FAK and S6K1 Inhibitor, Neferine, Dually Induces Autophagy and Apoptosis in Human Neuroblastoma Cells
Abstract
:1. Introduction
2. Results
2.1. Neferine Suppresses Cell Proliferation in Human Neuroblastoma Cells
2.2. Neferine Induces G2/M Cell Cycle Arrest in Human Neuroblastoma Cells
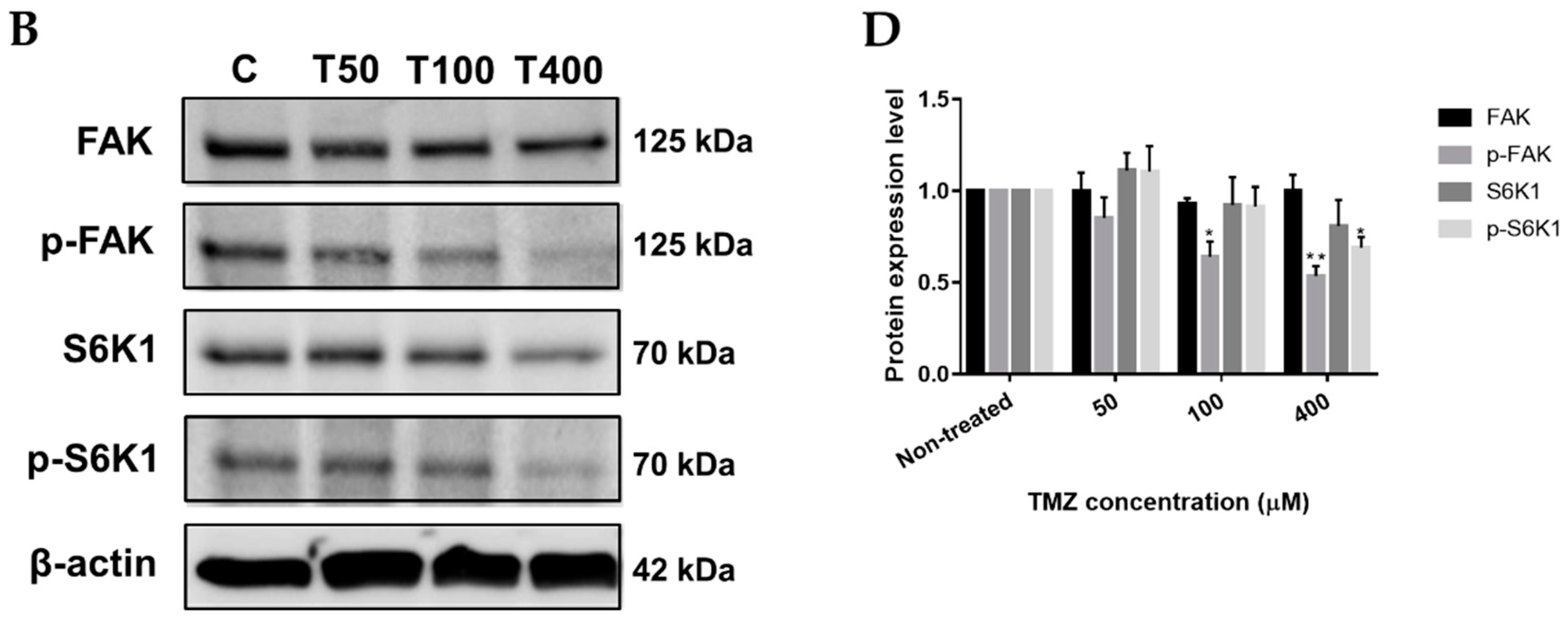
2.3. Neferine Inhibits p-FAK and p-S6K1 Protein Levels in Human Neuroblastoma Cells
2.4. Neferine triggers autophagy in human neuroblastoma cells
2.5. Neferine Induces Apoptosis in Human Neuroblastoma Cells
2.6. Neferine Inhibits Migration in Human Neuroblastoma Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Cell Culture
4.3. MTT Assay
4.4. Cell Cycle Analysis
4.5. Western Blot Analysis
4.6. Wound Healing Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Weinstein, J.L.; Katzenstein, H.M.; Cohn, S.L. Advances in the diagnosis and treatment of neuroblastoma. Oncologist 2003, 8, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Villablanca, J.G.; Seeger, R.C.; Stram, D.O.; Harris, R.E.; Ramsay, N.K.; Swift, P.; Shimada, H.; Black, C.T.; Brodeur, G.M.; et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. New Engl. J. Med. 1999, 341, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Keshelava, N.; Seeger, R.C.; Reynolds, C.P. Drug resistance in human neuroblastoma cell lines correlates with clinical therapy. Eur. J. Cancer 1997, 33, 2002–2006. [Google Scholar] [CrossRef]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, C.; Cao, X.; Zhou, H.; Hong, Z.; Pan, Y. Preparative counter-current chromatography isolation of liensinine and its analogues from embryo of the seed of Nelumbo nucifera GAERTN. using upright coil planet centrifuge with four multilayer coils connected in series. J. Chromatogr. A 2004, 1041, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Law, B.Y.K.; Mok, S.W.F.; Xu, S.W.; Efferth, T.; Wong, V.K.W. Mode of action analyses of neferine, a bisbenzylisoquinoline alkaloid of lotus (Nelumbo nucifera) against multidrug-resistant tumor cells. Front. Pharmacol. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Poornima, P.; Weng, C.F.; Padma, V.V. Neferine from Nelumbo nucifera induces autophagy through the inhibition of PI3K/Akt/mTOR pathway and ROS hyper generation in A549 cells. Food Chem. 2013, 141, 3598–3605. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Shi, J.H.; Wang, Y.; Guo, J.; Zhao, J.H.; Dong, L. Neferine inhibits cultured hepatic stellate cell activation and facilitates apoptosis A possible molecular mechanism. Eur. J. Pharmacol. 2011, 650, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Selvi, S.K.; Vinoth, A.; Varadharajan, T.; Weng, C.F.; Padma, V.V. Neferine augments therapeutic efficacy of cisplatin through ROS-mediated non-canonical autophagy in human lung adenocarcinoma (A549 cells). Food Chem. Toxicol. 2017, 103, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Poornima, P.; Kumar, V.B.; Weng, C.F.; Padma, V.V. Doxorubicin induced apoptosis was potentiated by neferine in human lung adenocarcima, A549 cells. Food Chem. Toxicol. 2014, 68, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, V.; Lin, S.H.; Chang, Y.C.; Weng, C.F. Identification of novel FAK and S6K1 dual inhibitors from natural compounds via ADMET screening and molecular docking. Biomed. Pharmacother. 2016, 80, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, B.M.; Sorrentino, S.; Proietti, G.; Lama, G.; Dobrowolny, G.; Catizone, A.; Binda, E.; Larocca, L.M.; Sica, G. Levetiracetam enhances the temozolomide effect on glioblastoma stem cell proliferation and apoptosis. Cancer Cell Int. 2018, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Wurstle, S.; Schneider, F.; Ringel, F.; Gempt, J.; Lammer, F.; Delbridge, C.; Wu, W.; Schlegel, J. Temozolomide induces autophagy in primary and established glioblastoma cells in an EGFR independent manner. Oncol. Lett. 2017, 14, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomicic, M.T.; Meise, R.; Aasland, D.; Berte, N.; Kitzinger, R.; Kramer, O.H.; Kaina, B.; Christmann, M. Apoptosis induced by temozolomide and nimustine in glioblastoma cells is supported by JNK/c-Jun-mediated induction of the BH3-only protein BIM. Oncotarget 2015, 6, 33755–33768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, J.T.; Martin, K.H.; Slack, J.K.; Taylor, J.M.; Weed, S.A. Focal adhesion kinase: A regulator of focal adhesion dynamics and cell movement. Oncogene 2000, 19, 5606–5613. [Google Scholar] [CrossRef] [PubMed]
- Pullen, N.; Thomas, G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997, 410, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Klionsky, D.J. Autophagosome formation: Core machinery and adaptations. Nat. Cell Biol. 2007, 9, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods in Mol. Biol. 2008, 445, 77–88. [Google Scholar]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Cell Biol. 2010, 188, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Badger, T.M.; Ronis, M.J.; Wu, X. Non-isoflavone phytochemicals in soy and their health effects. J. Agric. Food Chem. 2010, 58, 8119–8133. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Sang, S.; Hong, J.; Yang, C.S. Anticancer and anti-inflammatory effects of cysteine metabolites of the green tea polyphenol, (-)-epigallocatechin-3-gallate. J. Agric. Food Chem. 2010, 58, 10016–10019. [Google Scholar] [CrossRef] [PubMed]
- Poornima, P.; Quency, R.S.; Padma, V.V. Neferine induces reactive oxygen species mediated intrinsic pathway of apoptosis in HepG2 cells. Food Chem. 2013, 136, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Z.; Xu, B.; Sun, Z.; Gong, Y.; Shao, C. Neferine, an alkaloid ingredient in lotus seed embryo, inhibits proliferation of human osteosarcoma cells by promoting p38 MAPK-mediated p21 stabilization. Eur. J. Pharmacol. 2012, 677, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Senderowicz, A.M.; Sausville, E.A. Preclinical and clinical development of cyclin-dependent kinase modulators. J. Natl. Cancer Inst. 2000, 92, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Simmons Kovacs, L.A.; Orlando, D.A.; Haase, S.B. Transcription networks and cyclin/CDKs: The yin and yang of cell cycle oscillators. Cell Cycle 2008, 7, 2626–2629. [Google Scholar] [CrossRef] [PubMed]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and beta-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res. 2014, 34, 1377–1386. [Google Scholar] [PubMed]
- Huang, W.S.; Kuo, Y.H.; Kuo, H.C.; Hsieh, M.C.; Huang, C.Y.; Lee, K.C.; Lee, K.F.; Shen, C.H.; Tung, S.Y.; Teng, C.C. CIL-102-Induced Cell Cycle Arrest and Apoptosis in Colorectal Cancer Cells via Upregulation of p21 and GADD45. PLoS ONE 2017, 12, e0168989. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhong, B.; Jiang, X.; Mao, F.; Liu, G.; Song, B.; Wang, C.Y.; Jiao, Y.; Wang, J.P.; Xu, Z.B.; et al. Actein induces autophagy and apoptosis in human bladder cancer by potentiating ROS/JNK and inhibiting AKT pathways. Oncotarget 2017, 8, 112498–112515. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Yong, Y.; Kim, C.G.; Lee, Y.H.; Lim, Y. Deoxypodophyllotoxin induces G2/M cell cycle arrest and apoptosis in HeLa cells. Cancer Lett. 2010, 287, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, Y.; Deng, H.; Liang, L.; Peng, J. Aloperine induces G2/M phase cell cycle arrest and apoptosis in HCT116 human colon cancer cells. Int. J. Mol. Med. 2014, 33, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.M.; Cance, W.G. Focal adhesion kinase and p53 signaling in cancer cells. Int. Rev. Cytol. 2007, 263, 103–153. [Google Scholar] [PubMed]
- Zhao, J.H.; Bian, Z.C.; Yee, K.; Chen, B.P.C.; Chien, S.; Guan, J.L. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol. Cell 2003, 11, 1503–1515. [Google Scholar] [CrossRef]
- Ding, Q.; Grammer, J.R.; Nelson, M.A.; Guan, J.L.; Stewart, J.E., Jr.; Gladson, C.L. p27Kip1 and cyclin D1 are necessary for focal adhesion kinase regulation of cell cycle progression in glioblastoma cells propagated in vitro and in vivo in the scid mouse brain. J. Biol. Chem. 2005, 280, 6802–6815. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Yang, S. Effects of combining erlotinib and RNA-interfered downregulation of focal adhesion kinase expression on gastric cancer. J. Int. Med. Res. 2016, 44, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, R.; Wang, Q.; Ouyang, Y.; Wang, Q.; Xiong, X. Picfeltarraenin IA inhibits lipopolysaccharide-induced inflammatory cytokine production by the nuclear factor-kappaB pathway in human pulmonary epithelial A549 cells. Oncol. Lett. 2016, 11, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.S.; Ohta, Y.; Rabinovitz, I.; Stossel, T.P.; Blenis, J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin a on an important regulatory site. Mol. Cell. Biol. 2004, 24, 3025–3035. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.M. Overexpression of s6 kinase 1 in brain tumours is associated with induction of hypoxia-responsive genes and predicts patients’ survival. J. Oncol. 2012, 2012, 416927. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, C.S.; Rowley, M.; Naderi, A.; Couch, F.J. The 17q23 amplicon and breast cancer. Breast Cancer Res. Treat. 2003, 78, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, K.S.; Paramasivan, P.; Weng, C.F.; Viswanadha, V.P. Neferine Potentiates the Antitumor Effect of Cisplatin in Human Lung Adenocarcinoma Cells Via a Mitochondria-Mediated Apoptosis Pathway. J. Cell. Biochem. 2017, 118, 2865–2876. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Yang, P.M.; Shun, C.T.; Wu, M.S.; Weng, J.R.; Chen, C.C. Autophagy potentiates the anti-cancer effects of the histone deacetylase inhibitors in hepatocellular carcinoma. Autophagy 2010, 6, 1057–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of Atg proteins in autophagosome formation. Ann. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.H.; Liu, F.; Yang, Z.L.; Fu, X.H.; Yang, Z.H.; Liu, Q.; Wang, L.; Wan, X.B.; Fan, X.J. Prognostic value of autophagy related proteins ULK1, Beclin 1, ATG3, ATG5, ATG7, ATG9, ATG10, ATG12, LC3B and p62/SQSTM1 in gastric cancer. Am. J. Transl. Res. 2016, 8, 3831–3847. [Google Scholar] [PubMed]
- Li, W.L.; Xiong, L.X.; Shi, X.Y.; Xiao, L.; Qi, G.Y.; Meng, C. IKKbeta/NFkappaBp65 activated by interleukin-13 targets the autophagy-related genes LC3B and beclin 1 in fibroblasts co-cultured with breast cancer cells. Exp. Ther. Med. 2016, 11, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Hong, S.H.; Kang, B.; Minai-Tehrani, A.; Cho, M.H. Overexpression of beclin1 induced autophagy and apoptosis in lungs of K-rasLA1 mice. Lung Cancer 2013, 81, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Ferreira, I.; Santos-Ferreira, T.; Sousa-Ferreira, L.; Auregan, G.; Onofre, I.; Alves, S.; Dufour, N.; Colomer Gould, V.F.; Koeppen, A.; Deglon, N.; et al. Overexpression of the autophagic beclin-1 protein clears mutant ataxin-3 and alleviates Machado-Joseph disease. Brain 2011, 134, 1400–1415. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhu, Q.; Dee, R.; Opheim, Z.; Mack, C.P.; Cyr, D.M.; Taylor, J.M. Focal Adhesion Kinase-mediated Phosphorylation of Beclin1 Protein Suppresses Cardiomyocyte Autophagy and Initiates Hypertrophic Growth. J. Biol. Chem. 2017, 292, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Philchenkov, A.; Zavelevich, M.; Kroczak, T.J.; Los, M. Caspases and cancer: Mechanisms of inactivation and new treatment modalities. Exp. Oncol. 2004, 26, 82–97. [Google Scholar] [PubMed]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, S.; Song, J. Caspase-dependent apoptosis and -independent poly(ADP-ribose) polymerase cleavage induced by transforming growth factor beta1. Int. J. Biochem. Cell Biol. 2004, 36, 223–234. [Google Scholar] [CrossRef]
- Cheng, Z.; DiMichele, L.A.; Rojas, M.; Vaziri, C.; Mack, C.P.; Taylor, J.M. Focal adhesion kinase antagonizes doxorubicin cardiotoxicity via p21(Cip1.). J. Mol. Cell. Cardiol. 2014, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; DiMichele, L.A.; Hakim, Z.S.; Rojas, M.; Mack, C.P.; Taylor, J.M. Targeted focal adhesion kinase activation in cardiomyocytes protects the heart from ischemia/reperfusion injury. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.A.; Thamkachy, R.; Ashokan, B.; Komalam, R.J.; Sreerekha, K.V.; Bharathan, A.; Santhoshkumar, T.R.; Rajasekharan, K.N.; Sengupta, S. Diaminothiazoles inhibit angiogenesis efficiently by suppressing Akt phosphorylation. J. Pharmacol. Exp. Ther. 2012, 341, 718–724. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds neferine and temozolomide are not available from the authors. |










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, D.-C.; Chang, Y.-C.; Lin, S.-R.; Fuh, Y.-M.; Tsai, M.-J.; Weng, C.-F. FAK and S6K1 Inhibitor, Neferine, Dually Induces Autophagy and Apoptosis in Human Neuroblastoma Cells. Molecules 2018, 23, 3110. https://doi.org/10.3390/molecules23123110
Pham D-C, Chang Y-C, Lin S-R, Fuh Y-M, Tsai M-J, Weng C-F. FAK and S6K1 Inhibitor, Neferine, Dually Induces Autophagy and Apoptosis in Human Neuroblastoma Cells. Molecules. 2018; 23(12):3110. https://doi.org/10.3390/molecules23123110
Chicago/Turabian StylePham, Dinh-Chuong, Yu-Chuan Chang, Shian-Ren Lin, Yuh-Ming Fuh, May-Jywan Tsai, and Ching-Feng Weng. 2018. "FAK and S6K1 Inhibitor, Neferine, Dually Induces Autophagy and Apoptosis in Human Neuroblastoma Cells" Molecules 23, no. 12: 3110. https://doi.org/10.3390/molecules23123110
APA StylePham, D.-C., Chang, Y.-C., Lin, S.-R., Fuh, Y.-M., Tsai, M.-J., & Weng, C.-F. (2018). FAK and S6K1 Inhibitor, Neferine, Dually Induces Autophagy and Apoptosis in Human Neuroblastoma Cells. Molecules, 23(12), 3110. https://doi.org/10.3390/molecules23123110






