1 ± 4.9 | 74.7 ± 13.2 | |
| 250 | 41.3 ± 3.5 **,t | 60.0 ± 7.3 | 73.3 ± 32.8 **,t | 124.0 ± 16.1 ~t | 32.0 ± 4.8 **,t | 77.3 ± 1.3 | |
| 400 | 32.7 ± 4.3 **,t | 30.4 ± 4.5 **,t | 79.3 ± 7.4 **,t | 106.7 ± 3.1 **,t | 25.3 ± 3.3 **,t | 57.3 ± 11.9 **,t | |
| 550 | 25.3 ± 3.4 **,t | 28.7 ± 9.4 **,t | 64.0 ± 16.5 **,t | 104.7 ± 8.3 **,t | 30.7 ± 3.4 **,t | 28.7 ± 2.8 **,t | |
| 850 | 0.3 ± 0.2 **,t | 0.5 ± 0.1 **,t | 11.3 ± 5.2 **,t | 111.3 ± 8.7 **,t | 2.1 ± 0.1 **,t | 12.1 ± 0.5 **,t | |
| S1h | 50 | 53.3 ± 1.3 | 54.7 ± 8.1 | 153.3 ± 4.8 | 177.3 ± 7.4 | 59.3 ± 5.9 | 58.2 ± 3.4 |
| 100 | 54.0 ± 7.5 | 50.2 ± 6.7 | 153.3 ± 9.8 | 173.3 ± 13.3 | 50.7 ± 4.3 | 58.0 ± 5.0 | |
| 250 | 40.0 ± 2.3 **,t | 61.3 ± 7.1 | 139.3 ± 9.9 **,t | 170.7 ± 9.3 | 49.3±6.1 ~t | 54.2 ± 2.8 | |
| 400 | 53.3 ± 3.5 **,t | 56.7 ± 4.8 **,t | 138.7 ± 6.4 **,t | 146.0 ± 13.6 ~t | 46.0 ± 5.2 ~t | 52.0 ± 3.1 ~t | |
| 550 | 17.3 ± 7.4 **,t | 38.7 ± 9.3 **,t | 117.3 ± 8.6 **,t | 150.7 ± 10.7 **,t | 44.7 ± 8.5 **,t | 38.0 ± 2.3 **,t | |
| 850 | 5.1 ± 0.3 **,t | 11.8 ± 0.6 **,t | 37.3 ± 12.5 **,t | 49.3 ± 13.8 **,t | 22.1 ± 1.3 **,t | 17.8 ± 0.9 **,t | |
| Vehicle | 43.3 ± 2.8 a | 49.3 ± 2.1 a | 143.2 ± 13.6 a | 153.6 ± 17.1 a | 45.9 ± 5.2 a | 59.7 ± 5.3 a | |
| Mutagen | 128.1 ± 8.1 b | 186.1 ± 15.1 c | 608.5 ± 14.9 d | 379.2 ± 23.5 c | 287.4 ± 17.2 e | 323.7 ± 20.4 f | |
| Sample | Compound Amount (μg∙plate−1) | Number of Revertant Colonies | |||||
|---|---|---|---|---|---|---|---|
| TA98 | TA100 | WP2uvrA | |||||
| −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | ||
| J1h | 10 | 46.0 ± 2.3 | 59.0 ± 3.2 | 133.3 ± 14.8 | 150.5 ± 15.4 | 49.3 ± 7.4 | 60.0 ± 2.3 |
| 50 | 44.0 ± 1.1 | 59.3 ± 1.8 | 146.7 ± 8.3 | 129.7 ± 5.4 | 48.2 ± 3.4 | 50.0 ± 2.5 | |
| 100 | 38.0 ± 3.0 | 53.7 ± 3.5 | 162.8 ± 10.2 | 149.2 ± 7.1 | 39.5 ± 5.0 | 67.0 ± 3.7 | |
| 150 | 40.0 ± 1.8 | 55.7 ± 6.4 | 163.3 ± 11.8 | 155.3 ± 2.4 | 38.0 ± 5.0 | 60.2 ± 5.4 | |
| 250 | 43.7 ± 3.3 | 58.8 ± 3.7 | 136.0 ± 8.3 | 152.0 ± 8.3 | 38.3 ± 1.8 | 58.8 ± 11.9 | |
| 350 | 45.2 ± 2.1 | 62.7 ± 6.3 | 153.3 ± 10.9 | 149.3 ± 3.2 | 44.2 ± 2.8 | 63.7 ± 3.7 | |
| A1h | 10 (10) | 55.1 ± 6.8 | 48.9 ± 5.2 | 134.4 ± 13.2 | 140.8 ± 19.7 | 52.0 ± 5.4 | 65.8 ± 7.2 |
| 50 (18) | 49.3 ± 3.8 | 68.0 ± 7.9 | 144.0 ± 14.0 | 133.3 ± 23.6 | 42.7 ± 3.5 | 77.3 ± 5.8 | |
| 75 (25) | 42.7 ± 3.7 | 62.0 ± 3.1 | 160.8 ± 14.3 | 130.0 ± 18.2 | 45.3 ± 5.3 | 59.0 ± 9.2 | |
| 100 (36) | 41.3 ± 3.5 | 60.7 ± 7.3 | 170.0 ± 11.5 | 148.0 ± 4.6 | 43.1 ± 4.9 | 74.7 ± 13.2 | |
| 150 (50) | 56.0 ± 11.1 | 60.0 ± 7.3 | 155.1 ± 8.8 | 155.6 ± 10.4 | 35.3 ± 1.6 | 68.0 ± 12.9 | |
| S1h | 10 (10) | 56.7 ± 6.8 | 64.0 ± 4.2 | 178.0 ± 13.1 | 138.0 ± 8.2 | 53.5 ± 3.6 | 59.3 ± 7.4 |
| 50 (18) | 53.3 ± 1.3 | 64.7 ± 8.1 | 153.3 ± 4.8 | 177.3 ± 7.4 | 59.3 ± 5.9 | 58.2 ± 3.4 | |
| 75 (25) | 64.0 ± 7.7 | 70.2 ± 4.3 | 177.3 ± 12.7 | 145.5 ± 10.3 | 54.2 ± 4.3 | 59.5 ± 3.7 | |
| 100 (36) | 64.0 ± 7.5 | 70.2 ± 6.7 | 168.3 ± 3.8 | 169.7 ± 17.8 | 50.7 ± 4.3 | 58.0 ± 5.0 | |
| 150 (50) | 61.3 ± 5.8 | 68.0 ± 4.9 | 155.3 ± 14.4 | 146.7 ± 6.7 | 52.0 ± 9.2 | 58.5 ± 1.4 | |
| Vehicle | 47.7 ± 2.5 a | 52.7 ± 2.7 a | 157.1 ± 8.1 a | 153.6 ± 17.1 a | 48.2 ± 3.2 a | 66.5 ± 3.9 a | |
| Mutagen | 149.7 ± 10.6 b,** | 266.1 ± 25.1 c,** | 995.8 ± 85.9 d,** | 379.2 ± 23.5 c,** | 323.4 ± 31.2 e,** | 347.4 ± 16.4 f,** | |
3. Experiment Section
3.1. Plant Material
3.2. Essential Oil Extractions
3.3. Essential Oil Analysis
3.4. Antimicrobial Assay
3.5. Mutagenicity Tests
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. Forsch. Komplementmed. 2009, 16, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Naveed, R.; Hussain, I.; Mahmood, M.S.; Akhtar, M. In vitro and in vivo evaluation of antimicrobial activities of essential oils extracted from some indigenous spices. Pak. Vet. J. 2013, 33, 413–417. [Google Scholar]
- Pietrella, D.; Angiolella, L.; Vavala, E.; Rachini, A.; Mondello, F.; Ragno, R.; Bistoni, F.; Vecchiarelli, A. Beneficial effect of mentha suaveolens essential oil in the treatment of vaginal candidiasis assessed by real-time monitoring of infection. BMC Complement. Altern. Med. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Ni, X.; Gong, J.; Yu, H.; Tsao, R.; Han, Y.; Chambers, J.R. Antimicrobial activity of essential oils and structurally related synthetic food additives towards Clostridium perfringens. J. Appl. Microbiol. 2009, 106, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Low, W.L.; Martin, C.; Hill, D.J.; Kenward, M.A. Antimicrobial efficacy of silver ions in combination with tea tree oil against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans. Int. J. Antimicrob. Agents 2011, 37, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.M. Mint. The Genus Mentha, 1st, ed.; Series: Medicinal and Aromatic Plants—Industrial Profiles; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Arthur, T.; Robert, N. An overview of its classification and relationships. In Mint The Genus Mentha; CRC Press: Boca Raton, FL, USA, 2006; Chapter 1. [Google Scholar]
- Harley, R.M.; Brighton, C.A. Chromosome numbers in the genus Mentha L. Bot. J. Linn. Soc. 1977, 74, 71–96. [Google Scholar] [CrossRef]
- Sobti, S. Chromosome numbers in species of Mentha. Proc. Indian AS-Math Sci. B 1965, 62, 145–148. [Google Scholar]
- Deschamps, C.; Zanatta, J.L.; Bizzo, H.R.; Oliveira, M.C.; Roswalka, L.C. Seasonal evaluation of essential oil yield of mint species. Ciênc. Agrotecnol. 2008, 32, 725–730. [Google Scholar] [CrossRef]
- Šarić-Kundalić, B.; Fialová, S.; Dobeš, C.; Ölzant, S.; Tekeľová, D.; Grančai, D.; Reznicek, G.; Saukel, J. Multivariate numerical taxonomy of Mentha species, hybrids, varieties and cultivars. Sci. Pharm. 2009, 77, 851–876. [Google Scholar] [CrossRef]
- Aziz, E.E.; Craker, L.E. Essential oil constituents of peppermint, pennyroyal, and apple mint grown in a desert agrosystem. J. Herbs Spices Med. Plants 2009, 15, 361–367. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Kürkçüoǧlu, M.; Demirci, B.; Özek, T.; Tarimcilar, G. Essential oils of Mentha species from Marmara region of Turkey. J. Essent. Oil Res. 2012, 24, 265–272. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Kürkçüoglu, M.; Tarimcilar, G.; Kaynak, G. Essential oils of Mentha species from northern turkey. J. Essent. Oil Res. 1999, 11, 579–588. [Google Scholar] [CrossRef]
- Brada, M.; Bezzina, M.; Marlier, M.; Lognay, G.C. Chemical composition of the leaf oil of Mentha rotundifolia (L.) from Algeria. J. Essent. Oil Res. 2006, 18, 663–665. [Google Scholar] [CrossRef]
- Brian, L. Oil composition of other mentha species and hybrids. In Mint. The Genus Mentha; CRC Press: Boca Raton, FL, USA, 2006; Chapter 8. [Google Scholar]
- Derwich, E.; Benziane, Z.; Boukir, A. Antibacterial activity and chemical composition of the leaf essential oil of mentha rotundifolia from Morocco. J. Environ. Agric. Food Chem. 2010, 9, 19–28. [Google Scholar]
- Derwich, E.; Benziane, Z.; Taouil, R.; Senhaji, O.; Touzani, M. Comparative essential oil composition of leaves of mentha rotundifolia and mentha pulegium a traditional herbal medicine in Morocco. Am. -Eurasian J. Sust. Agric. 2010, 4, 47–54. [Google Scholar]
- Karousou, R.; Balta, M.; Hanlidou, E.; Kokkini, S. “Mints”, smells and traditional uses in Thessaloniki (Greece) and other Mediterranean countries. J. Ethnopharmacol. 2007, 109, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.B.; Eddine, A.D. Antibacterial activity of essential oils of some algerian aromatic plants against multidrug resistant bacteria. J. Essent. Oil Bear. Plants 2010, 13, 362–370. [Google Scholar] [CrossRef]
- Sutour, S.; Bradesi, P.; Casanova, J.; Tomi, F. Composition and chemical variability of Mentha suaveolens ssp. suaveolens and M. suaveolens ssp. insularis from Corsica. Chem. Biodivers. 2010, 7, 1002–1008. [Google Scholar] [PubMed]
- Sutour, S.; Bradesi, P.; de Rocca-Serra, D.; Casanova, J.; Tomi, F. Chemical composition and antibacterial activity of the essential oil from Mentha suaveolens ssp. insularis (Req.) Greuter. Flavour Fragr. J. 2008, 23, 107–114. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Angiolella, L.; Vavala, E.; Sivric, S.; Diodata, D.A.F.; Ragno, R. Letter: In vitro activity of mentha suaveolens essential oil against cryptococcus neoformans and dermatophytes. Int. J. Essent. Oil Ther. 2010, 4, 35–36. [Google Scholar]
- Ragno, R.; Sivric, S.; Sartorelli, G.; Serilli, A.; Vavala, E.; Angiolella, L. In vitro activity of essential oil of Myrtus communis L. against Candida albicans. Int. J. Essent. Oil Ther. 2008, 2, 156–157. [Google Scholar]
- Vavala, E.; Ragno, R.; Sivric, S.; Sartorelli, G.; Filippi, A.; Palamara, A.T.; Angiolella, L. Antimycotic activity of Achillea ageratum L. essential oil. Int. J. Essent. Oil Ther. 2009, 3, 101–105. [Google Scholar]
- Miconazole (Oravig) for oropharyngeal candidiasis. Med. Lett. Drugs Ther. 2010, 52, 95–96.
- Stringaro, A.; Vavala, E.; Colone, M.; Pepi, F.; Mignogna, G.; Garzoli, S.; Cecchetti, S.; Ragno, R.; Angiolella, L. Effects of mentha suaveolens essential oil alone or in combination with other drugs in Candida albicans. Evid. -Based Complement. Altern. Med. 2014, 2014, 125904. [Google Scholar] [CrossRef] [PubMed]
- Vavala, E.; Passariello, C.; Pepi, F.; Colone, M.; Garzoli, S.; Ragno, R.; Pirolli, A.; Stringaro, A.; Angiolella, L. Antibacterial activity of essential oils mixture against PSA. Nat. Prod. Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.C.; Netto, J.C.; Pereira, P.S.; Pereira, A.M.; Taleb-Contini, S.H.; Franca, S.C.; Marques, M.O.; Beleboni, R.O. The role of polar phytocomplexes on anticonvulsant effects of leaf extracts of Lippia alba (Mill.) N.E. Brown chemotypes. J. Pharm. Pharmacol. 2009, 61, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Maietti, S.; Rossi, D.; Guerrini, A.; Useli, C.; Romagnoli, C.; Poli, F.; Bruni, R.; Sacchetti, G. A multivariate analysis approach to the study of chemical and functional properties of chemo-diverse plant derivatives: Lavender essential oils. Flavour Fragr. J. 2013, 28, 144–154. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.J.; Haroutounian, S.A. Essential oils of Satureja, Origanum, and Thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food. Chem. 2004, 52, 8261–8267. [Google Scholar] [CrossRef] [PubMed]
- Chorianopoulos, N.; Evergetis, E.; Mallouchos, A.; Kalpoutzakis, E.; Nychas, G.J.; Haroutounian, S.A. Characterization of the essential oil volatiles of Satureja thymbra and Satureja parnassica: Influence of harvesting time and antimicrobial activity. J. Agric. Food. Chem. 2006, 54, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Evandri, M.G.; Mazzanti, G. Antimutagenic and mutagenic activities of some terpenes in the bacterial reverse mutation assay. Mutat. Res. 2008, 653, 130–133. [Google Scholar]
- Di Sotto, A.; Maffei, F.; Hrelia, P.; Castelli, F.; Sarpietro, M.G.; Mazzanti, G. Genotoxicity assessment of β-caryophyllene oxide. Regul. Toxicol. Pharm. 2013, 66, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Mazzanti, G.; Carbone, F.; Hrelia, P.; Maffei, F. Inhibition by beta-caryophyllene of ethyl methanesulfonate-induced clastogenicity in cultured human lymphocytes. Mutat. Res. 2010, 699, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Durazzi, F.; Sarpietro, M.G.; Mazzanti, G. Antimutagenic and antioxidant activities of some bioflavours from wine. Food Chem. Toxicol. 2013, 60, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Maffei, F.; Hrelia, P.; di Giacomo, S.; Pagano, E.; Borrelli, F.; Mazzanti, G. Genotoxicity assessment of some cosmetic and food additives. Regul. Toxicol. Pharm. 2014, 68, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garzoli, S.; Pirolli, A.; Vavala, E.; Di Sotto, A.; Sartorelli, G.; Božović, M.; Angiolella, L.; Mazzanti, G.; Pepi, F.; Ragno, R. Multidisciplinary Approach to Determine the Optimal Time and Period for Extracting the Essential Oil from Mentha suaveolens Ehrh . Molecules 2015, 20, 9640-9655. https://doi.org/10.3390/molecules20069640
Garzoli S, Pirolli A, Vavala E, Di Sotto A, Sartorelli G, Božović M, Angiolella L, Mazzanti G, Pepi F, Ragno R. Multidisciplinary Approach to Determine the Optimal Time and Period for Extracting the Essential Oil from Mentha suaveolens Ehrh . Molecules. 2015; 20(6):9640-9655. https://doi.org/10.3390/molecules20069640
Chicago/Turabian StyleGarzoli, Stefania, Adele Pirolli, Elisabetta Vavala, Antonella Di Sotto, Gianni Sartorelli, Mijat Božović, Letizia Angiolella, Gabriela Mazzanti, Federico Pepi, and Rino Ragno. 2015. "Multidisciplinary Approach to Determine the Optimal Time and Period for Extracting the Essential Oil from Mentha suaveolens Ehrh " Molecules 20, no. 6: 9640-9655. https://doi.org/10.3390/molecules20069640
APA StyleGarzoli, S., Pirolli, A., Vavala, E., Di Sotto, A., Sartorelli, G., Božović, M., Angiolella, L., Mazzanti, G., Pepi, F., & Ragno, R. (2015). Multidisciplinary Approach to Determine the Optimal Time and Period for Extracting the Essential Oil from Mentha suaveolens Ehrh . Molecules, 20(6), 9640-9655. https://doi.org/10.3390/molecules20069640