Synthesis and Biological Evaluation of New Substituted Hantzsch Thiazole Derivatives from Environmentally Benign One-Pot Synthesis Using Silica Supported Tungstosilisic Acid as Reusable Catalyst
Abstract
:1. Introduction
2. Results and Discussion
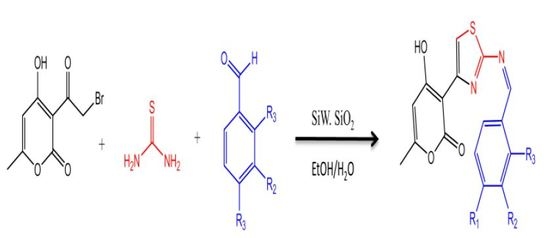
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Antioxidant Activity
2.2.2. In Vitro Antibacterial Activity
3. Experimental Section
3.1. Materials and Methods
3.2. General Procedure for the Preparation of Compounds (4a–4j)
3.3. Characterization Data of Synthesized Compounds
4. Pharmacological Assay
4.1. Antioxidant Activity Evaluation
4.2. Antibacterial Assay
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, Y.B.; Chen, H.L.; Wang, L.Y.; Chen, X.L.; Fu, B. A facile synthesis of 2,4-disubstituted thiazoles using MnO2. Molecules 2009, 14, 4858–4865. [Google Scholar] [CrossRef] [PubMed]
- Dawane, B.S.; Konda, S.G.; Kamble, V.T.; Chavan, S.A.; Bhosale, R.B.; Baseer, S. Multicomponent one-pot synthesis of substituted Hantzsch thiazole derivatives under solvent free conditions. E-J. Chem. 2009, 6, 358–362. [Google Scholar] [CrossRef]
- Sharshira, E.M.; Hamada, N.M.M. Synthesis, characterization and antimicrobial activities of some thiazole derivatives. Am. J. Org. Chem. 2012, 2, 69–73. [Google Scholar] [CrossRef]
- Sadjadikhah, S.S.; Maghsoodlou, M.T.; Hazeri, N.; Khorassani, S.M.; Najafi, S.G. One-Pot multicomponent synthesis of rightly substituted piperidines using p-toluene sulfonic acid monohydrate as catalyst. Monatsh. Chem. 2012, 143, 939–943. [Google Scholar] [CrossRef]
- Andreani, A.; Rambaldi, M.; Leoni, A.; Locatelli, A.; Bossa, R.; Chiericozzi, M.; Galatulas, I.; Salvatore, G. Synthesis and cardiotonic activity of imidazo[2,1-b]thiazoles bearing a lactam ring. Eur. J. Med. Chem. 1996, 31, 383–387. [Google Scholar] [CrossRef]
- Aggarwal, R.; Kumar, S.; Kaushik, P.; Kaushik, D.; Gupta, G.K. Synthesis and pharmacological evaluation of some novel 2-(5-hydroxy-5-trifluoromethyl-4,5-dihydropyrazol-1-yl)-4-(coumarin-3-yl)thiazoles. Eur. J. Med. Chem. 2013, 62, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Rashid, N.; Jones, P.G.; Hussain, R.; Bhatti, M.H. Spectroscopic characterization, crystal structure and antifungal activity of thiourea derivatives containing a thiazole moiety. Cent. Eur. J. Chem. 2010, 8, 550–558. [Google Scholar] [CrossRef]
- Liu, H.L.; Li, Z.; Anthonsen, T. Synthesis and fungicidal activity of 2-imino-3-(4-arylthiazol-2-yl)-thiazolidin-4-ones and their 5-arylidene derivatives. Molecules 2000, 5, 1055–1061. [Google Scholar] [CrossRef]
- Argyropoulou, I.; Geronikaki, A.; Vicini, P.; Zani, F. Synthesis and biological evaluation of sulfonamide thiazole and benzothiazole derivatives as antimicrobial agents. Arkivoc 2009, 6, 89–102. [Google Scholar]
- Amine, M.A.K.; Abdel Rahman, D.E.; El-Eryani, Y.A. Synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bioorg. Med. Chem. 2008, 16, 5377–5388. [Google Scholar] [CrossRef] [PubMed]
- Chowki, C.S.; Magdum, P.L.; Ladda, S.K. Synthesis and antitubercular activity of 6-nitro-2-[4-formyl-3-(substituted phenyl)pyrazol-1-yl]benzothiazoles. Int. J. Chem. Sci. 2008, 6, 1600–1605. [Google Scholar]
- Karegoudar, P.; Karthikeyan, M.S.; Prasad, D.J.; Mahalinga, M.; Holla, B.S.; Kumari, N.S. Synthesis of some novel 2,4-disubstituted thiazoles as possible antimicrobial agents. Eur. J. Med. Chem. 2008, 43, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.-S.T.; Kazak, A.M. Synthesis and antimicrobial activity of some new 1,3-thiazoles,1,3,4-thiadiazoles,1,2,4-triazoles and 1,3-thiazines incorporating acridine and 1,2,3,4-tetrahydroacridine moieties. Eur. J. Chem. 2010, 1, 6–11. [Google Scholar] [CrossRef]
- Badiger, N.P.; Khan, A.; Kalashetti, M.B.; Khazi, I.M. Synthesis and local anaesthetic activities of 2-aminothiazole/thiadiazole analogues of lidocaine. Med. Chem. Res. 2012, 21, 1544–1549. [Google Scholar] [CrossRef]
- Hutchinson, I.; Jennings, S.A.; Vishnuvajjala, B.R.; Westwell, A.D.; Stevens, M.F.G. Synthesis and pharmaceutical properties of antitumor 2-(4-aminophenyl)benzothiazole amino acid prodrugs. J. Med. Chem. 2002, 45, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Azarifar, D.; Shaebanzadeh, M. Synthesis and characterization of new 3,5-dinaphthyl substituted 2-pyrazolines and study of their antimicrobial activity. Molecules 2002, 7, 885–895. [Google Scholar] [CrossRef]
- Potewar, T.M.; Ingale, S.A.; Srinivasan, K.V. Catalyst-Free efficient synthesis of 2-aminothiazoles in water at ambient temperature. Tetrahedron 2008, 64, 5019–5022. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Mereddy, A.R. Microwave promoted synthesis of functionalized 2-aminothiazoles. Tetrahedron Lett. 2006, 47, 5171–5172. [Google Scholar] [CrossRef]
- Ding, Q.; Zhu, D.; Jin, H.; Chen, J.; Ding, J.; Wu, H. Eco-Friendly one-pot synthesis of 2,4-disubstituted thiazoles by grinding under catalyst- and solvent-free conditions. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 220–224. [Google Scholar] [CrossRef]
- Potewar, T.M.; Ingale, S.A.; Srinivasan, K.V. Efficient synthesis of 2,4-disubstituted thiazoles using ionic liquid under ambient conditions: A practical approach towards the synthesis of fanetizole. Tetrahedron 2007, 63, 11066–11069. [Google Scholar] [CrossRef]
- Penta, S.; Gadidasu, K.K.; Basavoju, S.; Rao, V.R. An efficient one-pot synthesis of pyrazolyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-6-yl)-2H-pyran-2-one derivatives via multicomponent approach and their potential antimicrobial and nematicidal activities. Tetrahedron Lett. 2013, 54, 5663–5666. [Google Scholar] [CrossRef]
- Rajendran, A.; karthikeyan, C.; Rajathi, K.; Ragupathy, D. An environmentally benign one pot synthesis of substituted quinolines catalysed by fluoroboric acid based ionic liquid. J. Chem. Sci. 2012, 124, 877–881. [Google Scholar] [CrossRef]
- Aoyama, T.; Murata, S.; Arai, I.; Araki, N.; Takido, T.; Suzuki, Y.; Kodomari, M. One-Pot synthesis using supported reagents system KSCN/SiO2-RNH3OAc/Al2O3: Synthesis of 2-aminothiazoles and N-allylthioureas. Tetrahedron 2006, 62, 3201–3213. [Google Scholar] [CrossRef]
- Jing, X.; Li, Z.; Pan, X.; Shi, Y.; Yan, C. NaIO4-Catalysed one-pot synthesis of dihydropyrimidinones at room temperature under solvent-free conditions. J. Iran. Chem. Soc. 2009, 6, 514–518. [Google Scholar] [CrossRef]
- Narender, M.; Reddy, M.S.; Sridhar, R.; Nageswara, Y.V.D.; Rao, K.R. Aqueous phase synthesis of thiazoles and aminothiazoles in the presence of β-cyclodextrin. Tetrahedron Lett. 2005, 46, 5953–5955. [Google Scholar] [CrossRef]
- Bai, N.; Sha, Y.W.; Meng, G. Efficient and eco-friendly preparation of 4-methyl-5-formylthiazole. Molecules 2008, 13, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Penta, S.; Vedula, R.R. Environmentally friendly one-pot synthesis of substituted thiazoles and thiazolylpyrazoles. Russ. J. Gen. Chem. 2012, 82, 1403–1406. [Google Scholar] [CrossRef]
- Ramachandran, R.; Jayanthi, S.; Jeong, Y.T. One-Pot synthesis of highly diversified tetrahydropyridines by tandem condensation of aldehydes, amines, and β-ketoesters. Tetrahedron 2012, 68, 363–369. [Google Scholar] [CrossRef]
- Sapi, J.; Laronze, J.Y. Indole based multicomponent reactions towards functionalized heterocycles. Arkivoc 2004, 7, 208–222. [Google Scholar] [CrossRef]
- Litvinov, Y.M.; Rodinovskaya, L.A.; Shestopalov, A.M. A new convenient four-component synthesis of 6-amino-2H,4H-pyrano [2,3-c]pyrazole-5-carbonitriles and one-pot synthesis of 6′-amino-5′-cyano-1,2-dihydrospiro-[(3H)-indole-3,4′-(4′H) pyrano[2,3-c]pyrazol]-2-ones. Russ. Chem. Bull. 2009, 58, 2362–2368. [Google Scholar] [CrossRef]
- Shahriza, W.A.; Esmati, S.; Nazari, N.G. Boric acid as mild and efficient catalyst for one-pot synthesis of 1-amidoalkyl-2-naphtols under solvent-free conditions. J. Chem. Sci. 2012, 124, 927–931. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Ding, M.W. A simple and one-pot synthesis of 2,3,4,5-tetrasubstituted 4,5-dihydro-3H-1,4-benzodiazepines. Tetrahedron 2013, 69, 9056–9062. [Google Scholar] [CrossRef]
- Santhosh1, P.; Chunduru1, V.S.R.; Rao, V.R. One-Pot synthesis of trisubstituted pyrazoles via multicomponent approach. Chem. Heterocycl. Compd. 2011, 47, 547–551. [Google Scholar] [CrossRef]
- Thirupaiah, B.; Vedula, R.R. Novel one-pot multicomponent synthesis of substituted 2,3-dihydro-2-(6-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)phthalazine-1,4-diones and substituted 3-[3-(N’-benzylidene-hydrazino)-7H-[1,2,4]Triazolo[3,4-b][1,3,4]thiadiazin-6-yl]-4-hydroxy-6-methyl-pyran-2-ones. Synth. Commun. 2014, 44, 513–519. [Google Scholar]
- Morin, P.; Hamad, B.; Sapaly, G.; Carneiro Rocha, M.G.; Pries de Oliveira, P.G.; Gonzalez, W.A.; Andrade Sales, E.; Essayem, N. Transesterification of rapeseed oil with ethanol: I. Catalysis with homogeneous Kegginheteropolyacids. Appl. Catal. A 2007, 330, 69–76. [Google Scholar] [CrossRef]
- Caetano, C.S.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Esterification of free fatty acids with methanol using heteropolyacids immobilized on silica. Catal. Commun. 2008, 9, 1996–1999. [Google Scholar] [CrossRef]
- Zieba, A.; Matachowski, L.; Gurgul, A.; Bielañska, E.; Drelinkiewicz, A. Transesterification reaction of triglycerides in the presence of Ag-doped H3PW12O40. J. Mol. Catal. A Chem. 2010, 316, 30–44. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Glycerol acetylation over dodecatungstophosphoric acid immobilized into a silica matrix as catalyst. Appl. Catal. B 2009, 91, 416–422. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Acetylation of glycerol over heteropolyacids supported on activated carbon. Catal. Commun. 2011, 12, 573–576. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Reddy, K.S. Ultrasound-Accelerated synthesis of chiral allylic alcohols promoted by indium metal. Tetrahedron 2003, 59, 5333–5336. [Google Scholar] [CrossRef]
- Gao, D.M.; Ma, W.L.; Li, T.R.; Huang, L.Z.; Du, Z.T. An improved synthesis of 1,2-diarylethanols under conventional heating and ultrasound irradiation. Molecules 2012, 17, 10708–10715. [Google Scholar] [CrossRef] [PubMed]
- Disselkamp, R.S.; Hart, T.R.; Williams, A.M.; White, J.F.; Peden, C.H.F. Ultrasound-Assisted hydrogenation of cinnamaldehyde. Ultrason. Sonochem. 2005, 12, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.S.; Junduan, F.; Ding, J.C.; Cheng, T.X.; Gao, W.X.; Chen, J.X.; Wu, H.Y. Tandem base-free synthesis of β-hydroxy sulphides under ultrasound irradiation. J. Chem. Sci. 2012, 124, 1057–1062. [Google Scholar] [CrossRef]
- Singh, A.K.; Shukla, S.K.; Quraishi, M.A. Ultrasound mediated green synthesis of hexa-hydro triazines. J. Mater. Environ. Sci. 2012, 2, 403–406. [Google Scholar]
- Ding, L.; Wang, W.; Zhang, A. Synthesis of 1,5-dinitroaryl-1,4-pentadien-3-ones under ultrasound irradiation. Ultrason. Sonochem. 2007, 14, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.N. Ultrasound promoted mild and facile one-pot, three component synthesis of 2H-indazoles by consecutive condensation, C-N and N-N bond formations catalysed by copper-doped silica cuprous sulphate (CDSCS) as an efficient heterogeneous nano-catalyst. Ultrason. Sonochem. 2017, 34, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Hikem-Oukacha, D.; Hamdi, M.; Silva, A.M.S.; Rachedi, Y. New synthesis and reactivity of 3-bromoacetyl-4-hydroxy-6-methyl-2H-pyran-2-one with binucleophilic amines. J. Heterocycl. Chem. 2011, 48, 63–68. [Google Scholar] [CrossRef]
- Boswell, G.E.; Musso, D.L.; Kelley, J.L.; Soroko, F.E.; Cooper, B.R. Synthesis and anti-tetrabenazine activity of C-3 analogues of dimethyl-2-phenylmorpholines. J. Heterocycl. Chem. 1996, 33, 33–39. [Google Scholar] [CrossRef]
- Lepeshkin, A.Y.; Turchin, K.F.; Gal´pern, E.G.; Stankevich, I.V.; Lyssenko, K.A.; Velezheva, V.S. Mechanism of dehydration of 2-CH2R- and 2-CHR2–4-hydroxy-Δ2-thiazolines as intermediates in the hantzsch thiazole synthesis and factors impeding the synthesis of 2-Me-, 2-Ar-, and -Het-substituted thiazoles and thiazolo[5,4-b]indoles. Russ. Chem. Bull. 2007, 56, 1447–1455. [Google Scholar] [CrossRef]
- Hikem-Oukacha, D.; Hamdi, M.; Silva, A.M.S.; Rachedi, Y. Synthesis and reactivity of 6-methyl-4H-furo[3,2c]pyran-3,4-dione. J. Heterocycl. Chem. 2011, 48, 31–37. [Google Scholar] [CrossRef]
- Hu, F.L.; Lu, R.L.; Huang, B.; Ming, L. Free radical scavenging activity of extracts prepared from fresh leaves of selected Chinese medicinal plants. Fitoterapia 2004, 75, 14–23. [Google Scholar] [PubMed]
- Wang, M.; Li, J.; Rangarajan, M.; Shao, Y.; La Voie, E.J.; Huang, C.T.; Ho, C.T. Antioxidative phenolic compounds from sage (Salvia officinalis). J. Agric. Food Chem. 1998, 46, 4869–4873. [Google Scholar] [CrossRef]
- Abdollah, G.P.; Iraj, H.; Hamze, A.S. Essential oil variation, antioxidant and antibacterial activity of mountain fennel (Zaravschanicamembranacea (Boiss.) M. Pimen.). Ind. Crops Prod. 2013, 50, 443. [Google Scholar]
Sample Availability: Samples of the compounds 4a–4j are available from the authors. |




| Entry | Solvent | Catalysis (%) | Time (h) | Temp (°C) | Yield (%) |
|---|---|---|---|---|---|
| 1 | Water | 15 | 24 | RT | 10 |
| 2 | Water | 15 | 6 | 100 | 45 |
| 3 | MeOH | 15 | 24 | RT | 18 |
| 4 | MeOH | 15 | 4 | 60 | 55 |
| 5 | EtOH | 15 | 24 | RT | 60 |
| 6 | EtOH | 15 | 2 | 65 | 87 |
| 7 | 1-Butanol | 15 | 6 | 110 | 60 |
| 8 | 2-propanol | 15 | 3 | 80 | 65 |
| 9 | EtOH/Water | 15 | 24 | RT | 70 |
| 10 | EtOH/Water | 15 | 2 | 65 | 87 |
| 11 | EtOH/Water | - | 7 | 65 | 50 |
| 12 | EtOH/Water | 5 | 2 | 65 | 74 |
| 13 | EtOH/Water | 10 | 2 | 65 | 79 |
| 14 | EtOH/Water | 18 | 2 | 65 | 87 |
| 15 | EtOH/Water | 20 | 2 | 65 | 87 |
| Product | Substitutions | Method A a | Method B b | ||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | Time (h) | Yield (%) | Time (h) | Yield (%) | |
| 4a | H | H | H | 2 | 87 | 1.5 | 90 |
| 4b | H | OH | H | 2 | 85 | 1.5 | 88 |
| 4c | OH | H | OH | 3.5 | 80 | 2 | 82 |
| 4d | NO2 | H | H | 2 | 82 | 1.5 | 85 |
| 4e | Cl | H | H | 2 | 84 | 1.5 | 87 |
| 4f | OH | H | H | 2 | 85 | 1.5 | 88 |
| 4g | H | OH | OH | 3.5 | 79 | 2 | 82 |
| 4h | H | H | OCH3 | 3.5 | 75 | 2 | 79 |
| 4i | OCH3 | H | H | 2 | 84 | 1.5 | 87 |
| 4j | H | OCH3 | H | 2 | 82 | 1.5 | 85 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouherrou, H.; Saidoun, A.; Abderrahmani, A.; Abdellaziz, L.; Rachedi, Y.; Dumas, F.; Demenceau, A. Synthesis and Biological Evaluation of New Substituted Hantzsch Thiazole Derivatives from Environmentally Benign One-Pot Synthesis Using Silica Supported Tungstosilisic Acid as Reusable Catalyst. Molecules 2017, 22, 757. https://doi.org/10.3390/molecules22050757
Bouherrou H, Saidoun A, Abderrahmani A, Abdellaziz L, Rachedi Y, Dumas F, Demenceau A. Synthesis and Biological Evaluation of New Substituted Hantzsch Thiazole Derivatives from Environmentally Benign One-Pot Synthesis Using Silica Supported Tungstosilisic Acid as Reusable Catalyst. Molecules. 2017; 22(5):757. https://doi.org/10.3390/molecules22050757
Chicago/Turabian StyleBouherrou, Houria, Aicha Saidoun, Ahmed Abderrahmani, Lamia Abdellaziz, Yahia Rachedi, Françoise Dumas, and Albert Demenceau. 2017. "Synthesis and Biological Evaluation of New Substituted Hantzsch Thiazole Derivatives from Environmentally Benign One-Pot Synthesis Using Silica Supported Tungstosilisic Acid as Reusable Catalyst" Molecules 22, no. 5: 757. https://doi.org/10.3390/molecules22050757
APA StyleBouherrou, H., Saidoun, A., Abderrahmani, A., Abdellaziz, L., Rachedi, Y., Dumas, F., & Demenceau, A. (2017). Synthesis and Biological Evaluation of New Substituted Hantzsch Thiazole Derivatives from Environmentally Benign One-Pot Synthesis Using Silica Supported Tungstosilisic Acid as Reusable Catalyst. Molecules, 22(5), 757. https://doi.org/10.3390/molecules22050757