Anti-Inflammatory Chemical Profiling of the Australian Rainforest Tree Alphitonia petriei (Rhamnaceae)
Abstract
:1. Introduction
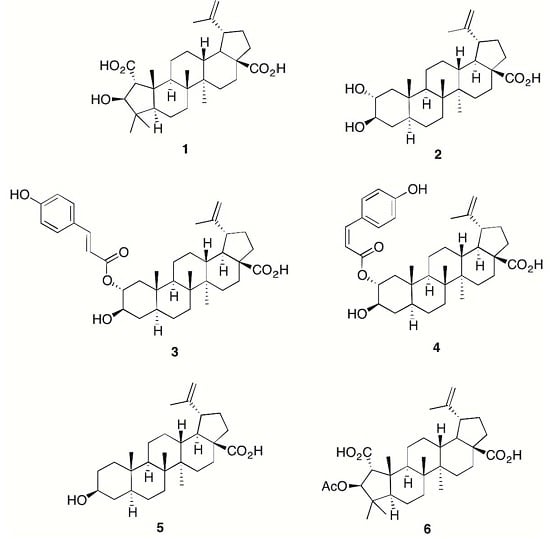
2. Results and Discussion
3. Method and Materials
3.1. General Information
3.2. Compound Isolation and Semisynthesis of 6
Compound 6 (Emmolic Acid Acetate)
3.3. Cell Lines and Cell Culture
3.3.1. Maintenance of RAW 264.7 Macrophages
3.3.2. Activation of RAW 264.7 Macrophages
3.3.3. Determination of Nitrite (as a Measure of Nitric Oxide Production) by the Griess Assay
3.3.4. Determination of TNF-α by ELISA
3.3.5. Determination of Cell Viability by the Alamar Blue Assay
3.4. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Panettieri, R.A., Jr. Airway smooth muscle: An immunomodulatory cell. J. Allergy Clin. Immunol. 2002, 110, S269–S274. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Weyand, C.M. Rheumatoid arthritis. Immunol. Rev. 2005, 204, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Kesmarky, G.; Feher, G.; Koltai, K.; Horvath, B.; Toth, K. Viscosity, hemostasis and inflammation in atherosclerotic heart diseases. Clin. Hemorheol. Microcirc. 2006, 35, 67–73. [Google Scholar] [PubMed]
- Eikelenboom, P.; Bate, C.; Gool, W.A.V.; Hoozemans, J.J.M.; Rozemuller, J.M.; Veerhuis, R.; Williams, A. Neuroinflammation in Alzheimer’s disease and prion disease. Glia 2002, 40, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Venigalla, M.; Sonego, S.; Gyengesi, E.; Sharman, M.J.; Münch, G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2015, 95, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Varley, J.; Brooks, D.J.; Edison, P. Imaging neuroinflammation in Alzheimer’s and other dementias: Recent advances and future directions. Alzheimer’s Dement. 2014, 11, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Durrenberger, P.F.; Fernando, F.S.; Kashefi, S.N.; Bonnert, T.P.; Seilhean, D.; Nait-Oumesmar, B.; Schmitt, A.; Gebicke-Haerter, P.J.; Falkai, P.; Grunblatt, E.; et al. Common mechanisms in neurodegeneration and neuroinflammation: A BrainNet Europe gene expression microarray study. J. Neural Transm. 2014, 122, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Meiners, I.; Hauschildt, S.; Nieber, K.; Münch, G. Pentoxyphylline and propentophylline are inhibitors of TNF-alpha release in monocytes activated by advanced glycation endproducts. J. Neural Transm. 2004, 111, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Münch, G.; Gasic-Milenkovic, J.; Dukic-Stefanovic, S.; Kuhla, B.; Heinrich, K.; Riederer, P.; Huttunen, H.J.; Founds, H.; Sajithlal, G. Microglial activation induces cell death, inhibits neurite outgrowth and causes neurite retraction of differentiated neuroblastoma cells. Exp. Brain Res. 2003, 150, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Holmquist, L.; Stuchbury, G.; Steele, M.; Münch, G. Hydrogen peroxide is a true first messenger. J. Neural Transm. Suppl. 2007, 72, 39–41. [Google Scholar]
- McCoy, M.K.; Tansey, M.G. TNF signaling inhibition in the CNS: Implications for normal brain function and neurodegenerative disease. J. Neuroinflam. 2008, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H.; Tsuda, M.; Adachi, H.; Ogura, T.; Sugimura, T.; Esumi, H. l-arginine-dependent formation of N-nitrosamines by the cytosol of macrophages activated with lipopolysaccharide and interferon-gamma. Carcinogenesis 1991, 12, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Singel, D.J.; Loscalzo, J. Biochemistry of nitric oxide and its redox-activated forms. Science 1992, 258, 1898–1902. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Warner, T.D. COX isoforms in the cardiovascular system: Understanding the activities of non-steroidal anti-inflammatory drugs. Nat. Rev. Drug Discov. 2006, 5, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Branch, G.; Burgess, D.; Dunstan, P.; Foo, L.; Green, G.; Mack, J.G.; Ritchie, E.; Taylor, W. Constituents of Alphitonia species. III. Alphitexolide, a new triterpene, and other extractives. Aust. J. Chem. 1972, 25, 2209–2216. [Google Scholar] [CrossRef]
- Setzer, W.N.; Petty, J.L.; Schmidt, J.M.; Setzer, M.C.; Bates, R.B.; Jackes, B.R. Bioactive lupane triterpenoids in Alphitonia petriei from Paluma, North Queensland, Australia. Curr. Top. Phytochem. 2004, 6, 145–148. [Google Scholar]
- Julian, P.L.; Pikl, J.; Dawson, R. The constituents of ceanothus americanus. I. Ceanothic acid. J. Am. Chem. Soc. 1938, 60, 77–79. [Google Scholar] [CrossRef]
- Boyer, J.P.; Eade, R.A.; Locksley, H.; Simes, J.J.H. Extractives of Australian timbers. II. Emmolic acid, a new triterpene acid from Emmenospermum alphitonoides F. Muell. Aust. J. Chem. 1958, 11, 236–245. [Google Scholar] [CrossRef]
- Yagi, A.; Okamura, N.; Haraguchi, Y.; Noda, K.; Nishioka, I. Studies on the constituents of Zizyphi fructus II. Structures of new p-coumaroylates of alphitolic acid. Chem. Pharm. Bull. 1978, 26, 1798–1802. [Google Scholar] [CrossRef]
- Plastina, P.; Bonofiglio, D.; Vizza, D.; Fazio, A.; Rovito, D.; Giordano, C.; Barone, I.; Catalano, S.; Gabriele, B. Identification of bioactive constituents of Ziziphus jujube fruit extracts exerting antiproliferative and apoptotic effects in human breast cancer cells. J. Ethnopharmacol. 2012, 140, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.G.; Ahmad, F.B.H.; Samzadeh-Kermani, A. Biological activity of betulinic acid: A review. Pharmacol. Pharm. 2012, 3, 119–123. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, X.; Zhang, P.; Liu, J.; Zhang, L.; Sun, H. Efficient access to isomeric 2,3-dihydroxy lupanes: First synthesis of alphitolic acid. Tetrahedron 2009, 65, 7975–7984. [Google Scholar] [CrossRef]
- Jeong, W.; Hong, S.S.; Kim, N.; Yang, Y.T.; Shin, Y.S.; Lee, C.; Hwang, B.Y.; Lee, D. Bioactive triterpenoids from Callistemon lanceolatus. Arch. Pharm. Res. 2009, 32, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.C.; Delporte, C.; Backhouse, N.; Erazo, S.; Letelier, M.E.; Cassels, B.K.; Silva, X.; Alegria, S.; Negrete, R. Topical anti-inflammatory activity of 2alpha-hydroxy pentacyclic triterpene acids from the leaves of Ugni molinae. Bioorg. Med. Chem. 2006, 14, 5673–5677. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.-Y.; Chiu, C.-F.; Chiu, S.-J.; Chen, Y.-W.; Hu, J.-L.; Wu, C.-Y.; Weng, J.-R. Alphitolic acid, an anti-inflammatory triterpene, induces apoptosis and autophagy in oral squamous cell carcinoma cells, in part, through a p53-dependent pathway. J. Funct. Foods 2015, 18, 368–378. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.

| Compound | NO Inhibition (IC50 in μM) (n = 8) | TNF-α Inhibition (IC50 in µM) (n = 3) (in μM) (IC50 in μM) | Cytotoxicity (LC50 in μM) (n = 8) | Therapeutic Index (TI) (Compared to NO) |
|---|---|---|---|---|
| 1 | >36 | >36 | >36 | |
| 2 | 17.6 ± 0.7 | 22.7 ± 1.1 | 18.6 ± 0.8 | 1 |
| 3 | 1.7 ± 0.3 | 10.9 ± 2.3 | 4.8 ± 0.9 | 2.8 |
| 4 | 3.5 ± 0.5 | 5.6 ± 0.4 | 8.0 ± 0.3 | 2.3 |
| 5 | 8.3 ± 1.0 | 23.5 ± 3.7 | 29.1 ± 4.4 | 3.5 |
| 6 | 14.7 ± 0.6 | >36 | 13.1 ± 2.5 | 0.9 |
| NSAID | ||||
| Diclofenac | 217 ± 43 | >333 | >333 | |
| Ibuprofen | 1155 ± 133 | 723 ± 261 | >1500 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, R.; Gunawardena, D.; Ahktar, M.A.; Low, M.; Reddell, P.; Münch, G. Anti-Inflammatory Chemical Profiling of the Australian Rainforest Tree Alphitonia petriei (Rhamnaceae). Molecules 2016, 21, 1521. https://doi.org/10.3390/molecules21111521
Raju R, Gunawardena D, Ahktar MA, Low M, Reddell P, Münch G. Anti-Inflammatory Chemical Profiling of the Australian Rainforest Tree Alphitonia petriei (Rhamnaceae). Molecules. 2016; 21(11):1521. https://doi.org/10.3390/molecules21111521
Chicago/Turabian StyleRaju, Ritesh, Dhanushka Gunawardena, Most Afia Ahktar, Mitchell Low, Paul Reddell, and Gerald Münch. 2016. "Anti-Inflammatory Chemical Profiling of the Australian Rainforest Tree Alphitonia petriei (Rhamnaceae)" Molecules 21, no. 11: 1521. https://doi.org/10.3390/molecules21111521
APA StyleRaju, R., Gunawardena, D., Ahktar, M. A., Low, M., Reddell, P., & Münch, G. (2016). Anti-Inflammatory Chemical Profiling of the Australian Rainforest Tree Alphitonia petriei (Rhamnaceae). Molecules, 21(11), 1521. https://doi.org/10.3390/molecules21111521