Abstract
Series of 4-(4-substituted benzylideneamino)-5-(3,4,5-trimethoxyphenyl)-4H-1,2,4-triazole-3-thiols were synthesized and their structures were confirmed. The synthesized Schiff bases were used as photostabilizers for polystyrene against photodegradation. Polystyrene polymeric films containing synthesized Schiff bases (0.5% by weight) were irradiated (λmax = 365 nm and light intensity = 6.43 × 10−9 ein·dm−3·s−1) at room temperature. The photostabilization effect of 1,2,4-triazole-3-thiols Schiff bases was determined using various methods. All the additives used enhanced the photostability of polystyrene films against irradiation compared with the result obtained in the absence of Schiff base. The Schiff bases can act as photostabilizers for polystyrene through the direct absorption of UV radiation and/or radical scavengers.
1. Introduction
Polystyrene (PS) is an aromatic synthetic polymer that is produced in millions of tons every year. It was reported that 55 million tons of polystyrene was produced in 2013 [1]. It is a widely used plastic produced from polymerization of styrene. Various types of polystyrene are known that mainly depend on the positions of phenyl groups along the polymeric chain [2,3,4]. Polystyrene is naturally colorless and transparent and can be used in the manufacture of containers, bottles, electronics, and insulation [2].
One of the main problems associated with the use of polymeric materials, either synthetic or semisynthetic, is their photodegradation [5]. Polystyrene undergoes photodegradation when exposed to harsh environments such as high temperatures and sunlight [6]. Polystyrene is not biodegradable, meaning that it will undergo very slow degradation which may require many years [7]. Photodegradation of polystyrene can lead to chain scission, cross-linking, and discoloration [8]. Therefore, polystyrene polymeric materials should be stabilized against photodegradation and photo-oxidation to prevent and/or reduce the effect weathering conditions. In addition, stabilization would maximize the long-term use of polystyrene to increase its economic viability. Various additives have been used to enhance the photostability of polymeric films such as plasticizers [9], heterocycles [10,11,12,13], aromatics [14,15,16], and organometallics [17,18,19]. Such photostabilizers in most cases can act as direct UV absorbers, radical scavengers, excited state quenchers and/or peroxide decomposers [8].
Recently, we have reported successful polymeric films photostabilization processes by the use of various additives at low concentrations [20,21,22,23,24,25] as part of our continuing interest in the field of polymeric materials [26,27,28,29,30]. Now, we report the photostabilization of polystyrene films in the presence of 1,2,4-triazole-3-thiol ring system Schiff bases.
2. Results and Discussion
2.1. Synthesis of Schiff Bases 1–4
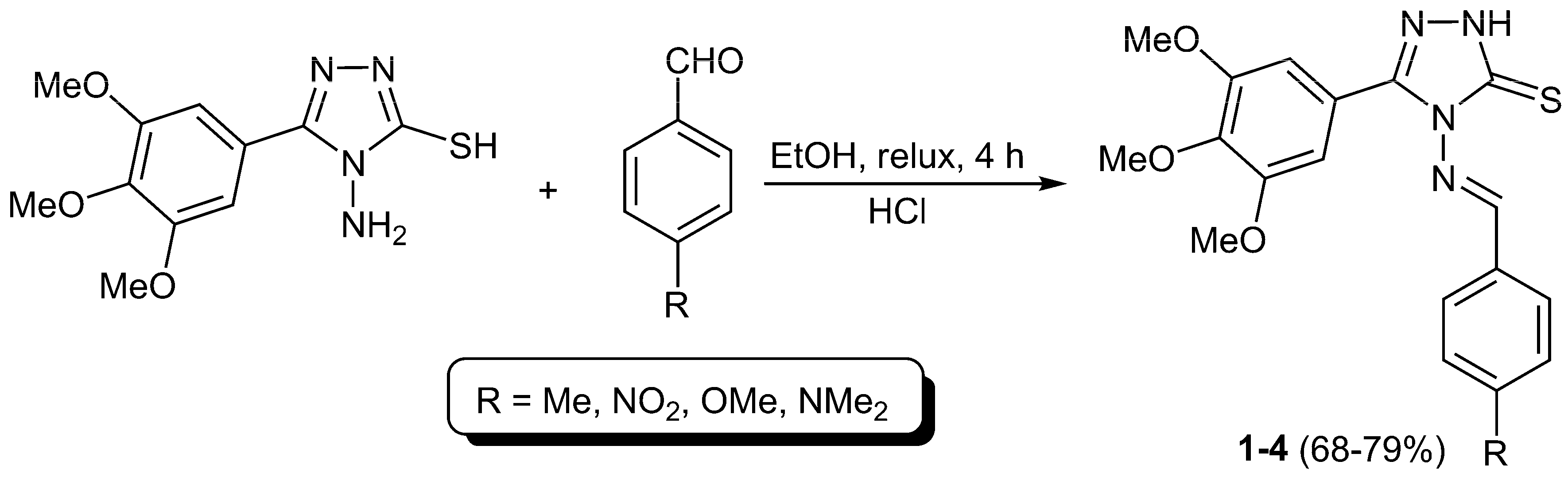
4-(4-Substituted benzylideneamino)-5-(3,4,5-trimethoxyphenyl)-4H-1,2,4-triazole-3-thiols 1–4 were synthesized in 68%–79% yields (Table 1) from reaction of 4-amino-5-(3,4,5-trimethoxyphenyl)-4H-1,2,4-triazole-3-thiol and various aromatic aldehydes (4-methylbenzaldehyde, 4-nitrobenzaldehyde, 4-methoxybenzaldehyde, and 4-(dimethylamino)benzaldehyde) in anhydrous ethanol in the presence of few drops of hydrochloric acid as a catalyst under reflux for 4 h (Scheme 1).

Table 1.
Physical properties and some FT-IR spectral data for Schiff bases 1–4.
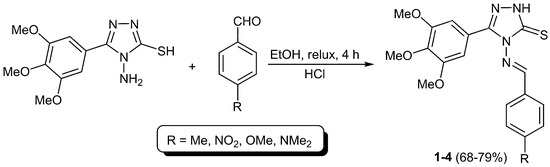
Scheme 1.
Synthesis of 4-(4-substituted benzylideneamino)-5-(3,4,5-trimethoxyphenyl)-4H-1,2,4-triazole-3-thiols 1–4.
2.2. Characterization of Schiff Bases 1–4
The structures of Schiff bases 1–4 have been confirmed by the IR and 1H-NMR spectral data. The FT-IR spectra showed characteristics stretching vibration bands for the CH=N bonds that resonate within the 1589–1614 cm−1 region. Also, they showed absorption bands at the 1240–1244 cm−1 region corresponding to the C=S bonds. Moreover, the FT-IR spectra of 1–4 showed the absence of the NH2 streching bands for aminotriazole or the C=O bands for aromatic aldehydes [31]. Some of the most common and abundant absorption bands for Schiff bases 1–4 are represented in Table 1.
The 1H-NMR spectra for 1–4 confirmed the presence of two different types of aromatic protons. They show two doublets (two protons each; J = 8.1–8.4 Hz) that resonated within the 7.73–8.30 ppm region, corresponding the aromatic protons from the aldehyde moeity. They also showed singlet signals (two protons) that are corresponding to the other aromatic protons (7.20–7.24 ppm), from the amine moeity. Also, they show singlet signals that resonate in the 9.56–9.13 ppm region due to the CH proton. This is a clear indication for the formation of the Schiff bases 1–4. However, the SH proton was only apparent in compound 4 and resonates as an exchangeable singlet signal at 14.09 ppm. The 1H-NMR spectral data for 1–4 are shown in Table 2.

Table 2.
1H-NMR spectral data for Schiff bases 1–4.
2.3. Measuring Photostabilization of Polystyrene Films by IR Spectroscopy
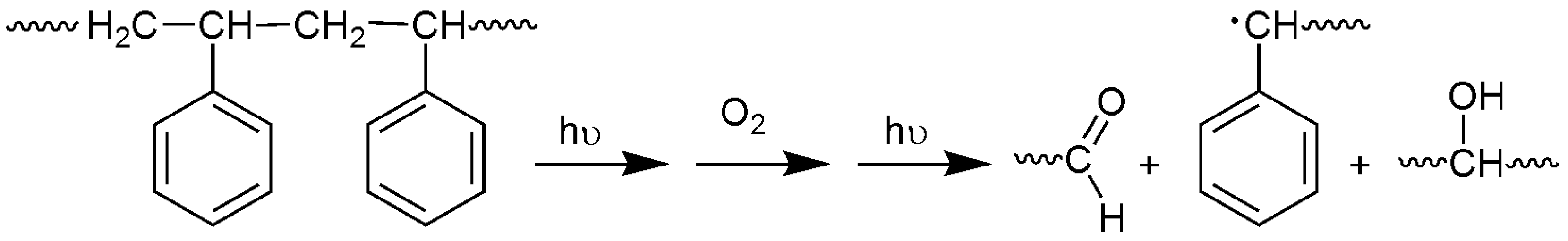
Ultraviolet radiation has harmful effects on polystyrene that lead to chemical changes within the polymeric chains. As a result, the polymeric materials could lose their mechanical properties and become discolored [32]. Photo-oxidation of polystyrene leads to the formation of several functional groups [33,34]. It has been reported that irradiation of polystyrene in the presence of oxygen can lead to the production of carbonyl and hydroxyl group moieties, for example, as shown in Figure 1 [35]. Initial degradation of PS leads to a polystyrene radical [–CH2CH(Ph)]. Such radicals can abstract a proton from other polymeric chains to produce various other radicals [–CH2C(Ph)–CH2–, –CH2C(Ph)–CH3 and –CH2CHC(H)–CH(Ph)–]. Theses radicals react with oxygen to produce the corresponding peroxy radicals [8]. Therefore, the changes in the IR spectrum of polystyrene due to irradiation can be used as a measure of photodegradation within the polymeric materials. The FT-IR spectra for PS films before and after irradiation (250 h) by UV light (λmax = 365 nm) are represented in Figure 2 and Figure 3, respectively.
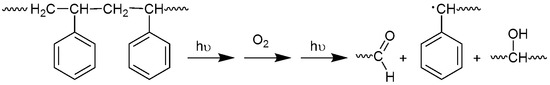
Figure 1.
Photooxidative degradation of PS.
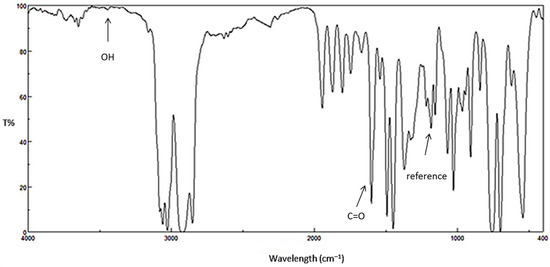
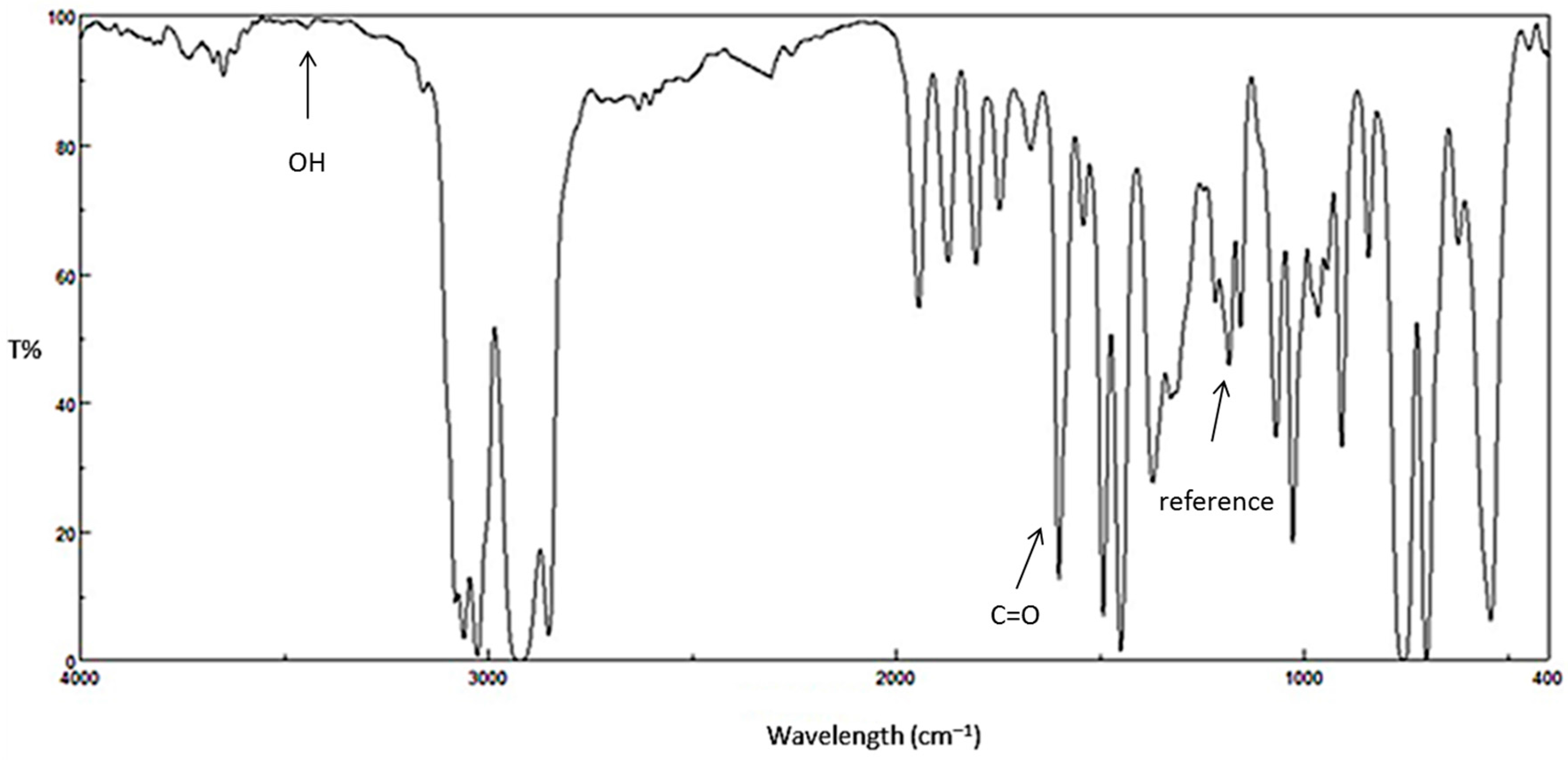
Figure 2.
FTIR spectrum for PS film before irradiation.
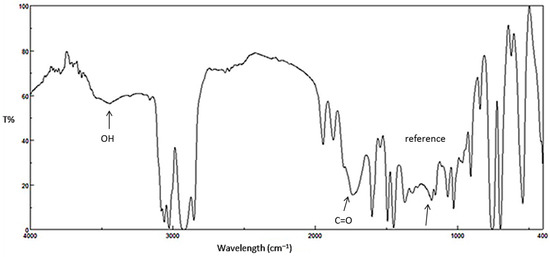
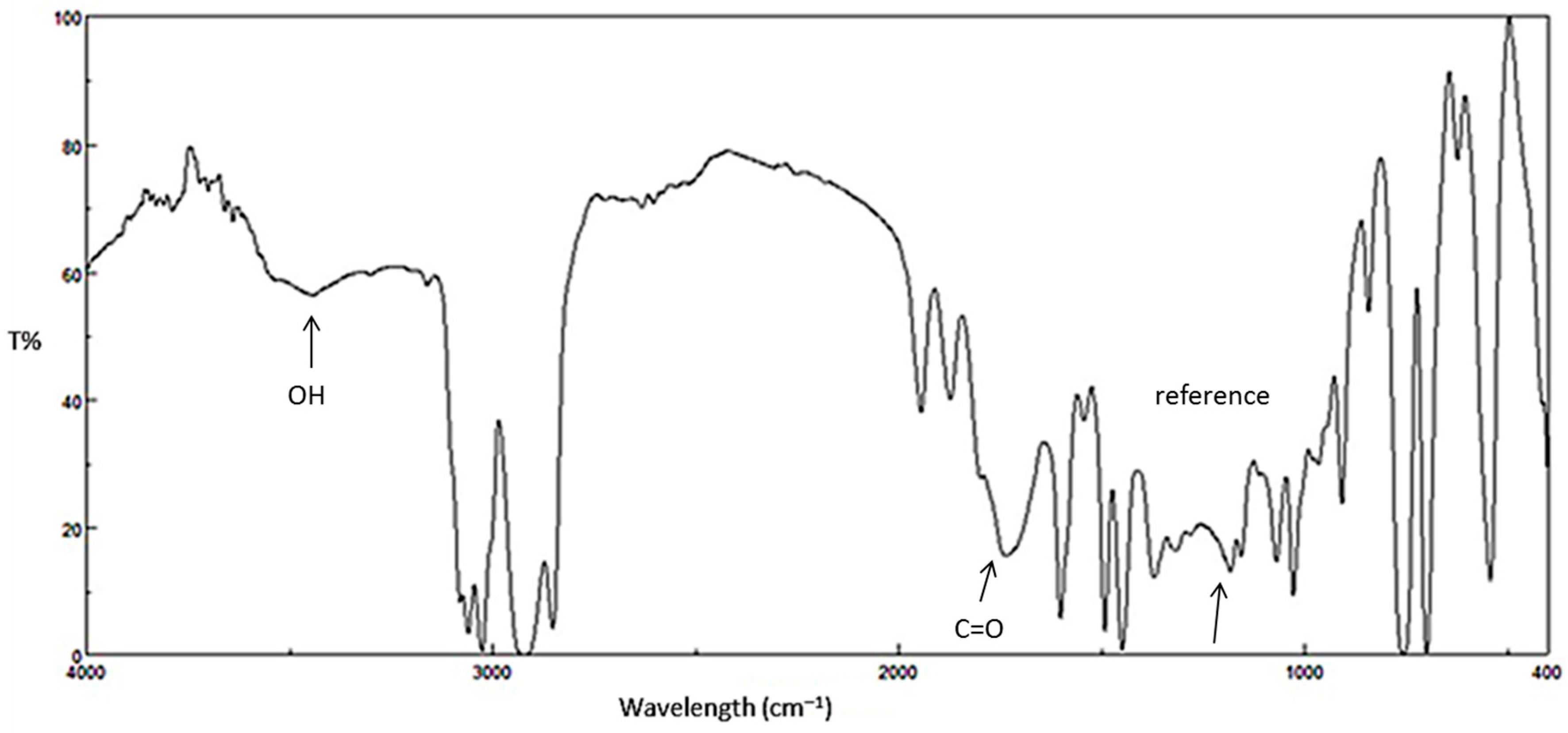
Figure 3.
FTIR spectrum for PS film after irradiation (250 h).
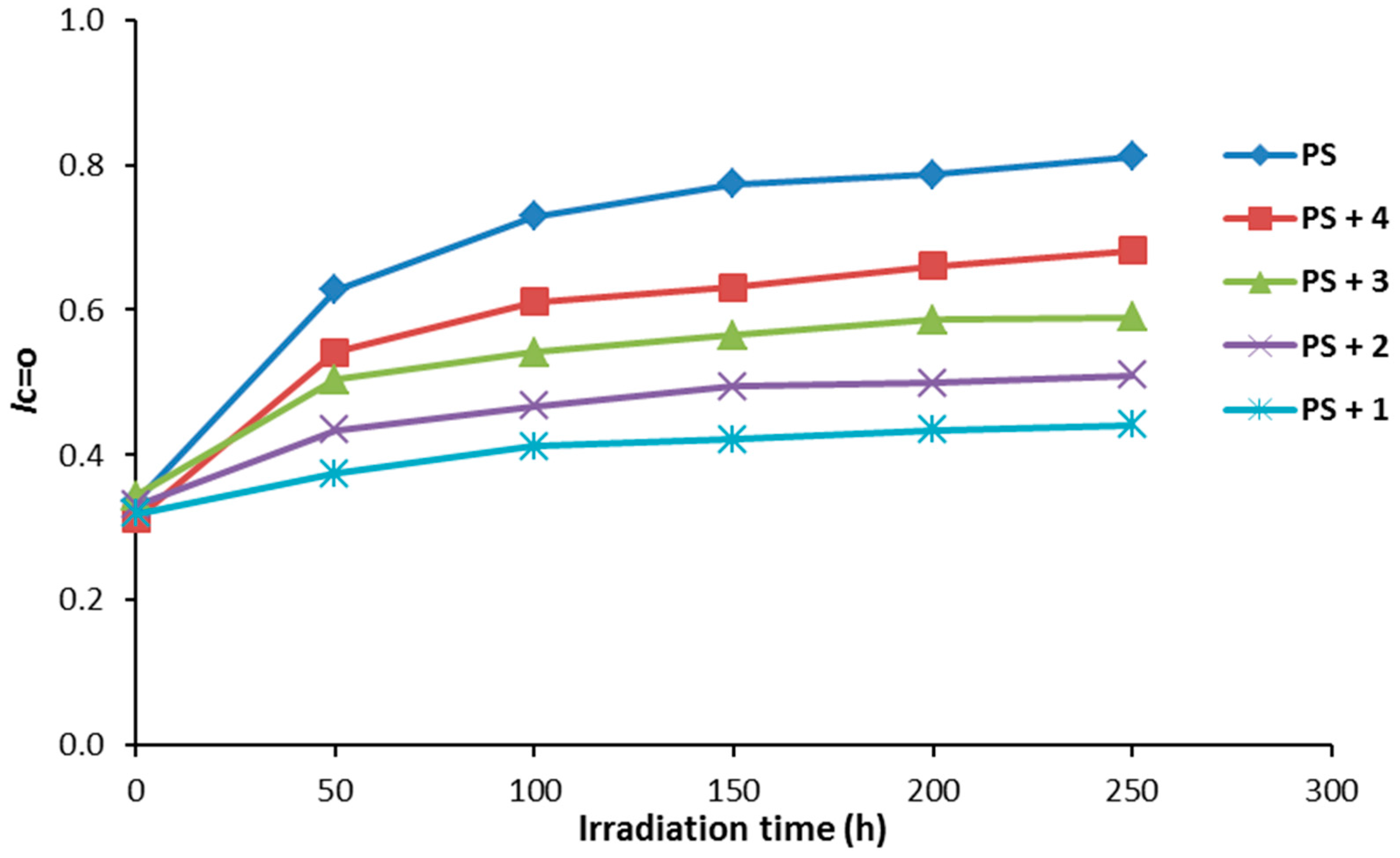
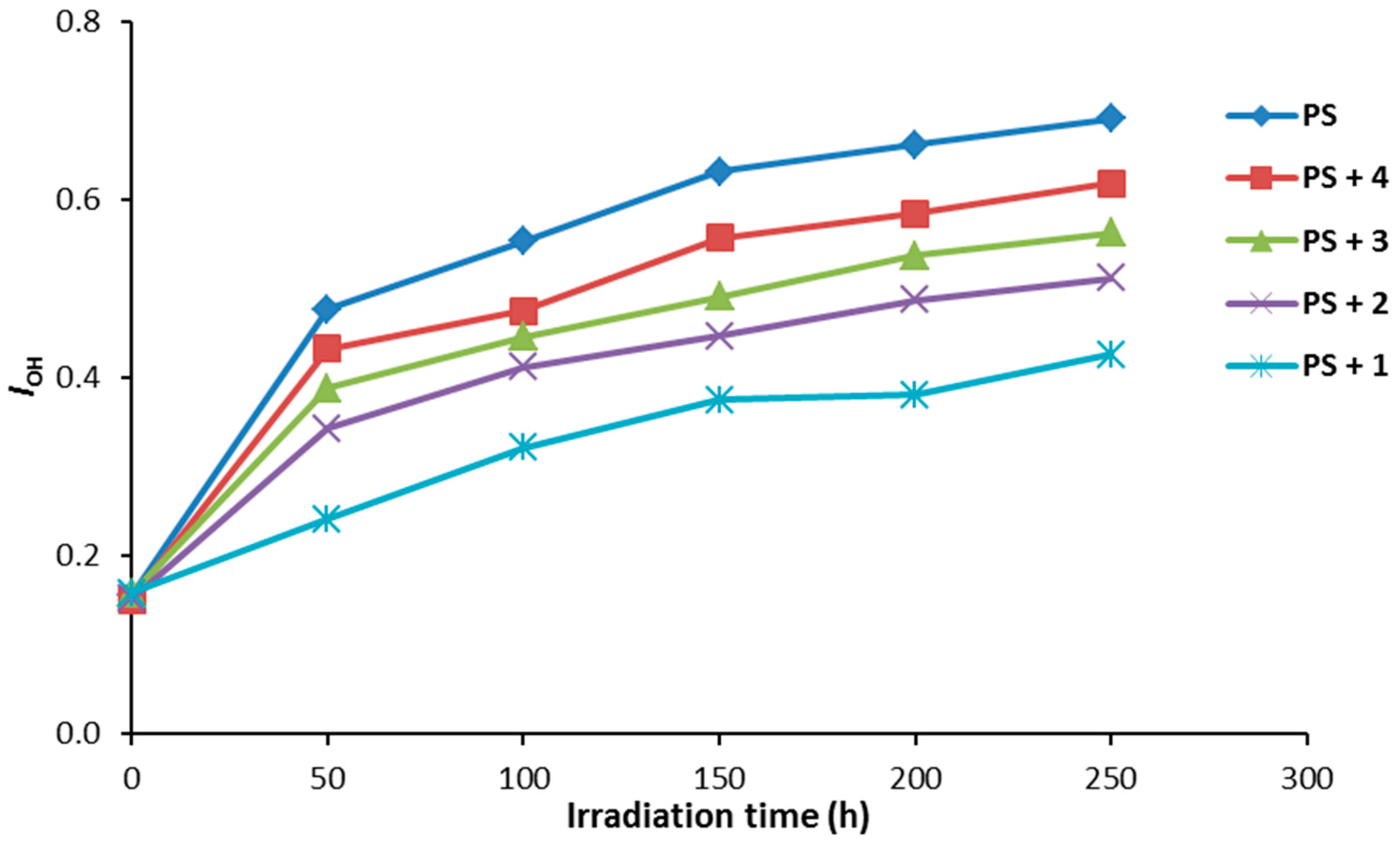
Compounds 1–4 (0.5% by weight) were mixed with PS to produce the polymeric films (40 μm thickness). The efficiency of the additives as photostabilizers for the photostabilization of PS films was investigated under irradiation for 250 h. The PS films were irradiated for 250 h and the carbonyl (ICO) and hydroxyl (IOH) indices were monitored using an IR spectrophotometer. The IR absorption bands that appeared at ca. 1720 and 3400 cm−1 can be assigned to the carbonyl and hydroxyl groups, respectively [36]. The increases in both ICO and IOH indexes, compared to the reference peak (1328 cm−1) can be used as an indicator for PS photodegradation. It should be noted that IC=O does not starts from zero because some photodegradation takes place during the preparation the PS films. The changes in the IC=O and IOH indices on irradiation of PS in the presence of Schiff bases 1–4 are represented in Figure 4 and Figure 5, respectively.
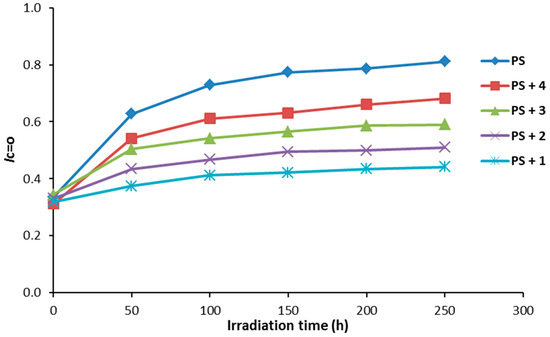
Figure 4.
The effect of irradiation on the ICO index for PS films.
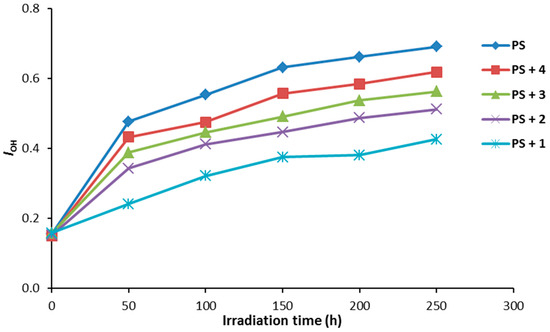
Figure 5.
The effect of irradiation on the IOH index for PS films.
As demonstrated through our findings, all the additives used stabilized the PS film against photodegradation. The changes in both the ICO and IOH indices were lower for the PS films containing the additives 1–4 compared to the ones for the PS (blank). Compound 1 was the most efficient additive for the photostabilization of PS film.
2.4. Measuring Photostabilization of Polystyrene Films by Weight Loss
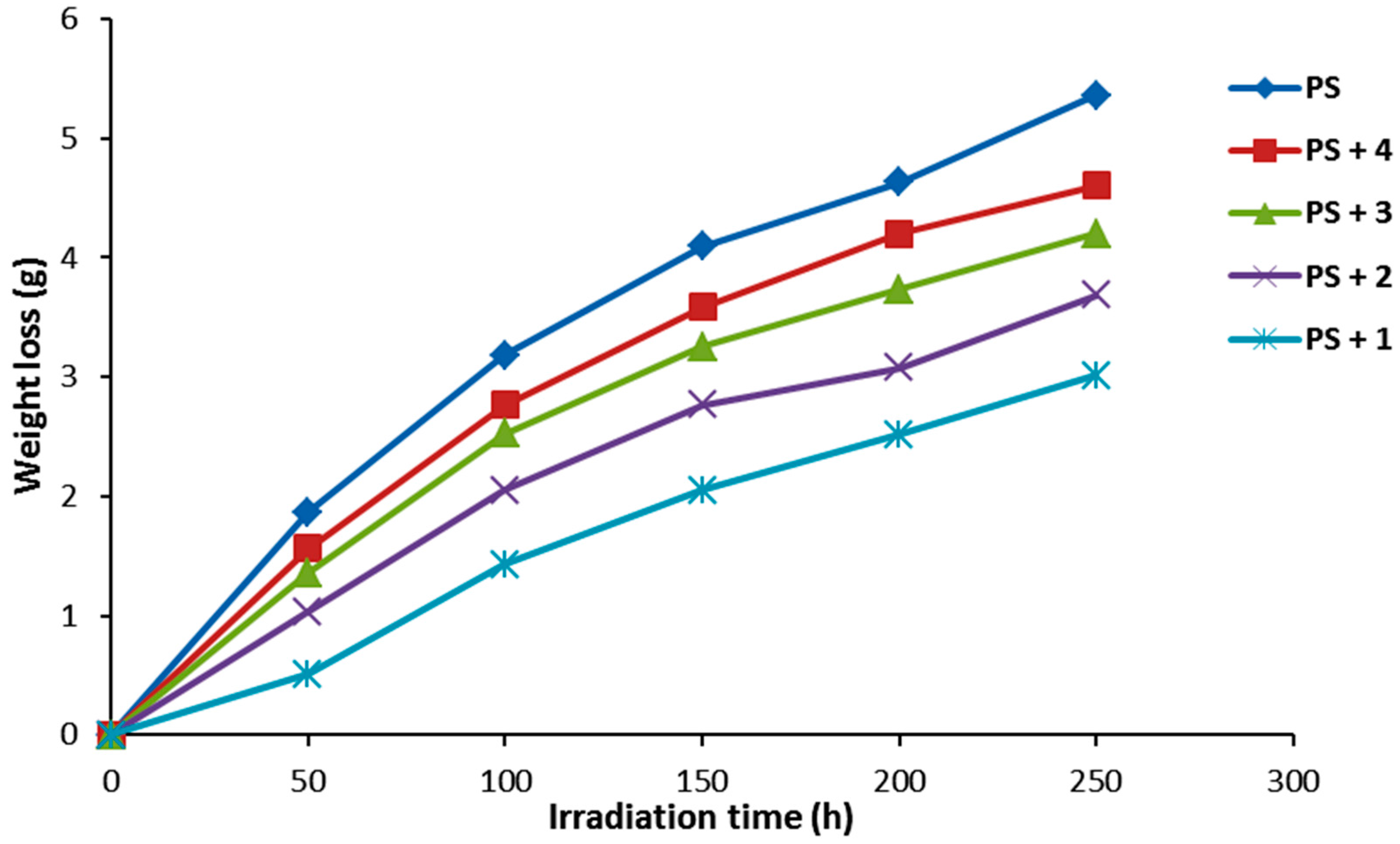
Photodegradation of PS produces low molecular weight fragments along with volatiles and leads to weight loss [37]. The efficiency of additive (0.5% by weight) photostabilizer for PS was determined in terms of PS weight loss under irradiation for 250 h. The effect of irradiation on the weight loss of PS films is represented in Figure 6. The PS film weight loss increases gradually with the irradiation time. Evidently, the weight loss was low for the PS films containing compounds 1–4 compared to that obtained for the PS film (blank) in the absence of such additives. The weight loss was lowest when compound 1 was used as the additive.
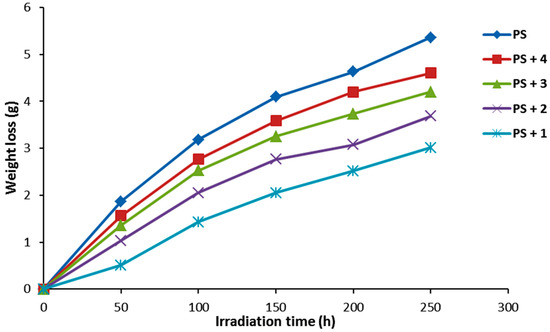
Figure 6.
The effect of irradiation on weight loss for PS films.
2.5. Measuring Photostabilization of Polystyrene Films by Variation in Molecular Weight
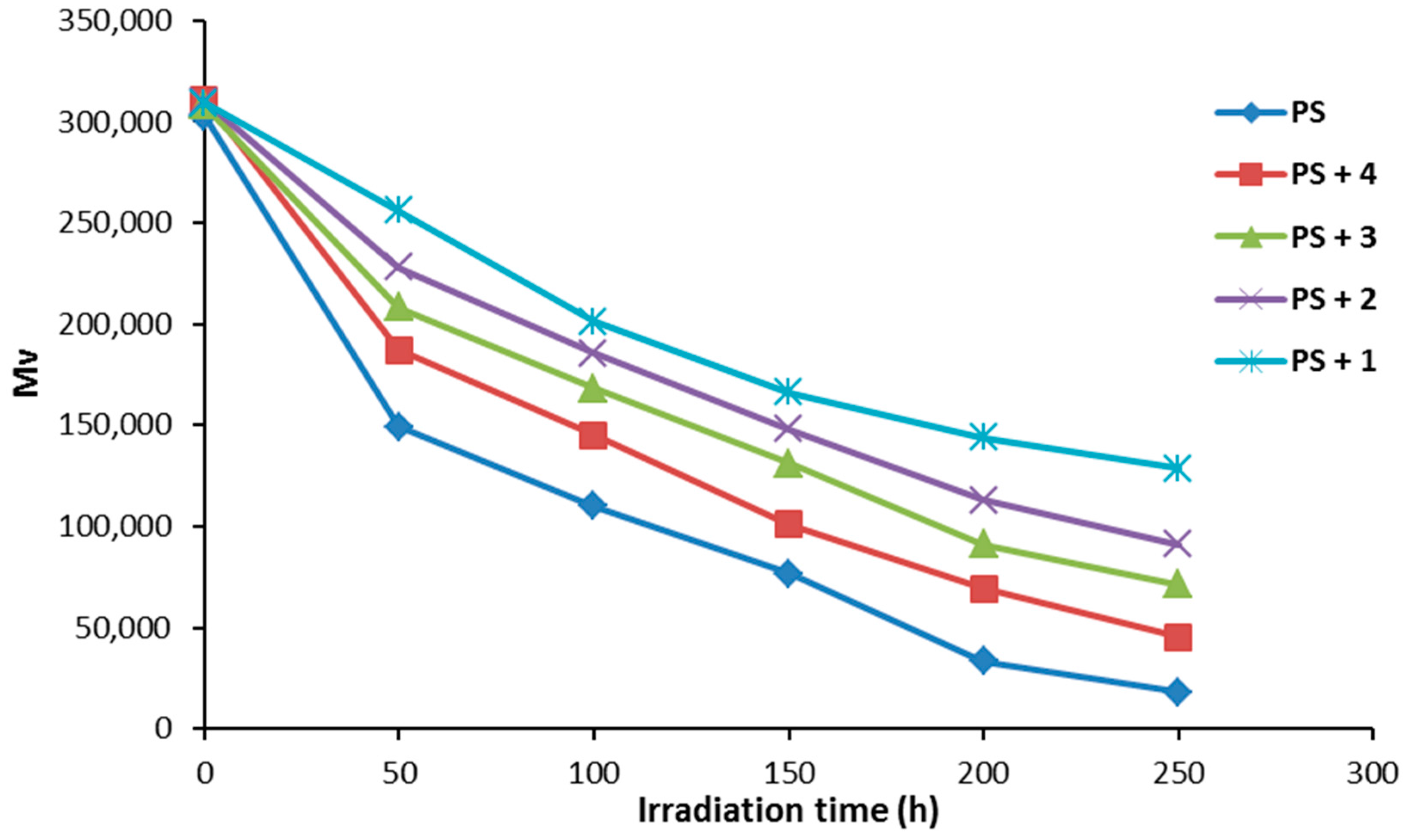
The variation of PS molecular weight during photolysis was investigated. Figure 7 shows the relative changes in the average molecular weight () for PS films (40 μm thickness) in the presence and absence of additives 1–4 (0.5% by weight). The decrease in for the PS films was sharp during the first 50 h and less noticeable thereafter. Photodegradation of PS films leads to a reduction in viscosity due to the formation of degraded polymeric chains of low molecular weight [38]. All additives were efficient against the photodegradation of PS films, compared to the PS films (blank) without additives. Compound 1 was once again the most efficient at photostabilizing the PS films.
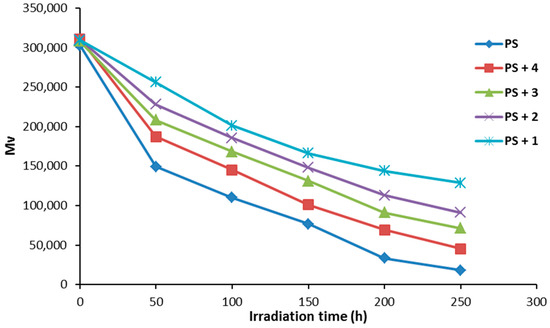
Figure 7.
The effect of irradiation on the viscosity average molecular weight for PS films.
The measurements of the initial viscosity average molecular weight () and a specific irradiation time () will allow the calculation of the average number of the chain scissions (S) as shown in Equation (1) [38].
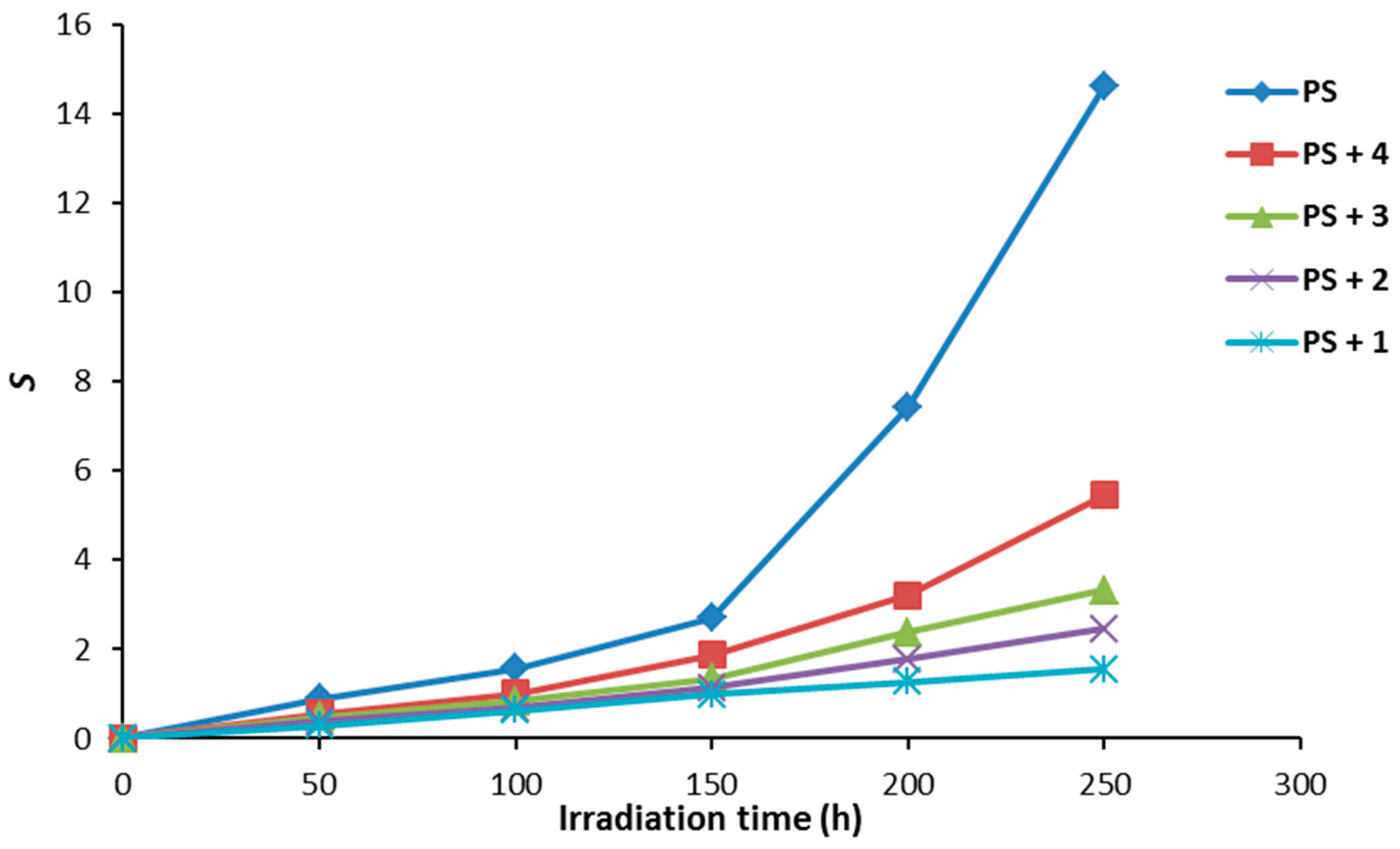
Figure 8 shows the effect of irradiation time on the S values. Irradiation of PS (blank) showed a higher degree cross-linking and/or branching compared to the PS films in the presence of additives 1–4. There was a sharp increase in the value of S for control PS between 150 and 250 h. Also, significant insoluble residues being formed during the irradiation process are an indication for polymeric chains crosslinking. On the other hand, the increase in the S value for PS containing Schiff base 1 was negligible compared to the others and low proportion of insoluble polymeric materials were observed.
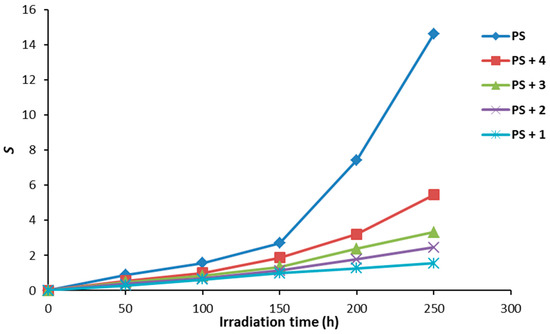
Figure 8.
The effect of irradiation on the main chain scissions (S) for PS films.
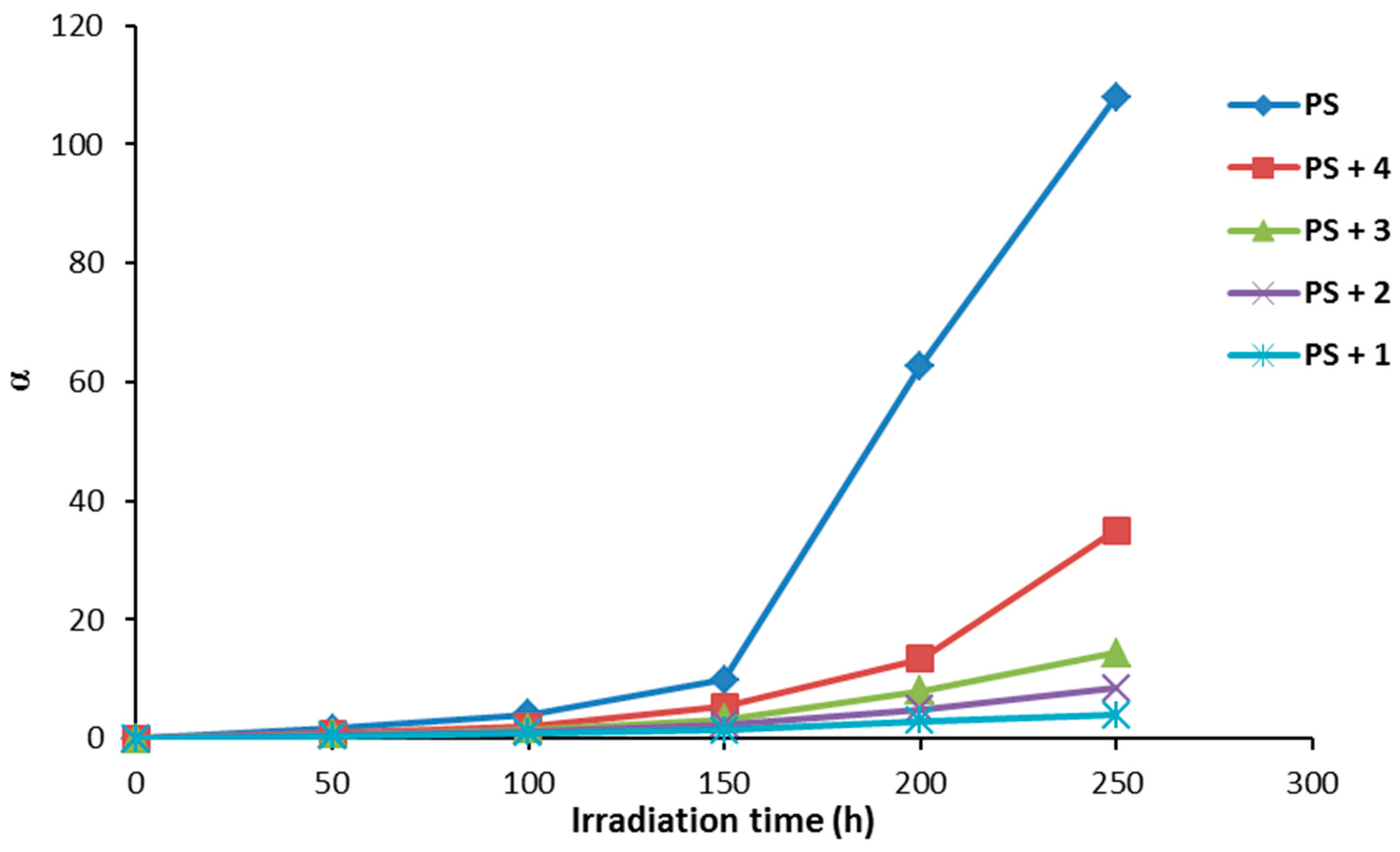
The degree of deterioration (α) for PS films provides a measure for the rapid break of randomly distributed weak bonds at the initial stage of photodegradation. Equation (2) can be used to calculate the α value that depends on the viscosity average molecular weight (), chain scissions (S), and molecular weight (m).
Figure 9 shows the effect of irradiation time on the degree of deterioration (α). The α values were very high for the PS films (blank) on irradiation compared to the samples containing additives 1–4. There was a sharp increase in the value of α when the irradiation time was between 150–250 h. The α values for PS films containing additives were very low compared to PS (blank). The α values were minimal for PS films in the presence of Schiff base 1 under irradiation.
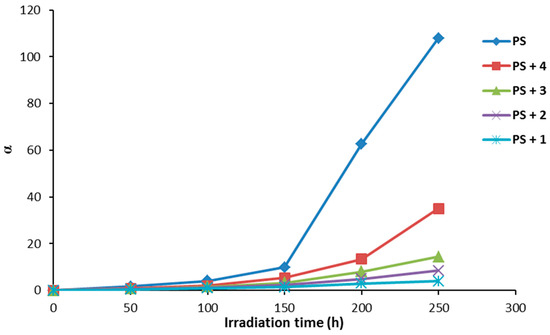
Figure 9.
The effect of irradiation on the degree of deterioration (α) for PS films
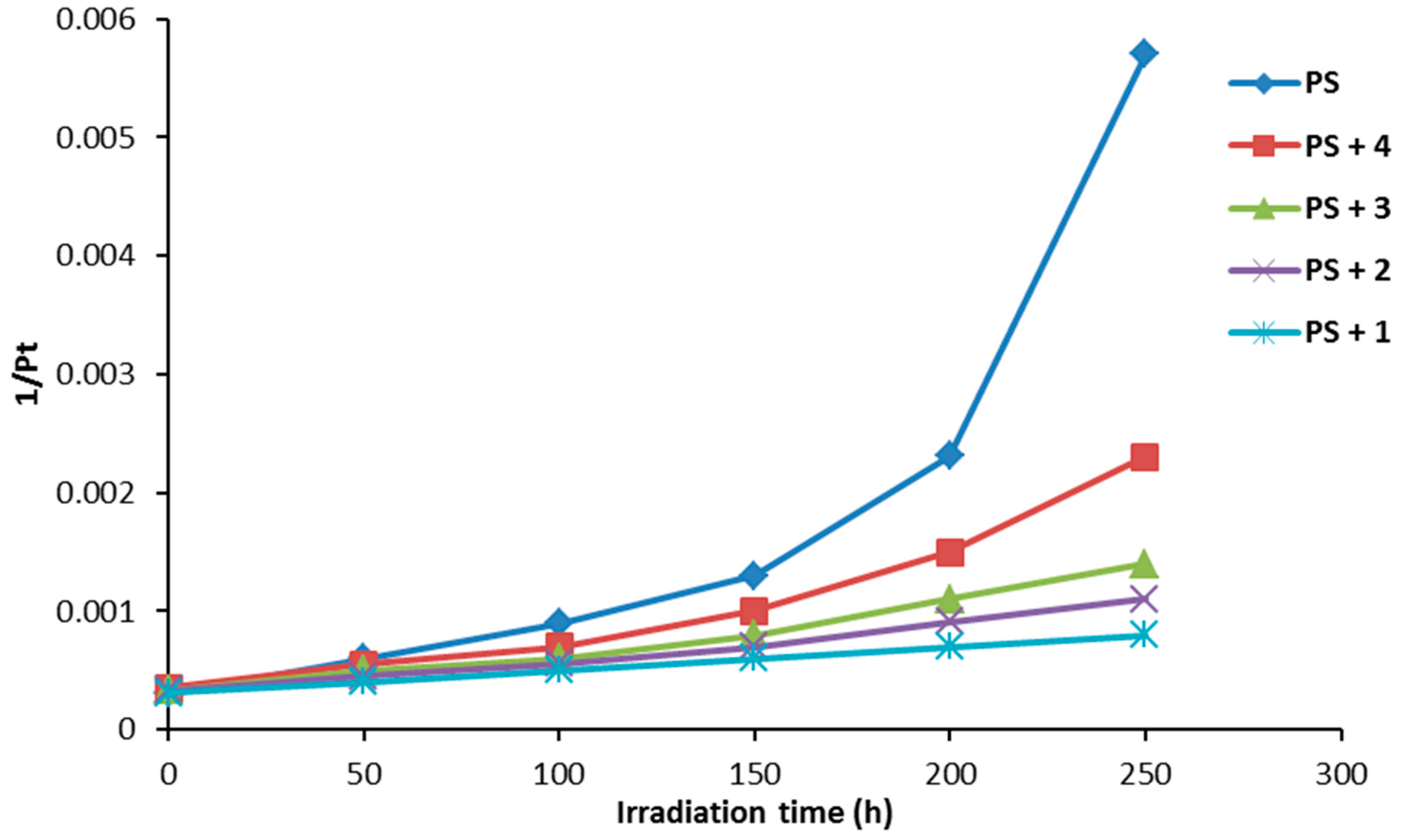
The degree of polymerization (DP) is the number of monomeric units in a homopolymer. It can be calculated using Equation (3) based on the average molecular weight (Mn) and molecular weight of the monomer unit (M0) [38,39].
Figure 10 shows the effect of irradiation on the reciprocal degree of polymerization (1/Pt). The curve indicates a sharp increase in 1/Pt with irradiation time for PS film (blank) compared to the ones obtained in the presence of additives 1–4. The changes in 1/Pt were very low when compound 1 (0.5% by weight) was mixed with the PS.
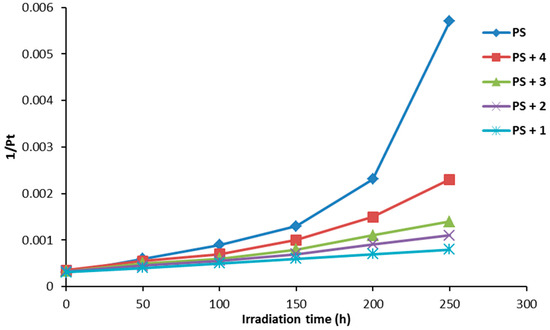
Figure 10.
The effect of irradiation on the average degree of polymerization (1/Pt) for PS films.
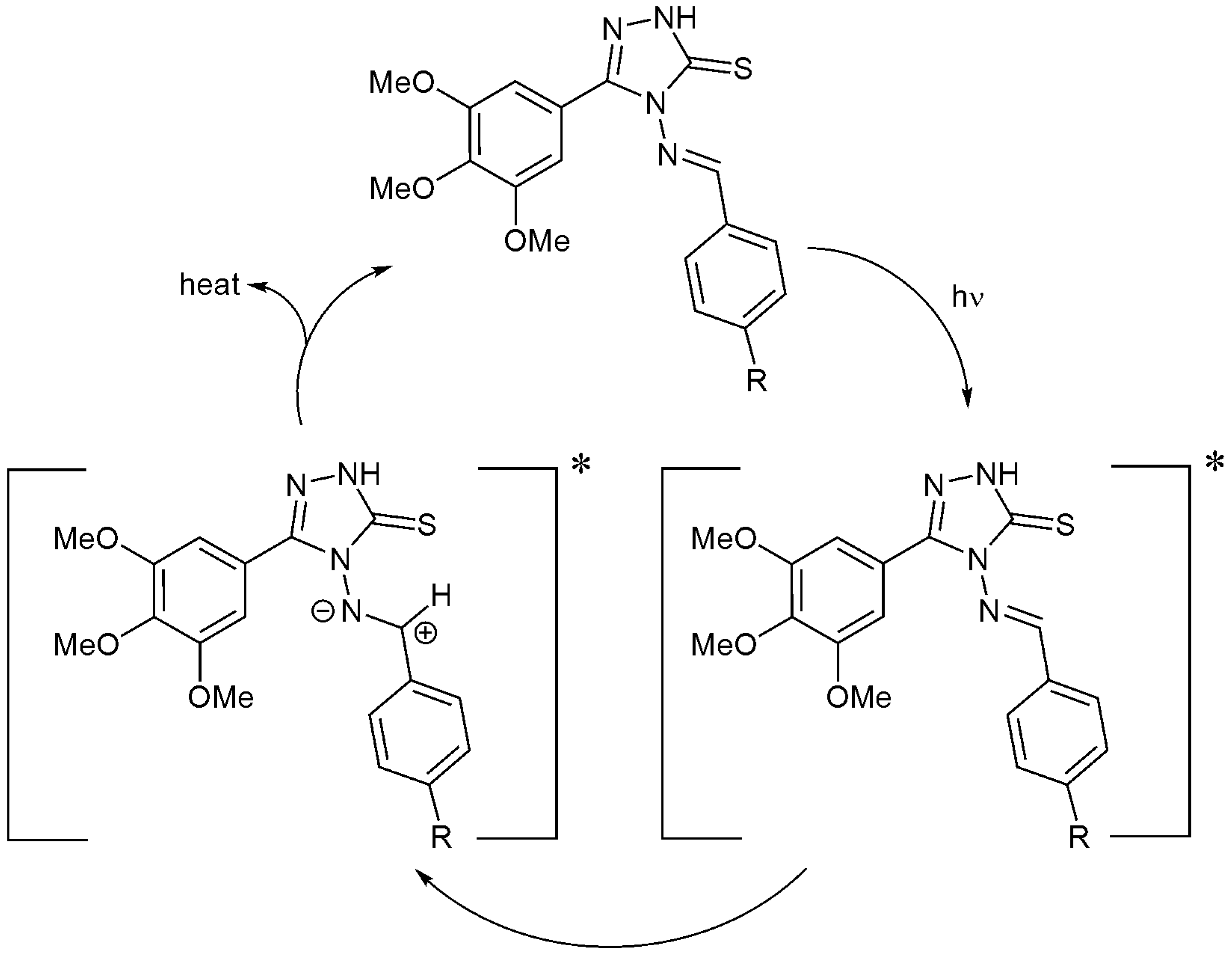
2.6. Photostabilization of Polystyrene Films Suggested Mechanisms
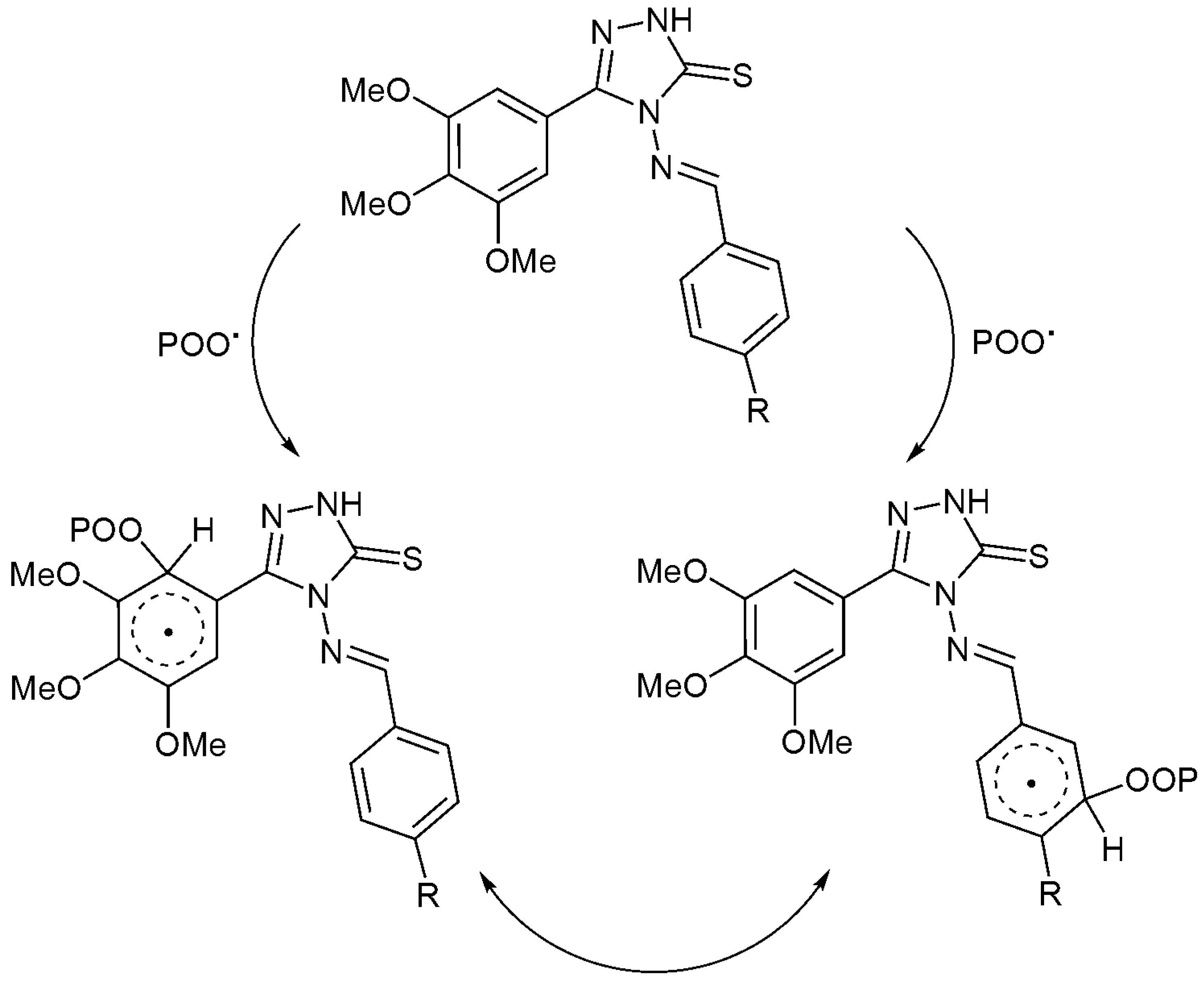
Schiff bases contain heterocycles and aryl moieties, in the presence of a chromophore (POO·), that could stabilize the PS samples by acting as radical scavengers. A complexation between the additive and the chromophore could be achieved in an excited state in which the energy can be transferred. The resonance within the aryl moieties could stabilize the unreactive charge transfer complexes to a level that is harmless to the polymeric chains (Scheme 2).
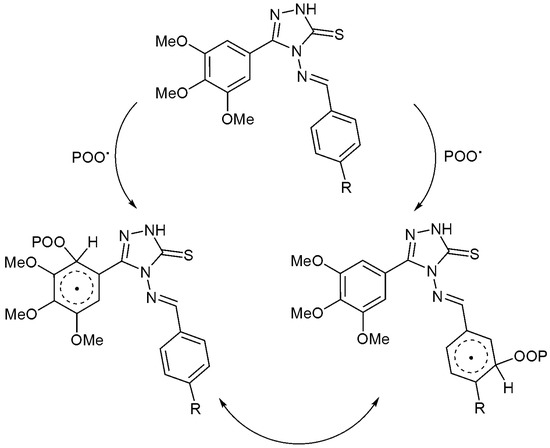
Scheme 2.
Additives 1–4 act as radical scavengers.
Heterocyclic and aryl moieties of the additives embedded within the polystyrene polymeric chains can absorb UV light directly [8]. Schiff bases 1–4 have both aryl and triazole ring systems that can act as UV absorbers. They can absorb the harmful UV light and convert it into heat that is harmless to polystyrene (Scheme 3).
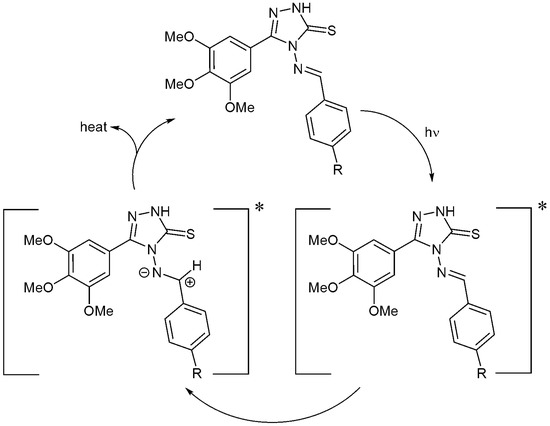
Scheme 3.
Additives 1–4 act as UV absorbers.
3. Experimental Section
3.1. General
Chemicals and reagents were obtained from BDH Chemicals (Poole, UK) and Sigma-Aldrich Chemical Company (Gillingham, UK). Melting points were recorded on a hot stage Gallenkamp melting point apparatus. The 1H-NMR spectra (300 MHz) were recorded on Bruker Ultrashield (Bruker, Coventry, UK) in DMSO-d6 with tetramethylsilane as an internal standard.
3.2. Synthesis of Schiff Bases 1–4
A mixture of 4-amino-5-(3,4,5-trimethoxiyphenyl)-1,2,4-triazole-2-thiol [40] (0.2 g, 0.7 mmol) and appropriate aromatic aldehyde (0.07 mmol) in absolute ethanol (10 mL) containing one drop of concentrated hydrochloric acid was refluxed for 4 h. The solid obtained on cooling was filtered, washed with hot ethanol, and dried to give Schiff bases 1–4 in 68%–79% yields.
3.3. Films Preparation
A mixture of polystyrene and Schiff bases was dissolved in chloroform and the films were prepared using the evaporation technique at 25 °C. A Digital Vernier Caliper 2610A micrometer (Vogel GmbH, Kevelaer, Germany) was used to fix the thickness of PS films as 40 μm.
3.4. Accelerated Testing Technique
The PS films were irradiated with UV light (λmax = 365 nm and light intensity = 6.43 × 10−9 ein·dm−3·s−1) at room temperature using an accelerated weather-meter QUV tester (Philips, Saarbrücken, Germany).
3.5. Photodegradation of PS Films by IR Spectrophotometer
The FTIR spectra (4000–400 cm−1) were recorded on FTIR 8300 Spectrophotometer (Shimadzu, Tokyo, Japan) for the PS films. The carbonyl and hydroxyl group indices (Is) can be calculated using Equation (4) [41]. The value of Is depends on the peak absorbance (As) of C=O or OH group and the reference peak absorbance (Ar) at 1328 cm−1.
3.6. Measuring the Photodegradation by Weight Loss
The weight loss percentage of PS films in photodegradation process was calculated using from the weight of PS sample before (W1) and after irradiation (W2) using Equation (5) [42].
3.7. Photodegradation of PS Films by Viscometery Method
The average molecular weight () of PS films was measured using Mark–Houwink relation, Equation (6), in which α and K are constants [41,43]. is directly proportional to the intrinsic viscosity, [η], of PS film.
Also, the average molecular weight () can be calculated using Equation (7).
4. Conclusions
Four Schiff bases with 4H-1,2,4-triazole-3-thiol ring systems have been synthesized and characterized. Polystyrene films containing Schiff bases at a concentration of 0.5% were found to more stable on irradiation compared to polystyrene without the additives. Such additives can be used for long-term polystyrene photostabilization. The additives could act as UV absorbers and/or radical scavengers.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for its funding for this research through the research group project RGP-239 and to Al-Mustansiriyah and Al-Nahrain Universities for continued support.
Author Contributions
Emad Yousif conceived and designed the experiments. Gassan Q. Ali, Ivan Hameed R. Tomi, Raghad Haddad, and Alaa J. Al-Qaisi performed the experiments and analyzed the data. Gamal A. El-Hiti and Emad Yousif wrote the paper. Gamal A. El-Hiti provided the funds and revised the paper. All the authors discussed the results and improved the final text of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wierckx, N.; Prieto, M.A.; Pomposiello, P.; de Lorenzo, V.; O’Connor, K.; Blank, L.M. Plastic waste as a novel substrate for industrial biotechnology. Microb. Biotechnol. 2015, 8, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Wünsch, J.R. Polystyrene: Synthesis, Production and Applications; Rapra Technology Ltd.: Shawbury, Shrewsbury, Shropshire, UK, 2000. [Google Scholar]
- Maul, J.; Frushour, B.G.; Kontoff, J.R.; Eichenauer, H.; Ott, K.-H.; Schade, C. Polystyrene and Styrene Copolymers. In Ullmann's Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- De Rosa, C.; Auriemma, F. Structure and physical properties of syndiotactic polypropylene: A highly crystalline thermoplastic elastomer. Prog. Polym. Sci. 2006, 31, 145–237. [Google Scholar]
- Rabek, T.F. Photodegradation of Polymers: Physical Characteristics and Applications; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- White, J.R.; Turnbull, A. Weathering of polymers: mechanisms of degradation and stabilization: Testing strategies and modelling. J. Mater. Sci. 1994, 29, 584–613. [Google Scholar] [CrossRef]
- Hernández, C.G.; González, R.; Soto, J.J.; Rosales, I. Photo-Oxidation of Polystyrene Film Irradiated with UV-B; Springer: Gewerbestasse, Switzerland, 2016. [Google Scholar]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Titow, W.V. PVC Technology, 4th ed.; Springer: Essex, UK, 1984. [Google Scholar]
- Sastre, R.; Catalina, F.; Mateo, J.L.; Claramunt, R.; Santa-Maria, M.D.; Catalán, J. Mechanism of photostabilization of polystyrene film by dihydroxyphenyl-pirazoles. J. Polym. Sci. A Polym. Chem. 1990, 28, 3661–3668. [Google Scholar] [CrossRef]
- Haddad, R.; Yousif, E.; Yusop, R.M. Ultra violet spectra studies of polystyrene films in presence of some transition metal complexes with 4-amino-5-pyridyl)-4H-1,2,4-triazole-3-thiol. Oriental J. Chem. 2014, 30, 1565–1569. [Google Scholar] [CrossRef]
- Goldshtein, J.; Margel, S. Synthesis and characterization of polystyrene/2-(5-chloro-2H-benzotriazole-2-yl)-6-(1,1-dimethylethyl)-4-methyl-phenol composite microspheres of narrow size distribution for UV irradiation protection. Colloid Polym. Sci. 2011, 289, 1863–1874. [Google Scholar] [CrossRef]
- Yousif, E.; Salimon, J.; Salih, N. New stabilizer for polystyrene based on 2-N-salicylidene-5-(substituted)-1,3,4-thiadiazole compounds. J. Saudi Chem. Soc. 2011, 16, 299–306. [Google Scholar] [CrossRef]
- Abd, M.A.; Zahra, A.A.I.A.; Shenta, A.A. Photostabilization of polystyrene films by anthraquinones derivatives and their complexes with copper(II), oxovanadium(IV) and nickel(II) ions. J. Basrah Res. 2009, 35, 81–97. [Google Scholar]
- Sabaa, M.W.; Oraby, E.H.; Naby, A.S.; Mohammed, R.R. Anthraquinone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photo-degradation. Eur. Polym. J. 2005, 41, 2530–2543. [Google Scholar] [CrossRef]
- Tomohito, K.; Masahiko, O.; Guido, G.; Tadaaki, M.; Toshiaki, Y. Antibacterial effect of thiocyanate substituted poly (vinyl chloride). J. Polym. Res. 2011, 18, 945–947. [Google Scholar]
- Folarin, O.M.; Sadiku, E.R. Thermal stabilizers for poly(vinyl chloride): A review. Int. J. Phys. Sci. 2011, 6, 4323–4330. [Google Scholar]
- Chen, X.; Li, C.; Zhang, L.; Xu, S.; Zhou, Q.; Zhu, Y.; Qu, X. Main factors in preparation of antibacterial particles/PVC composite. China Particuol. 2004, 2, 226–229. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, C.; Pavlinek, V.; Saha, P.; Wang, H. Surface-modified antibacterial TiO2/Ag+ nanoparticles: Preparation and properties. Appl. Surf. Sci. 2006, 252, 4154–4160. [Google Scholar] [CrossRef]
- Mohammed, R.; El-Hiti, G.A.; Ahmed, A.; Yousif, E. Poly(vinyl chloride) doped by 2-(4-isobutylphenyl)propanoate metal complexes: enhanced resistance to UV irradiation. Arab J. Sci. Eng. 2016. [Google Scholar] [CrossRef]
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Hussain, Z.; Altaie, A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in the presence of sulfamethoxazole Schiff’s bases. Polymers 2015, 7, 2190–2204. [Google Scholar] [CrossRef]
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride). Int. J. Polym. Sci. 2015. [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Haddad, R.; Balakit, A.A. Photochemical stability and photostabilizing efficiency of poly(methyl methacrylate) based on 2-(6-methoxynaphthalen-2-yl)propanoate metal ion complexes. Polymers 2015, 7, 1005–1019. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Screening and evaluation of poly(3-hydroxybutyrate) with Rhodococcus equi using different carbon sources. Arab J. Sci. Eng. 2016. [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus 2016. [CrossRef] [PubMed]
- Smith, K.; Al-Zuhairi, A.J.; El-Hiti, G.A.; Alshammari, M.B. Comparison of cyclic and polymeric disulfides as catalysts for the regioselective chlorination of phenols. J. Sulfur Chem. 2015, 36, 74–85. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; El-Hiti, G.A. Poly(propylene sulfide)-borane: Convenient and versatile reagent for organic synthesis. Tetrahedron 2012, 68, 7834–7839. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; Pardasani, R.T.; El-Hiti, G.A. New polymeric sulfide-borane complexes: Convenient hydroborating and reducing reagents. J. Sulfur Chem. 2011, 32, 287–295. [Google Scholar] [CrossRef]
- Sumangala, V.; Poojary, B.; Chidananda, N.; Arulmoli, T.; Shenoy, S. Synthesis and biological evaluation of some Schiff bases of 4-amino-5-(4-methylsulfonyl)benzyl-2,4-dihydro-3H-[1,2,4]-triazole-3-thione. Med. Chem. Res. 2013, 22, 2921–2928. [Google Scholar] [CrossRef]
- Kiatkamjornwong, S.; Sonsuk, M.; Wittayapichet, S.; Prasassarakich, P.; Vejjanukroh, P.-C. Degradation of styrene-g-cassava starch filled polystyrene plastics. Polym. Degrad. Stab. 1999, 66, 323–334. [Google Scholar] [CrossRef]
- Rabek, J.F. Polymer Photodegradation: Mechanisms and Experimental Methods; Chapman and Hall: London, UK, 1995. [Google Scholar]
- Mori, F.; Koyama, M.; Oki, Y. Studies on photodegradation of poly(vinyl chloride), part 3. Macromol. Mater. Eng. 1979, 75, 113–122. [Google Scholar]
- Pinto, L.F.A.; Goi, B.E.; Schmitt, C.C.; Neumann, M.G. Photodegradation of polystyrene films containing UV-visible sensitizers. J. Res. Updates Polym. Sci. 2013, 2, 39–47. [Google Scholar]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley and Sons, Ltd.: Chichester, UK, 2004. [Google Scholar]
- Rabie, S.T.; Ahmed, A.E.; Sabaa, M.W.; Abd El-Ghaffar, M.A. Maleic diamides as photostabilizers for polystyrene. J. Ind. Eng. Chem. 2013, 19, 1869–1878. [Google Scholar] [CrossRef]
- Jellinek, H.H.G. Aspects of Degradation and Stabilization of Polymers; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Allcock, H.R.; Lamp, F.W.; Mark, J.E. Contemporary Polymer Chemistry, 3rd ed.; Pearson Prentice-Hall: Upper Saddle River, NJ, USA, 2003; pp. 316–325. [Google Scholar]
- Ameri, A.; Khodarahmi, G.; Hassanzadeh, F.; Forootanfar, H.; Hakimelahi, H. Novel aldimine-type Schiff bases of 4-amino-5-[(3,4,5-trimethoxyphenyl)methyl]-1,2,4-triazole-3-thione/thiol: Docking study, synthesis, biological Evaluation, and anti-tubulin activity. Arch. Pharm. Chem. Life Sci. 2016, 349, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Rabek, J.; Ranby, B. Photodegradation, Photo-oxidation and Photostabilization of Polymers; John Wiley: New York, NY, USA, 1975. [Google Scholar]
- Sabaa, M.W.; Oraby, E.H.; Naby, A.S.; Mohamed, R.R. N-Phenyl-3-substituted-5-pyrazolone derivatives as organic stabilizer for rigid PVC against photodegradation. J. Appl. Polym. Sci. 2005, 101, 1543–1555. [Google Scholar] [CrossRef]
- Mark, J. Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007. [Google Scholar]
- Sample Availability: Samples from the Schiff bases are not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).