Abstract
Objective: To screen for influenza virus neuraminidase inhibition and to provide a reference for the clinical treatment of influenza using traditional Chinese medicines (TCM). In this study, 421 crude extracts (solubilized with petroleum ether, ethanol, ethyl acetate, and aqueous solvents) were obtained from 113 TCM. The medicine extracts were then reacted with oseltamivir, using 2’-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (MUNANA) as the substrate, to determine influenza virus neuraminidase activity using a standard fluorimetric assay. It was found that Chinese medicine extracts from Pyrola calliantha, Cynanchum wilfordii, Balanophora involucrata and Paeonia delavayi significantly inhibited neuraminidase activity at a concentration of 40 μg/mL. Dose-dependent inhibitory assays also revealed significant inhibition. The IC50 range of the TCM extracts for influenza virus neuraminidase was approximately 12.66–34.85 μg/mL, respectively. Some Chinese medicines have clear anti-influenza viral effects that may play an important role in the treatment of influenza through the inhibition of viral neuraminidase. The results of this study demonstrated that plant medicines can serve as a useful source of neuraminidase (NA) inhibitors and further investigation into the pharmacologic activities of these extracts is warranted.
1. Introduction
Influenza (flu) is an infectious disease that seriously affects human life and health [1,2]. According to the World Health Organization (WHO) statistics, influenza annually causes an estimated 250,000–500,000 deaths and approximately three to five million cases of severe illness worldwide. Influenza poses a range of serious threats to public health by inducing substantial economic losses and social problems throughout the world [3,4].
Influenza A viruses, including the H5N1, H3N2 and H1N1 subtypes, pose a potential pandemic threat to public health [1]. According to World Health Organization (WHO) statistics, as of January 2014, there have been a total of 650 confirmed human cases of H5N1 virus, with 386 deaths (59% mortality rate) in 15 countries since 2003 [5].
At present, there are two available classes of anti-influenza viral drugs: NA inhibitors (oseltamivir, zanamivir, peramivir and laninamivir) and M2 ion channel inhibitors (amantadine and rimantadine) [6]. NA inhibitors were developed because of the genetic stability of the influenza virus active NA enzymatic center [7]. NA is an influenza virus surface glycoprotein that is recognized as an attractive target for the development of antiviral drugs [8,9]. Currently, neuraminidase inhibitors (NAIs) are in wide use for the treatment of influenza [10]. However, the efficacy of these drugs has declined due to viral mutations conferring resistance to some NAIs [11]. Because of this challenge, many researchers are now focused on the development of new anti-influenza treatments or combination therapies to enhance the efficacy of anti-influenza drugs [12,13].
Although synthetic NAIs, such as seltamivir and zanamivir, have been designed to halt viral replication, adverse side effects, such as nausea, vomiting, diarrhea, abdominal pain, have been observed [14,15]. Hence, naturally existing NAIs have attracted considerable interest for treating influenza [16,17]. Additionally, compound indigowoad root granules and ginseng polysaccharides have been recognized as antiviral agents with activity against the influenza virus [9]. Many Chinese traditional patent medicines, such as Shuanghuanglian oral liquid, Qingkailing oral liquid, Qingre Jiedu oral liquid and Reduning injection, have also displayed relatively high NA inhibitory activities.
In this study, 421 crude extracts (solubilized with petroleum ether, ethanol, ethyl acetate, and aqueous solvents) were obtained from 113 traditional Chinese medicines. Some plant medicines have clear anti-influenza viral effects. The results of this study will provide important information for the isolation of active constituents and for the clinical use of TCM for treating and preventing influenza.
2. Materials and Methods
2.1. Plant Materials
All TCM were collected from Yun Nan and Si Chuan provinces by Professor Linfang Huang. The identities of all samples were authenticated by Professor Yulin Li. The selected specimens were deposited in the herbarium of the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences.
2.2. Chemicals
Chemicals used included 2’-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (MUNANA, Sigma, St. Louis, MO, USA), MES, (Sigma, St. Louis, MO, USA), CaCl2, NaOH, absolute ethyl alcohol (pure analytical grade), and other chemicals, all of which were of extra pure analytical grade.
2.3. Plant Extraction
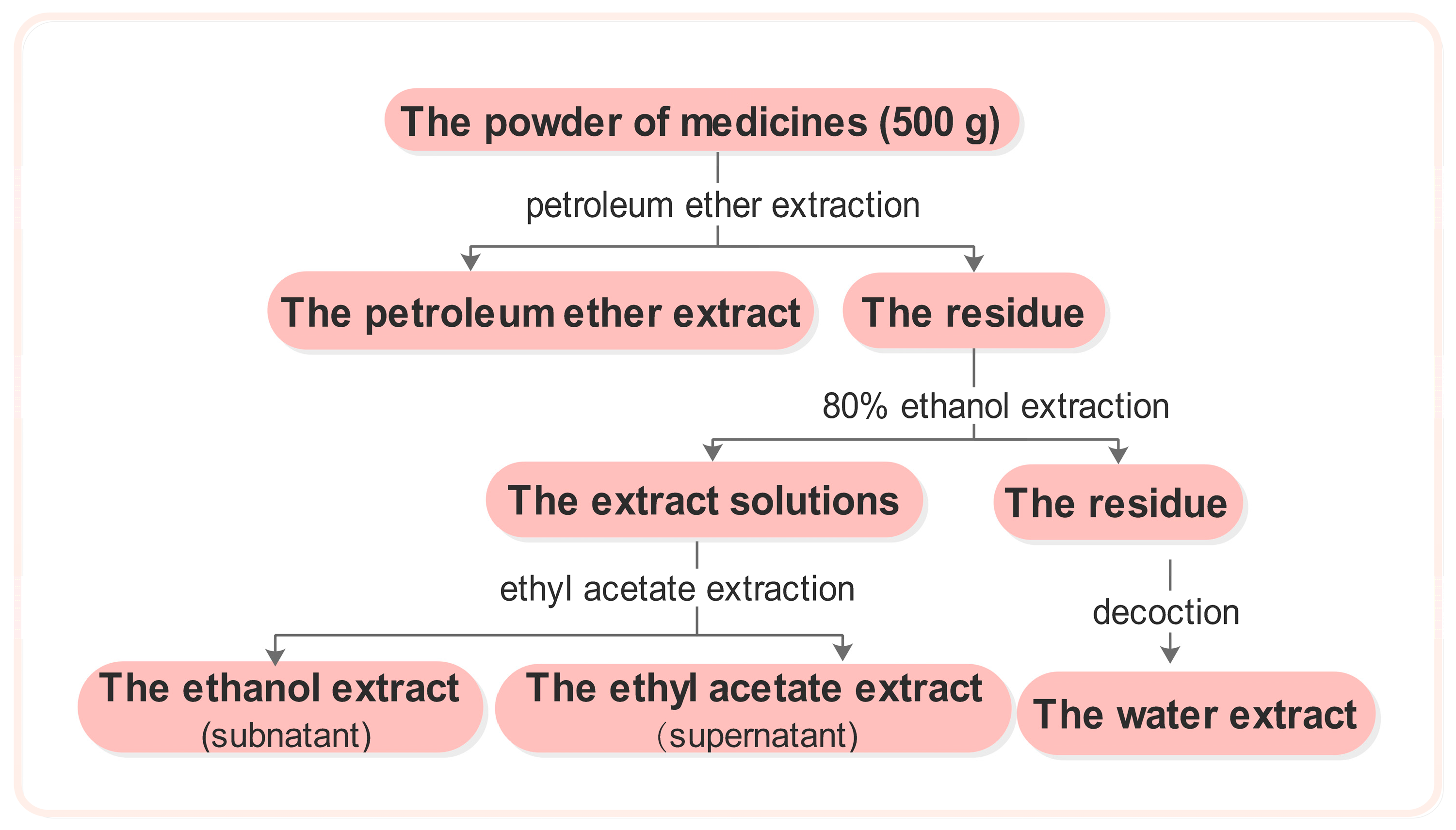
The medicinal plant material was crushed into coarse powder. Five hundred grams of powder was soaked in petroleum ether for 24 h, after which a percolation extraction was performed. The filter was retrieved and the petroleum ether was evaporated. The residue was washed with 80% ethanol and subjected twice to reflux extraction with triple the volume of 80% ethanol. The extract solutions were then combined and ethanol was reclaimed at reduced pressures until no alcohol was detected. Extraction was then performed twice with an equal volume of ethyl acetate. The upper solution was then extracted and concentrated to obtain the ethyl acetate extract, whereas the lower solution was concentrated to dryness to yield the ethanol extract. The residue was evaporated to dryness and was then extracted twice with an amount of water equal to triple the mass of the materials. The aqueous extract solutions were combined and concentrated to dryness, and the water extract was then obtained (Figure 1).
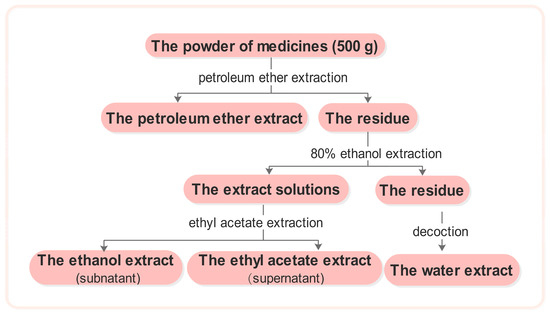
Figure 1.
The extract flow chart of the 113 traditional Chinese medicines.
2.4. Neuraminidase Inhibition Assay
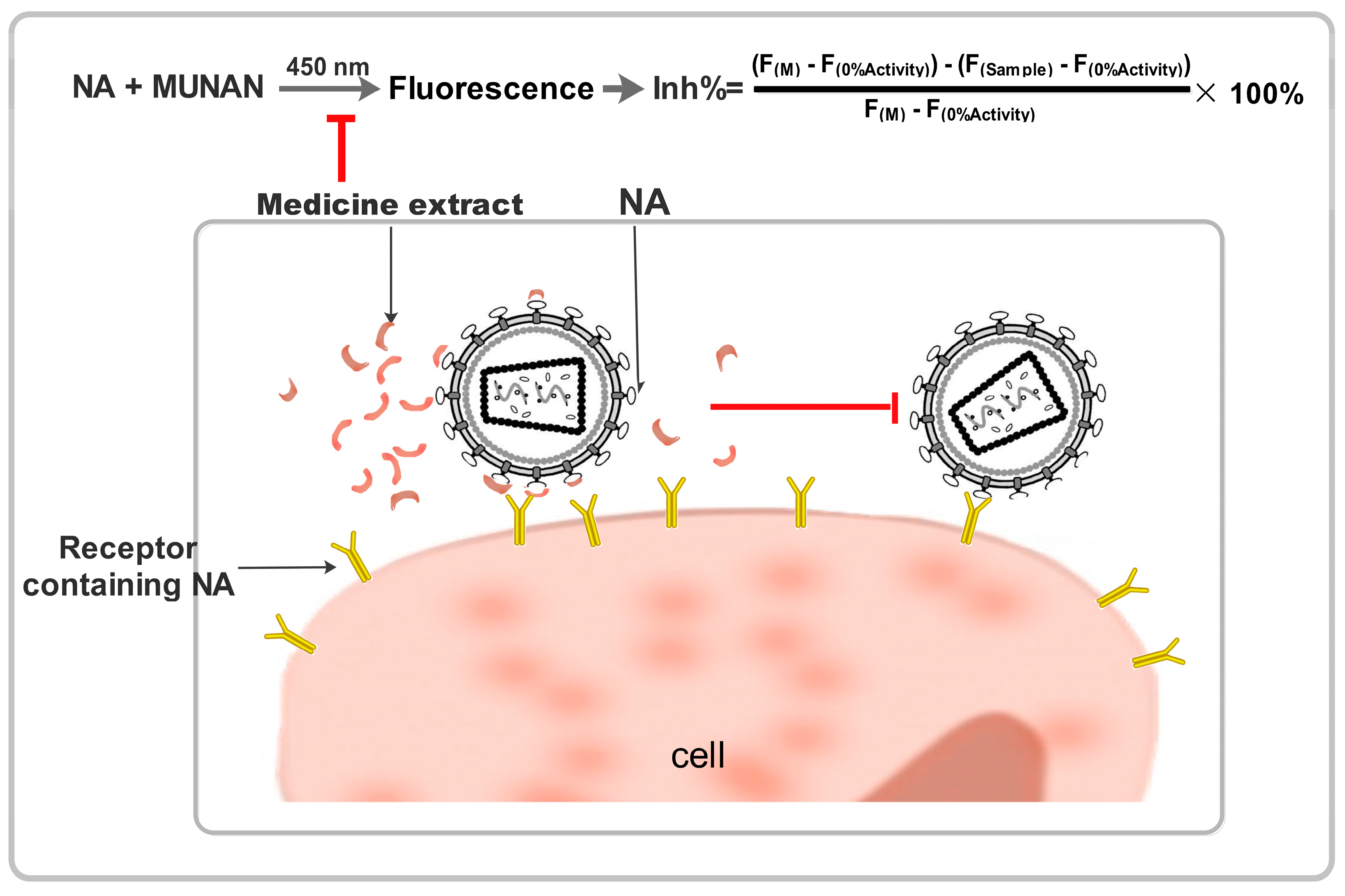
The substrate 2’-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (MUNANA) was combined with oseltamivir or traditional Chinese medicine extracts to examine influenza virus NA activity using a standard fluorimetric assay. In this assay, the substrate and NA reacted to yield a fluorescent product that could be quantified [6,18] (Figure 2).
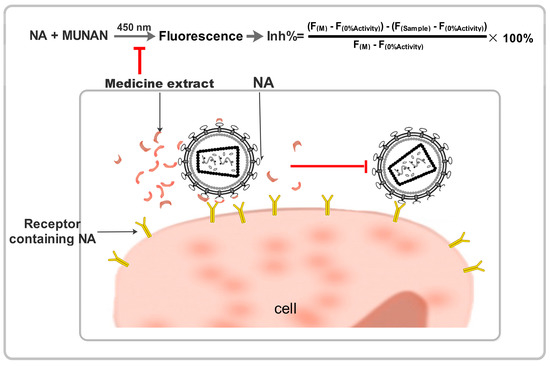
Figure 2.
Schematic diagrams showing the neuraminidase inhibiting effect of Chinese medicine.
The reaction mixture containing test extract compounds and either NA enzyme or a viral suspension in 33 mM MES buffer and 4 mM calcium chloride (pH 6.5) was incubated for 40 min at 37 °C. After incubation, the reaction was terminated by adding 34 mM NaOH. Fluorescence was quantified at an excitation wavelength of 360 nm and an emission wavelength of 450 nm. The 50% inhibitory concentration (IC50) was defined as the concentration of NA inhibitor necessary to reduce NA activity by 50% relative to a reaction mixture containing virus but no inhibitor. The data were expressed as the mean of six independent experiments.
3. Results and Discussion
The inhibitory activities on NA for the TCM species examined were evaluated and the percentage inhibitions are shown in Table 1.

Table 1.
Effects of selected traditional Chinese medicines (extracts) on inhibition of NA.
Four extracts using petroleum ether, ethyl acetate, ethanol and aqueous extracts were prepared from each of the 113 dried medicines. The TCM extracts were analyzed for NA inhibitory activity. Twenty-six of the extracts (from Citrus reticulata Blanco, Angelica pubescens and Radix Anemones Rivularis species) were found to promote NA activity, whereas 395 extracts showed different degrees of NA inhibitory activity. Twenty-six extracts were found to inhibit NA by greater than 50%, including the 11 ethanol extracts of Curcuma longa L., Rhus chinensis Mill., Fagopyrum dibotrys and Fagopyrum dibotrys species. Furthermore, the 12 ethyl acetate extracts of Balanophora involucrata, Balanophora involucrata, Paeonia delavayi Franch, and Cynanchum wilfordii (Maxim.) Hemsl.; the three petroleum ether extracts of Carthamus tinctorius L., Fagopyrum dibotrys, Polygonum aubertii Henry; and the three aqueous extracts of Cynanchum wilfordii, Paeonia delavayi Franch and Rhus chinensis Mill. exhibited significant NA inhibition at 40 μg/mL.
The dose-dependent NA inhibitory activities of 10 medicines that exhibited the most NA inhibition were studied further. The IC50 inhibition values are presented in Table 2. Among these 10 TCM, the most potent NA inhibition was exhibited by the ethyl acetate extract of Paeonia delavayi Franch (IC50 = 12.66 μg/mL).

Table 2.
IC50 values for NA inhibitors of the petroleum ether, ethanol, ethyl acetate, and aqueous extracts from 10 traditional Chinese medicines.
Influenza is a serious threat to human health. Thus, there is an urgent need to develop anti-influenza drugs. Some herbal medicines are used as a treatment for influenza. Traditional Chinese medicines may have an important role in the research and development of new drugs for influenza treatment. Screening for bioactive compounds from medicinal plants is an important strategy. NAIs from TCM are important resources for potential therapeutic agents directed against influenza.
This paper evaluated the in vitro activity of commonly used TCM against influenza virus neuraminidase. Here, we screened novel NAI extracted from 113 medicines using a fluorimetric assay. These results suggest that Rhus chinensis and Paeonia delavayi offer great potential for the treatment of influenza. Most of the ethyl acetate extracts showed strong NA inhibitory activities. This is the first time that medicine extracts have been tested on a large scale for their ability to inhibit NA. In addition, the 10 TCM that exhibited the most NAI in this study have not been traditionally used to treat influenza. Among these 10 medicine extracts, the Paeonia delavayi ethyl acetate extracts were the most potent in the NAI assays.
According to the Chinese pharmacopoeia (2015, [19]) and other references, all 10 TCM have the effects of heat-clearing and detoxification. It is believed that heat-clearing and detoxification are connected with eliminating the virus, while the support of healthy energy is concerned with enhancing immunity. Influenza is treated by drugs to relieve the ‘exterior syndrome’, and heat-clearing drugs are used as antibiotics [15].
Interestingly, some medicines (Isatis indigotica, Forsythia suspensa, Lonicera japonica and Scutellaria baicalensis) that have traditionally been prescribed to treat influenza were found to have low anti-NA activity at 40 μg/mL. The inhibition by Isatis indigotica was less than 5%. The data indicated that the anti-influenza effect of this medicine is not influenced by the effect of inhibiting NA.
4. Conclusions
The results of this study indicate that many plant medicines offer great potential for the treatment of influenza. The full therapeutic range of traditional Chinese medicines has been relatively unexplored. The results of this report warrant further investigation of TCM extracts for potential therapeutic agents to use in the treatment of influenza. The anti-influenza activity of NAIs has been well established by numerous in vitro and in vivo studies. However, there is scarcity in the volume of the cell experiments and in vivo studies undertaken to explore these TCM potentials for anti-influenza activity. In the future, we will make an effort to identify the bioactive components of the extracts and explore the antiviral activity of these compounds with in vivo and in vitro experiments.
Acknowledgments
The study was supported by grants from the National Natural Science Foundation of China (No. 81274013 and No. 81473315), and the Key National Natural Science Foundation of China (No. 81130069).
Author Contributions
Lin-Fang Huang and Xiao-wei Xu conceived and designed the experiments; Ai-lin Liu and Xian-Ying Yang performed the experiments; Ai-lin Liu and Xian-Ying Yang. analyzed the data; Ai-lin Liu and Shu-jing Liu contributed reagents/materials/analysis tools; Lin-Fang Huang and Xian-Ying Yang wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lei, H.; Peng, X.; Zhao, D.; Ouyang, J.; Jiao, H.; Shu, H.; Ge, X. Lactococcus lactis displayed neuraminidase confers cross protective immunity against influenza A viruses in mice. Virology 2015, 476, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.A.; Dao, T.T.; Tung, B.T.; Choi, H.; Kim, E.; Park, J.; Lim, S.I.; Oh, W.K. Influenza A (H1N1) neuraminidase inhibitors from Vitis amurensis. Food Chem. 2011, 124, 437–443. [Google Scholar] [CrossRef]
- AbdelGhafar, A.N.; Chotpitayasunondh, T.; Gao, Z.; Hayden, F.G.; Hien, N.D.; de Jong, M.D.; Naghdaliyev, A.; Peiris, J.; Shindo, N.; Soeroso, S. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 2008, 358, 261–273. [Google Scholar]
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl. J. Med. 2009, 2009, 2605–2615. [Google Scholar]
- Shigemori, T.; Nagayama, M.; Yamada, J.; Miura, N.; Yongkiettrakul, S.; Kuroda, K.; Katsuragi, T.; Ueda, M. Construction of a convenient system for easily screening inhibitors of mutated influenza virus neuraminidases. FEBS Open Bio. 2013, 3, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Moscona, A. Neuraminidase inhibitors for influenza. N. Eng. J. Med. 2005, 353, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Colman, P.M. Influenza virus neuraminidase: Structure, antibodies, and inhibitors. Protein Sci. 1994, 3, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Gubareva, L.V.; Kaiser, L.; Hayden, F.G. Influenza virus neuraminidase inhibitors. Lancet 2000, 355, 827–835. [Google Scholar] [CrossRef]
- Kubo, S.; Tomozawa, T.; Kakuta, M.; Tokumitsu, A.; Yamashita, M. Laninamivir prodrug CS-8958, a long-acting neuraminidase inhibitor, shows superior anti-influenza virus activity after a single administration. Antimicrob. Agents Chemother. 2010, 54, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Jones, M.; Doshi, P.; del Mar, C. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. Bmj 2009, 339, b5106. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, I.; Democratis, J.; Lackenby, A.; McNally, T.; Smith, J.; Pareek, M.; Ellis, J.; Bermingham, A.; Nicholson, K.; Zambon, M. Neuraminidase inhibitor resistance after oseltamivir treatment of acute influenza A and B in children. Clin. Infect. Dis. 2009, 48, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Kiso, M.; Mitamura, K.; Sakai-Tagawa, Y.; Shiraishi, K.; Kawakami, C.; Kimura, K.; Hayden, F.G.; Sugaya, N.; Kawaoka, Y. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 2004, 364, 759–765. [Google Scholar] [CrossRef]
- Triana-Baltzer, G.B.; Gubareva, L.V.; Klimov, A.I.; Wurtman, D.F.; Moss, R.B.; Hedlund, M.; Larson, J.L.; Belshe, R.B.; Fang, F. Inhibition of neuraminidase inhibitor-resistant influenza virus by DAS181, a novel sialidase fusion protein. PLoS ONE 2009, 4, e7838. [Google Scholar] [CrossRef] [PubMed]
- Sheu, T.G.; Deyde, V.M.; Okomo-Adhiambo, M.; Garten, R.J.; Xu, X.; Bright, R.A.; Butler, E.N.; Wallis, T.R.; Klimov, A.I.; Gubareva, L.V. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob. Agents Chemother. 2008, 52, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, W.; Zhao, A.; Wang, X. Anti-influenza agents from plants and traditional Chinese medicine. Phytother. Res. 2006, 20, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.F.; Fung, C.P.; Perng, D.W.; Wang, F.D. Effects of corticosteroid and neuraminidase inhibitors on survival in patients with respiratory distress induced by influenza virus. J. Microbiol. Immunol. Infect. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.B.; Kim, J.H.; Park, S.J.; Chang, J.S.; Rho, M.C.; Bae, K.H.; Park, K.H.; Lee, W.S. Inhibition of neuraminidase activity by polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorganic Med. Chem. Lett. 2010, 20, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Okomo-Adhiambo, M.; Mishin, V.; Sleeman, K.; Saguar, E.; Guevara, H.; Reisdorf, E.; Griesser, R.; Spackman, K.; Mendenhall, M.; Carlos, M. Standardizing the influenza neuraminidase inhibition assay among United States public health laboratories conducting virological surveillance. Antivir. Res. 2016, 128, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Editorial Committee of Chinese Pharmacopoeia. Chinese Pharmacopoeia; Medical Science and Technology Press: Beijing, China, 2015. [Google Scholar]
- Sample Availability: Samples of crude extracts are available from authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).