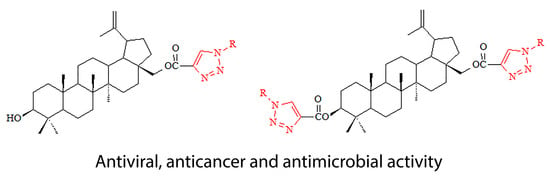
Novel Triazole Hybrids of Betulin: Synthesis and Biological Activity Profile
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antiviral Activity
2.2.1. Characterization of Antiviral Activity against Viruses Belonging to Different Taxonomic Groups
2.2.2. Antiproliferative Effect of Triazole Derivatives
2.3. Anticancer Activity
2.4. Antimicrobial Activity
3. Materials and Methods
3.1. General Techniques
3.2. Synthesis of 28-O-Propynoylbetulin 3 and 3,28-O,O’-di(propynoyl)betulin 4
3.3. General Procedure for the Synthesis of Triazole Derivatives of Betulin 5
3.4. General Procedure for the Synthesis of Bistriazole Derivatives of Betulin 6
3.5. Biological Activities
3.5.1. Cells and Viruses
3.5.2. Cytotoxicity Assay: Determination of Cytotoxic Concentration CC50
3.5.3. Virucidal Activity
3.5.4. Pretreatment Assays
3.5.5. Time of Addition Assay
3.5.6. Measurement of Inhibition Activity on Tumor Cell Proliferation
3.5.7. Anticancer Activity
3.5.8. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lambert, C. Alkyne chemistry in crop protection. Bioorg. Med. Chem. 2009, 17, 4047–4063. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, B.; Surendra Reddy, P.; Sailendra Kumar, N. Cu(I)-catalyzed one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles via nucleophilic displacement and 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006, 47, 3055–3058. [Google Scholar] [CrossRef]
- El Moncef, A.; El Hadrami, E.M.; Ben-Tama, A.; Ramirez de Arellano, C.; Zaballos-Garcia, E.; Stiriba, S.-E. Synthesis and characterization of new 1,4 and 1,5-disubstituted glucopyranosyl 1,2,3-triazole by 1,3-dipolar cycloaddition. J. Mol. Struct. 2009, 929, 6–9. [Google Scholar] [CrossRef]
- Crowley, J.D.; Bandeen, P.H.; Hanton, L.R. A one pot multi-component CuAAC “click” approach to bidentate and tridentate pyridyl-1,2,3-triazole ligands: Synthesis, X-ray structures and copper(II) and silver(I) complexes. Polyhedron 2010, 29, 70–83. [Google Scholar] [CrossRef]
- Zheng, Z.-J.; Wang, D.; Xu, Z.; Xu, L.-W. Synthesis of bi- and bis-1,2,3-triazoles by copper-catalyzed Huisgen cycloaddition: A family of valuable products by click chemistry. Beilstein J. Org. Chem. 2015, 11, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Vatmurge, N.S.; Hazra, B.G.; Pore, V.S.; Shirazi, F.; Chavan, P.S.; Deshpande, M.V. Synthesis and antimicrobial activity of β-lactam-bile acid conjugates linked via triazole. Bioorg. Med. Chem. Lett. 2008, 18, 2043–2047. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, A.; Upadhyay, S.; Singh, J.; Sahu, V. A novel approach to the synthesis of 1,2,3-triazoles and their SAR studies. Med. Chem. Res. 2010, 19, 589–602. [Google Scholar] [CrossRef]
- Demaray, J.A.; Thuener, J.E.; Dawson, M.N.; Sucheck, S.J. Synthesis of triazole-oxazolidinones via a one pot reaction and evaluation of their antimicrobial activity. Bioorg. Med. Chem. Lett. 2008, 18, 4868–4871. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Wan, K.; Zhou, C.-H. Synthesis of novel sulfanilamide-derived 1,2,3-triazoles and their evaluation for antibacterial and antifungal activities. Eur. J. Med. Chem. 2010, 45, 4631–4639. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.H.; Shameem, S.A.; Ganai, B.A. Antimicrobial studies of unsymmetrical bis-1,2,3-triazoles. Org. Chem. Lett. 2012, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pokhodylo, N.; Shyyka, O.; Matiychuk, V. Synthesis and anticancer activity of new 1,2,3-triazole-4-carboxamide derivatives. Med. Chem. Res. 2014, 23, 2426–2438. [Google Scholar] [CrossRef]
- Chandrashekhar, M.; Nayak, V.L.; Ramakrishna, S.; Mallavadhani, U.V. Novel triazole hybrids of myrrhanone C, a natural polypodane triterpene: Synthesis, cytotoxic activity and cell based studies. Eur. J. Med. Chem. 2016, 114, 293–307. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-W.; Dong, C.-Z.; Zhao, J.-Y.; Ma, L.-L.; Li, Y.-H.; Aisa, H.A. 1,2,3-Triazole containing derivatives of rupestonic acid: Click-chemical synthesis and antiviral activities against influenza viruses. Eur. J. Med. Chem. 2014, 76, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Whiting, M.; Tripp, J.C.; Lin, Y.-C.; Lindstrom, W.; Olson, A.J.; Elder, J.H.; Sharpless, K.B.; Fokin, V.V. Rapid discovery and structure-activity profiling of novel inhibitors of human immunodeficiency virus type 1 protease enabled by the copper(I)-catalyzed synthesis of 1,2,3-triazoles and their further functionalization. J. Med. Chem. 2006, 49, 7697–7710. [Google Scholar] [CrossRef] [PubMed]
- Buckle, D.R.; Rockell, C.J.M.; Smith, H.; Spicer, B.A. Studies on 1,2,3-triazoles. 10. Synthesis and antiallergic properties of 9-oxo-1H,9H-benzothiopyrano[2,3-d]-1,2,3-triazoles and their S-oxides. J. Med. Chem. 1984, 27, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Shanmugavelan, P.; Nagarajan, S.; Sathishkumar, M.; Ponnuswamy, A.; Yogeeswari, P.; Sriram, D. Efficient synthesis and in vitro antitubercular activity of 1,2,3-triazoles as inhibitors of Mycobacterium tuberculosis Bioorg. Med. Chem. Lett. 2011, 21, 7273–7276. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.; Boechat, N.; Rangel, E.A.; Silva, F.D.C.D.; Souza, A.M.T.D.; Rodrigues, C.R.; Castro, H.C.; Junior, I.N.; Lourenco, M.C.S.; Wardell, S.M.S.V.; Ferreira, V. Synthesis, tuberculosis inhibitory activity, and SAR study of N-substituted-phenyl-1,2,3-triazole derivatives. Bioorg. Med. Chem. 2006, 14, 8644–8653. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Yadav, A.K.; Kumar, B.; Gaikwad, A.; Sinha, S.K.; Chaturvedi, V.; Tripathi, R.P. Preparation and reactions of sugar azides with alkynes: Synthesis of sugar triazoles as antitubercular agents. Carbohydr. Chem. 2008, 343, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, A.; Rajagopal, K.A. Synthesis of some novel triazole derivatives as anti-nociceptive and anti-inflammatory agents. Acta Pharm. 2009, 59, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Simone, R.D.; Chini, M.G.; Bruno, I.; Riccio, R.; Mueller, D.; Werz, O.; Bifulco, G. Structure-based discovery of inhibitors of microsomal prostaglandin E2 synthase -1,5-lipoxygenase and 5-lipoxygenase-activating protein: Promising hits for the development of new anti-inflammatory agents. J. Med. Chem. 2011, 54, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Dherr, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Barthel, A.; Sczepek, R.; Siewert, B.; Schwarz, S. Synthesis, encapsulation and antitumor activity of new betulin derivatives. Arch. Pharm. Chem. Life Sci. 2011, 1, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Majeed, R.; Sangwan, P.L.; Chinthakindi, P.K.; Khan, I.; Dangroo, N.A.; Thota, N.; Hamid, A.; Sharma, P.R.; Saxena, A.K.; Koul, S. Synthesis of 3-O-propargylated betulinic acid and its 1,2,3-triazoles as potential apoptotic agents. Eur. J. Med. Chem. 2013, 63, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Dang Thi, T.A.; Kim Tuyet, N.T.; Pham The, P.; Than Nguyen, H.; Ba Thi, C.; Doan Duy, T.; D’hooghe, M.; Van Nguyen, T. Synthesis and cytotoxic evaluation of novel ester-triazole-linked triterpenoid-AZT conjugates. Bioorg. Med. Chem. Lett. 2014, 24, 5190–5194. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Guru, S.K.; Rath, S.K.; Chinthakindi, P.K.; Singh, B.; Koul, S.; Bhushan, S.; Sangwan, P.L. A novel triazole derivatives of betulinic acid induces extrinsic and intrinsic apoptosis in human leukemia HL-60 cells. Eur. J. Med. Chem. 2016, 108, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Bori, I.D.; Hung, H.-Y.; Qian, K.; Chen, C.-H.; Morris-Natschke, S.L.; Lee, K.-H. Anti-AIDS agents 88. Anti-HIV conjugates of betulin and betulinic acid with AZT prepared via click chemistry. Tetrahedron Lett. 2012, 53, 1987–1989. [Google Scholar] [CrossRef] [PubMed]
- Vasilevsky, S.F.; Govdi, A.I.; Sorokina, I.V.; Tolstikova, T.G.; Baev, D.S.; Tolstikov, G.A.; Mamatuyk, V.I.; Alabugin, I.V. Rapid access to new bioconjugates of betulonic acid via click chemistry. Bioorg. Med. Chem. Lett. 2011, 21, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, R.; Shi, Y.; Si, L.; Jiao, P.; Fan, Z.; Han, X.; Wu, X.; Zhou, X.; Zhang, Y.; et al. Design, synthesis and biological evaluation of novel l-ascorbic acid-conjugated pentacyclic triterpene derivatives as potential influenza virus entry inhibitors. Eur. J. Med. Chem. 2016, 110, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Boryczka, S.; Bębenek, E.; Wietrzyk, J.; Kempińska, J.; Jastrzębska, M.; Kusz, J.; Nowak, M. Synthesis, structure and cytotoxic activity of new acetylenic derivatives of betulin. Molecules 2013, 18, 4526–4543. [Google Scholar] [CrossRef] [PubMed]
- Mooney, L.M.; Al-Sakkaf, K.A.; Brown, B.L.; Dobson, P.R.M. Apoptotic mechanism in T47D and MCF-7 human breast cancer cells. Br. J. Cancer 2002, 87, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Orchel, A.; Kulczycka, A.; Chodurek, E.; Bębenek, E.; Borkowska, P.; Boryczka, S.; Kowalski, J.; Dzierżewicz, Z. Influence of betulin and 28-O-propynoylbetulin on proliferation and apoptosis of human melanoma cells (G-361). Postepy Hig. Med. Dosw. 2014, 68, 191–197. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards/Clinical and Laboratory Standards Institute (NCCLS/CLSI). Methods for Dilution Antibacterial Susceptibility Test for Bacteria That Grow Aerobically: Approved Standard, 8th ed.; CLSI document M07-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- National Committee for Clinical Laboratory Standards/Clinical and Laboratory Standards Institute (NCCLS/CLSI). Reference Method for Broth Dilution Anifungial Susceptibility Testing of Yeasts: Approved Standard, 3rd ed.; CLSI document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Cunha, L.C.S.; Silva, M.L.A.; Furtado, N.A.J.C.; Vinhólis, A.H.C.; Martins, C.H.G.; Filho, A.A.S.; Cunha, W.R. Antibacterial activity of triterpene acids and semi-synthetic derivatives against oral pathogens. Z. Naturforsch. 2007, 62, 668–672. [Google Scholar] [CrossRef]
- Ayala-Núñez, N.V.; Lara Villegas, H.H.; del Carmen Ixtepan Turrent, L.; Rodríguez Padilla, C. Silver nanoparticles toxicity and bactericidal effect against methicillin-resistant Staphylococcus aureus: Nanoscale does matter. Nanobiotechnology 2009, 5, 2–9. [Google Scholar] [CrossRef]
- Tada, H.; Shiho, O.; Kuroshima, K.-I.; Koyama, M.; Tsukamoto, K. An improved colorimetric assay for interleukin 2. J. Immunol. Methods 1986, 93, 157–165. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–6 are available from the authors. |



| 1H NMR δ (ppm) | 13C NMR δ (ppm) | HSQC | HMBC |
|---|---|---|---|
| H-33 7.88 | C-33 127.16 | 7.88–127.16 | 7.88–140.61/54.49 |
| H-34 5.51 | C-34 54.49 | 5.51–54.49 | 5.51–133.77 |
| Compound | CC50 (μg/mL) | EC50 (μg/mL) | SI |
|---|---|---|---|
| 5e | 10.3 | 1.0 | 10.3 |
| 5f | 100.0 | 5.0 | 20.0 |
| 5h | 27.7 | 0.5 | 55.5 |
| 5j | 100.0 | 5.0 | 20.0 |
| 6f | 100.0 | 5.0 | 20.0 |
| 6h | 29.8 | 0.25 | 0.25 |
| Betulin 1 | 18.5 | 0.5 | 37.0 |
| Ribavirin | 100.0 | 10.0 | 10.0 |
| Compound | Concentration (μg/mL) | Max. Inhibition (% of Control) |
|---|---|---|
| 5a | 25 | 40 |
| 5b | 10 | 40 |
| 5d | 10 | 40 |
| 5e | 50 | 75 |
| 5f | 25 | 35 |
| 5j | 10 | 40 |
| 6h | 50 | 70 |
| Betulin 1 | 2.5 | 30 |
| Ribavirin | 1.5 | 30 |
| Compound | Human Cell Line /IC50 ± SD [μM] | ||||
|---|---|---|---|---|---|
| T47D | MCF-7 | SNB-19 | Colo-829 | C-32 | |
| Betulin 1 | Neg | Neg | 17.7 ± 1.2 | 15.3 ± 2.2 | Neg |
| 3 | 110.8 ± 0.9 | 102.1 ± 1.4 | 4.2 ± 0.3 | 1.7 ± 0.08 | 16.7 ± 0.8 |
| 4 | Neg | Neg | 13.1 ± 1.3 | 9.7 ± 1.5 | 121.2 ± 4.1 |
| 5a | 10.5 ± 0.09 | 58.8 ± 1.5 | 96.2 ± 1.2 | 78.5 ± 8.3 | Neg |
| 5b | 8.5 ± 0.02 | Neg | 66.1 ± 0.7 | 129.2 ± 7.7 | Neg |
| 5c | 1.4 ± 0.01 | Neg | 0.70 ± 0.08 | 9.7 ± 0.1 | Neg |
| 5d | 52.5 ± 1.4 | Neg | 13.0 ± 0.09 | 100.0 ± 5.5 | Neg |
| 5e | Neg | Neg | Neg | 35.1 ± 6.2 | 75.2 ± 3.7 |
| 5f | Neg | Neg | Neg | 109.5 ± 7.2 | Neg |
| 5g | 135.2 ± 2.6 | Neg | 92.3 ± 0.9 | 11.4 ± 0.2 | Neg |
| 5h | 1.3 ± 0.05 | 15.5 ± 1.1 | 9.9 ± 0.7 | 6.2 ± 0.6 | 80.3 ± 5.8 |
| 5i | 3.63 ± 0.3 | 6.94 ± 0.8 | 0.9 ± 0.03 | 0.6 ± 0.13 | 7.9 ± 0.9 |
| 5j | Neg | Neg | Neg | 3.3 ± 1.1 | Neg |
| 5k | 7.2 ± 0.2 | 37.2 ± 0.7 | 6.3 ± 0.9 | 8.7 ± 0.5 | 46.9 ± 2.5 |
| 6a | Neg | Neg | 24.4 ± 1.3 | 80.7 ± 1.8 | Neg |
| 6b | 0.05 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 | Neg | Neg |
| 6c | 1.1 ± 0.01 | 60.7 ± 0.6 | 0.06 ± 0.01 | Neg | Neg |
| 6d | 0.9 ± 0.05 | 0.9 ± 0.08 | 0.5 ± 0.02 | 102.6 ± 4.7 | 100.4 ± 2.8 |
| 6e | Neg | Neg | Neg | Neg | Neg |
| 6f | Neg | Neg | Neg | Neg | Neg |
| 6g | Neg | Neg | Neg | 97.6±3.3 | Neg |
| 6h | 62.4 ± 1.1 | 11.7 ± 1.2 | 68.3 ± 0.9 | 6.5 ± 1.0 | 9.1 ± 0.2 |
| Cisplatin | 24.9 ± 1.1 | 5.5 ± 1.0 | 2.3 ± 0.05 | 16.8 ± 1.7 | 12.3 ± 2.1 |
| Strains | MIC (μM) | MBC (μM) |
|---|---|---|
| Escherichia coli ATCC 25922 | 1.95 | 7.8 |
| Klebsiella pneumoniae ATCC 700603 | 0.95 | 3.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bębenek, E.; Jastrzębska, M.; Kadela-Tomanek, M.; Chrobak, E.; Orzechowska, B.; Zwolińska, K.; Latocha, M.; Mertas, A.; Czuba, Z.; Boryczka, S. Novel Triazole Hybrids of Betulin: Synthesis and Biological Activity Profile. Molecules 2017, 22, 1876. https://doi.org/10.3390/molecules22111876
Bębenek E, Jastrzębska M, Kadela-Tomanek M, Chrobak E, Orzechowska B, Zwolińska K, Latocha M, Mertas A, Czuba Z, Boryczka S. Novel Triazole Hybrids of Betulin: Synthesis and Biological Activity Profile. Molecules. 2017; 22(11):1876. https://doi.org/10.3390/molecules22111876
Chicago/Turabian StyleBębenek, Ewa, Maria Jastrzębska, Monika Kadela-Tomanek, Elwira Chrobak, Beata Orzechowska, Katarzyna Zwolińska, Małgorzata Latocha, Anna Mertas, Zenon Czuba, and Stanisław Boryczka. 2017. "Novel Triazole Hybrids of Betulin: Synthesis and Biological Activity Profile" Molecules 22, no. 11: 1876. https://doi.org/10.3390/molecules22111876
APA StyleBębenek, E., Jastrzębska, M., Kadela-Tomanek, M., Chrobak, E., Orzechowska, B., Zwolińska, K., Latocha, M., Mertas, A., Czuba, Z., & Boryczka, S. (2017). Novel Triazole Hybrids of Betulin: Synthesis and Biological Activity Profile. Molecules, 22(11), 1876. https://doi.org/10.3390/molecules22111876