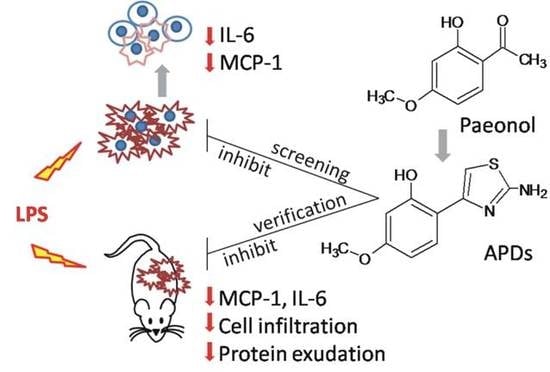
Evaluation of LPS-Induced Acute Lung Injury Attenuation in Rats by Aminothiazole-Paeonol Derivatives
Abstract
:1. Introduction
2. Results
2.1. Synthesis
2.2. MCP-1 and IL-6 Secretion in LPS-Activated A549 Cells
2.3. Cell Viabilities for APDs Treatments
2.4. Comparison of BALF Collected from ALI-Bearing Rats
2.5. Comparison of Compound 4 and Compound 4.HCl by Histopathological Examinations
3. Discussion
4. Materials and Methods
4.1. Chemicals and General Procedures
4.2. Procedure for the Preparation of Morpholine-O-Paeonol (2)
4.3. Procedure for the Preparation of Morpholine-O-Paeonol Aminothiazole (3)
4.4. Procedure for the Preparation of Aminothiazole-Paeonol (4)
4.5. Procedure for the Preparation of Aminothiazole-Paeonol salt (4.HCl)
4.6. Procedures for the Preparation of Aminothiazole-Paeonol Derivatives 5a–5g
4.7. Cell Culture
4.8. Cytotoxicity of APDs
4.9. Preliminary Screening of APDs Against LPS-Activated A549 Cells
4.10. Animal Model of Acute Lung Injury
4.11. Total Cell Counts in BALF
4.12. Total Proteins, IL-6 and MCP-1 in BALF
4.13. Histopathological Examination
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghosh, N.; Ali, A.; Ghosh, R.; Das, S.; Mandal, S.C.; Pal, M. Chronic Inflammatory Diseases: Progress and Prospect with Herbal Medicine. Curr. Pharm. Des. 2016, 22, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Ren, Y.; Wang, Y.; Ma, H.; Xu, J.; Zhou, C.; Yin, Z.; Luo, L. Anti-inflammatory effect of SQC-beta-CD on lipopolysaccharide-induced acute lung injury. J. Ethnopharmacol. 2008, 118, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhu, L.; Wang, J.; He, H.; Chang, X.; Gao, J.; Shumin, W.; Yan, T. Anti-inflammatory effects of water extract of Taraxacum mongolicum hand.-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathway. J. Ethnopharmacol. 2015, 168, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Dai, M. Paeonol from Paeonia suffruticosa prevents TNF-alpha-induced monocytic cell adhesion to rat aortic endothelial cells by suppression of VCAM-1 expression. Phytomedicine 2009, 16, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Oh, H.M.; Min, Y.N.; Park, S.H.; Lee, M.J.; Kim, J.S.; Yean, M.H.; Kang, S.S.; Kim, Y.S.; Chang, K.C.; et al. Paeonol suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells by blocking p38, ERK and nuclear factor-kappaB signaling pathways. Int. Immunopharmacol. 2007, 7, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Kang, O.H.; Lee, Y.S.; Choi, J.G.; Oh, Y.C.; Jang, H.J.; Kim, M.S.; Kim, J.H.; Jeong, S.I.; Kwon, D.Y. Inhibition of LPS-induced iNOS, COX-2 and inflammatory mediator expression by paeonol through the MAPKs inactivation in RAW 264.7 cells. Am. J. Chin. Med. 2009, 37, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Lin, A.H.; Lee, H.F.; Ko, H.K.; Lee, T.S.; Kou, Y.R. Paeonol attenuates cigarette smoke-induced lung inflammation by inhibiting ROS-sensitive inflammatory signaling. Mediators Inflamm. 2014, 2014, 651890. [Google Scholar] [CrossRef] [PubMed]
- Jamal, J.; Mustafa, M.R.; Wong, P.F. Paeonol protects against premature senescence in endothelial cells by modulating Sirtuin 1 pathway. J. Ethnopharmacol. 2014, 154, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.K.; Wu, C.L.; Tsai, T.H.; Hsieh, C.L. Anti-inflammatory and anticoagulative effects of paeonol on LPS-induced acute lung injury in rats. Evid. Based Complement. Alternat. Med. 2012, 2012, 837513. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.K.; Yang, C.Y.; Tsai, T.H.; Hsieh, C.L. Moutan cortex radicis improves lipopolysaccharide-induced acute lung injury in rats through anti-inflammation. Phytomedicine 2012, 19, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.D.; Yang, Z.Y.; Zhang, F.H.; Du, B.; Wang, P.; Li, T.R. Evaluation of the antioxidant, DNA interaction and tumor cell cytotoxicity activities of Copper(II) complexes with Paeonol Schiff-base. Inorg. Chem. Commun. 2010, 13, 727–729. [Google Scholar] [CrossRef]
- Huang, T.J.; Chuang, H.; Liang, Y.C.; Lin, H.H.; Horng, J.C.; Kuo, Y.C.; Chen, C.W.; Tsai, F.Y.; Yen, S.C.; Chou, S.C.; et al. Design, synthesis, and bioevaluation of paeonol derivatives as potential anti-HBV agents. Eur. J. Med. Chem. 2015, 90, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Pao, K.C.; Zhao, J.F.; Lee, T.S.; Huang, Y.P.; Han, C.C.; Huang, L.C.S.; Wu, K.H.; Hsu, M.H. Low-dose paeonol derivatives alleviate lipid accumulation. RSC Adv. 2015, 5, 5652–5656. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Kapoor, M.; Huang, Y.P.; Lin, H.H.; Liang, Y.C.; Lin, Y.L.; Huang, S.C.; Liao, W.N.; Chen, J.K.; Huang, J.S.; et al. Synthesis and Evaluation of Aminothiazole-Paeonol Derivatives as Potential Anticancer Agents. Molecules 2016, 21, 145. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, L.A.; Rodenhuis, N.; Wikstrom, H.; Pugsley, T.A.; Serpa, K.A.; Meltzer, L.T.; Heffner, T.G.; Wise, L.D.; Lajiness, M.E.; Huff, R.M.; et al. Thiazoloindans and thiazolobenzopyrans: a novel class of orally active central dopamine (partial) agonists. J. Med. Chem. 2000, 43, 3549–3557. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Chuang, S.H.; Huang, L.Y.; Lai, C.L.; Lin, Y.H.; Yang, J.Y.; Liu, C.W.; Yang, S.C.; Lin, H.S.; Chang, C.C.; et al. Discovery of 4-aryl-N-arylcarbonyl-2-aminothiazoles as Hec1/Nek2 inhibitors. Part I: optimization of in vitro potencies and pharmacokinetic properties. J. Med. Chem. 2014, 57, 4098–4110. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sikdar, P.; Bairagi, M. Recent developments of 2-aminothiazoles in medicinal chemistry. Eur. J. Med. Chem. 2016, 109, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [PubMed]
- Fujishima, S.; Gando, S.; Daizoh, S.; Kushimoto, S.; Ogura, H.; Mayumi, T.; Takuma, K.; Kotani, J.; Yamashita, N.; Tsuruta, R.; et al. Infection site is predictive of outcome in acute lung injury associated with severe sepsis and septic shock. Respirology 2016, 21, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Brower, R.G.; Carson, S.; Douglas, I.S.; Eisner, M.; Hite, D.; Holets, S.; Kallet, R.H.; Liu, K.D.; MacIntyre, N.; et al. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am. J. Respir. Crit. Care Med. 2011, 184, 561–568. [Google Scholar] [PubMed]
- Xiong, B.; Wang, C.; Tan, J.; Cao, Y.; Zou, Y.; Yao, Y.; Qian, J.; Rong, S.; Huang, Y.; Huang, J. Statins for the prevention and treatment of acute lung injury and acute respiratory distress syndrome: A systematic review and meta-analysis. Respirology 2016, 21, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Kor, D.J.; Carter, R.E.; Park, P.K.; Festic, E.; Banner-Goodspeed, V.M.; Hinds, R.; Talmor, D.; Gajic, O.; Ware, L.B.; Gong, M.N. Effect of Aspirin on Development of ARDS in At-Risk Patients Presenting to the Emergency Department: The LIPS-A Randomized Clinical Trial. JAMA 2016. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef] [PubMed]
- Rocco, P.R.; Nieman, G.F. ARDS: what experimental models have taught us. Intensive Care Med. 2016, 42, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Ren, H.; Xu, N.; Xia, L.; Chen, D.; Zhang, J. Eupatorium lindleyanum DC. sesquiterpenes fraction attenuates lipopolysaccharide-induced acute lung injury in mice. J. Ethnopharmacol. 2016, 185, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, D.; Ricard, J.D. Acute lung injury and bacterial infection. Clin. Chest Med. 2005, 26, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Sapru, A.; Wiemels, J.L.; Witte, J.S.; Ware, L.B.; Matthay, M.A. Acute lung injury and the coagulation pathway: Potential role of gene polymorphisms in the protein C and fibrinolytic pathways. Intensive Care Med. 2006, 32, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, S. Pathophysiology and biomarkers of acute respiratory distress syndrome. J. Intensive Care Med. 2014, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Lien, D.C.; Wagner Jr, W.W.; Capen, R.L.; Haslett, C.; Hanson, W.L.; Hofmeister, S.E.; Henson, P.M.; Worthen, G.S. Physiological neutrophil sequestration in the lung: visual evidence for localization in capillaries. J. Appl. Physiol. 1987, 62, 1236–1243. [Google Scholar] [PubMed]
- Worthen, G.S.; Schwab, B.; Elson, E.L.; Downey, G.P. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science 1989, 245, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.U.; Kohler, G.; Headley, S.; Tolley, E.; Stentz, F.; Postlethwaite, A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest 1995, 108, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, M.; Wendt, C.H. Biomarkers in acute lung injury. Translational Research 2012, 159, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.S.; Jones, M.L.; Flory, C.M. Analysis of monocyte chemoattractant protein 1-mediated lung injury using rat lung organ cultures. Am. J. Pathol. 1993, 143, 894–906. [Google Scholar] [PubMed]
- Han, Z.; Boyle, D.L.; Chang, L.; Bennett, B.; Karin, M.; Yang, L.; Manning, A.M.; Firestein, G.S. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Investig. 2001, 108, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.A.; Standiford, T.J. Cytokine immunotherapy during bacterial pneumonia: from benchtop to bedside. Semin. Respir. Infect. 2001, 16, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.K.; Chang, J.H.; Chien, M.H.; Tsao, S.M.; Yu, M.C.; Bai, K.J.; Tsao, T.C.; Yang, S.F. Plasma monocyte chemoattractant protein-1 level as a predictor of the severity of community-acquired pneumonia. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Ho, C.C.; Chang, H.; Lin, J.F.; Yang, C.S.; Tsai, M.H.; Tsai, H.T.; Lin, P. Particulate nature of inhaled zinc oxide nanoparticles determines systemic effects and mechanisms of pulmonary inflammation in mice. Nanotoxicology 2015, 9, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.; Rogel, M.R.; Baker, M.A.; Troken, J.R.; Urich, D.; Morales-Nebreda, L.; Sennello, J.A.; Kutuzov, M.A.; Sitikov, A.; Davis, J.M.; et al. Vimentin regulates activation of the NLRP3 inflammasome. Nat. Commun. 2015, 6, 6574. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, Y.; Xie, T.; Liu, N.; Chen, H.; Geng, Y.; Kurkciyan, A.; Mena, J.M.; Stripp, B.R.; Jiang, D.; et al. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat. Med. 2016, 22, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- O'Grady, N.P.; Preas, H.L.; Pugin, J.; Fiuza, C.; Tropea, M.; Reda, D.; Banks, S.M.; Suffredini, A.F. Local inflammatory responses following bronchial endotoxin instillation in humans. Am. J. Respir. Crit. Care Med. 2001, 163, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Lin, L.Y.; Yang, J.S.; Chan, M.C.; Hsueh, C.M. Attenuation of lipopolysaccharide-induced acute lung injury by treatment with IL-10. Respirology 2009, 14, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.K. Anti-inflammatory and anticoagulative effects of Moutan Cortex Radices and paeonol on LPS-induced acute lung injury in rats. Ph.D. Thesis, China Medical University, Taichung, Taiwan, 10 October 2012. [Google Scholar]
- Tatsumi, S.; Mabuchi, T.; Abe, T.; Xu, L.; Minami, T.; Ito, S. Analgesic effect of extracts of Chinese medicinal herbs Moutan cortex and Coicis semen on neuropathic pain in mice. Neurosci. Lett. 2004, 370, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Ohta, T.; Kawaguchi, A.; Matsuda, H. Bioactive constituents of Chinese natural medicines. V. Radical scavenging effect of Moutan Cortex. (1): Absolute stereostructures of two monoterpenes, paeonisuffrone and paeonisuffral. Chem. Pharm. Bull. 2000, 48, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.H.; Cao, S.W.; Yu, Y.Y. Synthesis, characterization and biological evaluation of paeonol thiosemicarbazone analogues as mushroom tyrosinase inhibitors. Int. J. Biol. Macromol. 2013, 62, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Diaz, E.; Festic, E.; Gajic, O.; Levitt, J.E. Emerging pharmacological therapies for prevention and early treatment of acute lung injury. Semin. Respir. Crit. Care Med. 2013, 34, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Bosma, K.J.; Taneja, R.; Lewis, J.F. Pharmacotherapy for prevention and treatment of acute respiratory distress syndrome: current and experimental approaches. Drugs 2010, 70, 1255–1282. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Matthay, M.A. Acute lung injury: epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Scholl, A.; Averhoff, P.; Zychlinsky, A. How do neutrophils and pathogens interact? Curr. Opin. Microbiol. 2004, 7, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.S.; Ley, K. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R7–R28. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [PubMed]
- Strieter, R.M.; Kunkel, S.L. Acute lung injury: the role of cytokines in the elicitation of neutrophils. J. Investig. Med. 1994, 42, 640–651. [Google Scholar] [PubMed]
- Kobayashi, A.; Hashimoto, S.; Kooguchi, K.; Kitamura, Y.; Onodera, H.; Urata, Y.; Ashihara, T. Expression of inducible nitric oxide synthase and inflammatory cytokines in alveolar macrophages of ARDS following sepsis. Chest 1998, 113, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Shinbori, T.; Walczak, H.; Krammer, P.H. Activated T killer cells induce apoptosis in lung epithelial cells and the release of pro-inflammatory cytokine TNF-alpha. Eur. J. Immunol. 2004, 34, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Balamayooran, G.; Batra, S.; Balamayooran, T.; Cai, S.; Jeyaseelan, S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during Escherichia coli infection. Infect. Immun. 2011, 79, 2567–2577. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |







| Drugs (Symbols) | Secretion of MCP-1 in Conditioned Media (pg/mL) | |
|---|---|---|
| Low Dose Treatment | High Dose Treatment | |
| Dexamethasone (Dexa) | 2669 ± 578 * | 2538 ± 343 * |
| Paeonol (P) | 3938 ± 106 | 3584 ± 329 |
| 2 (P-2) | 4332 ± 332 | 3610 ± 505 |
| 3 (P-3) | 3618 ± 267 | 4446 ± 201 |
| 4 (P-4) | 2724 ± 254 * | 2077 ± 111 * |
| 4.HCl (P-4.HCl) | 3323 ± 261 | 2826 ± 362 * |
| 5a (P-5a) | 3980 ± 424 | 3225 ± 76 |
| 5b (P-5b) | 3983 ± 120 | 3786 ± 131 |
| 5c (P-5c) | 4238 ± 464 | 3793 ± 176 |
| 5d (P-5d) | 4140 ± 416 | 4118 ± 372 |
| 5e (P-5e) | 4002 ± 125 | 3893 ± 312 |
| 5f (P-5f) | 4050 ± 2.3 | 4334 ± 544 |
| 5g (P-5g) | 4354 ± 155 | 4628 ± 89 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, P.-K.; Yang, C.-Y.; Huang, S.-C.; Hung, Y.-W.; Jeng, K.-C.; Huang, Y.-P.; Chuang, H.; Huang, N.-C.; Li, J.-P.; Hsu, M.-H.; et al. Evaluation of LPS-Induced Acute Lung Injury Attenuation in Rats by Aminothiazole-Paeonol Derivatives. Molecules 2017, 22, 1605. https://doi.org/10.3390/molecules22101605
Fu P-K, Yang C-Y, Huang S-C, Hung Y-W, Jeng K-C, Huang Y-P, Chuang H, Huang N-C, Li J-P, Hsu M-H, et al. Evaluation of LPS-Induced Acute Lung Injury Attenuation in Rats by Aminothiazole-Paeonol Derivatives. Molecules. 2017; 22(10):1605. https://doi.org/10.3390/molecules22101605
Chicago/Turabian StyleFu, Pin-Kuei, Chi-Yu Yang, Su-Chin Huang, Yu-Wen Hung, Kee-Ching Jeng, Ying-Pei Huang, Hong Chuang, Nai-Chun Huang, Jui-Ping Li, Ming-Hua Hsu, and et al. 2017. "Evaluation of LPS-Induced Acute Lung Injury Attenuation in Rats by Aminothiazole-Paeonol Derivatives" Molecules 22, no. 10: 1605. https://doi.org/10.3390/molecules22101605
APA StyleFu, P.-K., Yang, C.-Y., Huang, S.-C., Hung, Y.-W., Jeng, K.-C., Huang, Y.-P., Chuang, H., Huang, N.-C., Li, J.-P., Hsu, M.-H., & Chen, J.-K. (2017). Evaluation of LPS-Induced Acute Lung Injury Attenuation in Rats by Aminothiazole-Paeonol Derivatives. Molecules, 22(10), 1605. https://doi.org/10.3390/molecules22101605