Response of Plant Secondary Metabolites to Environmental Factors
Abstract
:1. Introduction
2. Response of Plant SMs to Light Irradiation
2.1. Effect of Photoperiod on Plant Secondary Metabolites
2.2. Effect of Light Intensity on Plant Secondary Metabolites
2.3. Effect of Light Quality on Plant Secondary Metabolites
3. Response of Plant SMs to Temperature
4. Response of Plant SMs to Soil Water
5. Response of Plant SMs to Soil Salinity
6. Response of Plant SMs to Soil Fertility
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kossel, A. Ueber die chemische Zusammensetzung der Zelle. Archiv für physiologie 1891, 4, 181–186. [Google Scholar]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary plant metabolism. eLS 2008. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Harborne, J.B. Classes and functions of secondary products from plants. In Chemicals from Plants, Perspectives on Secondary Plant Products; Walton, N.J., Brown, D.E., Eds.; Imperial College Press: London, UK, 1999; pp. 1–25. [Google Scholar]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Balandrin, M.F.; Klocke, J.A.; Wurtele, E.S.; Bollinger, W.H. Natural plant chemicals: sources of industrial and medicinal materials. Science 1985, 228, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.M. Plant utilization: Patterns and prospects. Econ. Bot. 1985, 39, 241–265. [Google Scholar] [CrossRef]
- Balandrin, M.F.; Klocke, J.A. Medicinal, aromatic, and industrial materials from plants. In Medicinal and Aromatic Plants I; Springer: Berlin/Heidelberg, Germany, 1988; pp. 3–36. [Google Scholar]
- Asada, M.; Shuler, M.L. Stimulation of ajmalicine production and excretion from Catharanthus roseus: Effects of adsorption in situ, elicitors and alginate immobilization. Appl. Microbiol. Biot. 1989, 30, 475–481. [Google Scholar] [CrossRef]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.B. Mechanism of action of taxol. Trends Pharmacol. Sci. 1992, 13, 134–136. [Google Scholar] [CrossRef]
- Parke, D.V.; Rahman, H. The effects of some terpenoids and other dietary anutrients on hepatic drug-metabolizing enzymes. Biochem. J. 1969, 113, 12. [Google Scholar] [CrossRef]
- Markman, M.; Mekhail, T.M. Paclitaxel in cancer therapy. Expert Opin. Pharmacother. 2002, 3, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Manandhar, S. In vitro propagation of carrot (Daucus carota) L. Sci. World 2007, 5, 51–53. [Google Scholar]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Qu, C.; Chen, Z. Antitumor effect of water decoctions of taxus cuspidate on pancreatic cancer. Evid. Based Complement. Alternat. Med. 2014, 2014, 291675. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Q.; Fu, Y.Y.; Li, B.H.; Zhang, M.L.; Yang, X.L.; Xin, C.W.; Huang, P. PSY-1, a Taxus chinensis var. mairei extract, inhibits cancer cell metastasis by interfering with MMPs. Nat. Prod. Commun. 2014, 9, 241–245. [Google Scholar] [PubMed]
- El-Khattouti, A.; Selimovic, D.; Haïkel, Y.; Megahed, M.; Gomez, C.R.; Hassan, M. Identification and analysis of CD133+ melanoma stem-like cells conferring resistance to taxol: An insight into the mechanisms of their resistance and response. Cancer Lett. 2014, 343, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Guo, F.; Chang, Y.; Jiang, H.; Wang, Q. Optimization of extraction of evodiamine and rutaecarpine from fruit of Evodia rutaecarpa using modified supercritical CO2. J. Chromatogr. A 2010, 1217, 7833–7839. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Li, Z.H.; Wang, Q.; Pan, C.D.; Jiang, D.A.; Wang, G.G. Autotoxicity and allelopathy of 3, 4-dihydroxyacetophenone isolated from Picea schrenkiana needles. Molecules 2011, 16, 8874–8893. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Yan, L.Y.; Li, X.X.; Liu, B.; Zhang, H.; Wang, Q. Optimization of process parameters of extraction of amentoflavone, quercetin and ginkgetin from Taxus chinensis using supercritical CO2 plus co-solvent. Molecules 2014, 19, 17682–17696. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Yang, L.; Cui, W.X.; Zhang, M.X.; Li, Z.H.; Liu, B.; Wang, Q. Optimization of supercritical fluid extraction of total alkaloids, peimisine, peimine and peiminine from the bulb of Fritillaria thunbergii Miq, and evaluation of antioxidant activities of the extracts. Materials 2016, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Antognoni, F.; Zheng, S.; Pagnucco, C.; Baraldi, R.; Poli, F.; Biondi, S. Induction of flavonoid production by UV-B radiation in Passiflora quadrangularis callus cultures. Fitoterapia 2007, 78, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Bertolucci, S.K.V.; Pereira, A.B.D.; Pinto, J.E.B.; Oliveira, A.B.; Braga, F.C. Seasonal variation on the contents of coumarin and kaurane-type diterpenes in Mikania laevigata and M. glomerata leaves under different shade levels. Chem. Biodivers. 2013, 10, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Guo, J.; Xia, Q.; Zhao, G.; Zhou, H.; Xie, F. Metabolic profiling of chinese tobacco leaf of different geographical origins by GC-MS. J. Agric. Food Chem. 2013, 61, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, N.; Gómez-Caravaca, A.M.; Roldán, C.; León, L.; De la Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Chemometric analysis for the evaluation of phenolic patterns in olive leaves from six cultivars at different growth stages. J. Agric. Food Chem. 2015, 63, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, Z.S.; Cheng, F.; Ruan, X.; Jiang, D.A.; Pan, C.D.; Wang, Q. Seasonal dynamics of metabolites in needles of Taxus wallichiana var. mairei. Molecules 2016, 21, 1403. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Ferrandino, A.; Lovisolo, C. Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ. Exp. Bot. 2014, 103, 138–147. [Google Scholar] [CrossRef]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir). Plant Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Metlen, K.L.; Aschehoug, E.T.; Callaway, R.M. Plant behavioural ecology: Dynamic plasticity in secondary metabolites. Plant Cell Environ. 2009, 32, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Musilova, L.; Ridl, J.; Polivkova, M.; Macek, T.; Uhlik, O. Effects of secondary plant metabolites on microbial populations: changes in community structure and metabolic activity in contaminated environments. Int. J. Mol. Sci. 2016, 17, 1205. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.F.; Takaki, M.; Azevedo, R.A. Plant pigments: The many faces of light perception. Acta Physiol. Plant. 2011, 33, 241–248. [Google Scholar] [CrossRef]
- Zoratti, L.; Karppinen, K.; Escobar, A.L.; Häggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zheng, J.; Laaksonen, O.; Tahvonen, R.; Kallio, H. Effects of latitude and weather conditions on phenolic compounds in currant (Ribes spp.) cultivars. J. Agric. Food Chem. 2013, 61, 3517–3532. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.O. Some effects of photoperiod on the biosynthesis of phenylpropane derivatives in Xanthium. Plant Physiol. 1965, 40, 273. [Google Scholar] [CrossRef] [PubMed]
- Camm, E.L.; McCallum, J.; Leaf, E.; Koupai-abyazani, M.R. Cold-induced purpling of Pinus contorta seedlings depends on previous daylength treatment. Plant Cell Environ. 1993, 16, 761–764. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Cavaco, T.; Carvalho, L.M.; Duque, P. Effect of photoperiod on flavonoid pathway activity in sweet potato (Ipomoea batatas (L.) Lam.) leaves. Food Chem. 2010, 118, 384–390. [Google Scholar] [CrossRef]
- Uleberg, E.; Rohloff, J.; Jaakola, L.; Trôst, K.; Junttila, O.; Häggman, H.; Martinussen, I. Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 2012, 60, 10406–10414. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Yamada, Y. Alkaloid biogenesis: Molecular aspects. Annu. Rev. Plant Biol. 1994, 45, 257–285. [Google Scholar] [CrossRef]
- Vincent, R.M.; Lopez-Meyer, M.; McKnight, T.D.; Nessler, C.L. Sustained harvest of camptothecin from the leaves of Camptotheca acuminata. J. Nat. Prod. 1997, 60, 618–619. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, K.W. Shade, leaf growth and crown development of quercus rubra, quercus velutina, prunus serotina and acer rubrum seedlings. Tree Physiol. 1994, 14, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Carpenter, S.B.; Constantin, R.J. Camptothecin production in Camptotheca acuminata seedlings in response to shading and flooding. Can. J. Bot. 1997, 75, 368–373. [Google Scholar] [CrossRef]
- Devkota, A.; Dall’Acqua, S.; Comai, S.; Innocenti, G.; Jha, P.K. Centella asiatica (L.) urban from Nepal: Quali-quantitative analysis of samples from several sites, and selection of high terpene containing populations for cultivation. Biochem. Syst. Ecol. 2010, 38, 12–22. [Google Scholar] [CrossRef]
- Alqahtani, A.; Tongkao-on, W.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K.; Li, G.Q. Seasonal variation of triterpenes and phenolic compounds in Australian Centella asiatica (L.) Urb. Phytochem. Anal. 2015, 26, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Khatib, A.; Shaari, K.; Abas, F.; Shitan, M.; Kneer, R.; Neto, V.; Lajis, N.H. Discrimination of three pegaga (Centella) varieties and determination of growth-lighting effects on metabolites content based on the chemometry of 1H nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2011, 60, 410–417. [Google Scholar]
- Arena, M.E.; Postemsky, P.D.; Curvetto, N.R. Changes in the phenolic compounds and antioxidant capacity of Berberis microphylla G. Forst. berries in relation to light intensity and fertilization. Sci. Hortic. 2017, 218, 63–71. [Google Scholar] [CrossRef]
- Kliewer, W.M. Influence of temperature, solar radiation and nitrogen on coloration and composition of Emperor grapes. Am. J. Enol. Vitic. 1977, 28, 96–103. [Google Scholar]
- Broeckling, C.D.; Huhman, D.V.; Farag, M.A.; Smith, J.T.; May, G.D.; Mendes, P.; Dixon, R.A.; Sumner, L.W. Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J. Exp. Bot. 2004, 56, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.L.; Chang, P.S.; Cheng, Y.C.; Lien, C.Y. Production of stilbenoids from the callus of Arachis hypogaea: A novel source of the anticancer compound piceatannol. J. Agric. Food Chem. 2005, 53, 3877–3881. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.; Jayabaskaran, C. Enhanced catharanthine and vindoline production in suspension cultures of Catharanthus roseus by ultraviolet-B light. J. Mol. Signal. 2008, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Gläßgen, W.E.; Rose, A.; Madlung, J.; Koch, W.; Gleitz, J.; Seitz, H.U. Regulation of enzymes involved in anthocyanin biosynthesis in carrot cell cultures in response to treatment with ultraviolet light and fungal elicitors. Planta 1998, 204, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T.; Proksch, P.; Gülz, P.G. Epicuticular flavonoid aglycones in the genus Cistus, Cistaceae. J. Plant Physiol. 1987, 131, 25–36. [Google Scholar] [CrossRef]
- Chaves, N.; Escudero, J.C.; Gutierrez-Merino, C. Role of ecological variables in the seasonal variation of flavonoid content of Cistus ladanifer exudate. J. Chem. Ecol. 1997, 23, 579–603. [Google Scholar] [CrossRef]
- Regvar, M.; Bukovnik, U.; Likar, M.; Kreft, I. UV-B radiation affects flavonoids and fungal colonisation in Fagopyrum esculentum and F. tataricum. Open Life Sci. 2012, 7, 275–283. [Google Scholar] [CrossRef]
- Warren, J.M.; Bassman, J.H.; Fellman, J.K.; Mattinson, D.S.; Eigenbrode, S. Ultraviolet-B radiation alters phenolic salicylate and flavonoid composition of Populus trichocarpa leaves. Tree Physiol. 2003, 23, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.R.; Tanner, G.J.; Caasi-Lit, M.; Whitecross, M.I.; Nayudu, M.; Mitchell, K.A. Possible protective role for 3′, 4′-dihydroxyflavones induced by enhanced UV-B in a UV-tolerant rice cultivar. Phytochemistry 1998, 49, 1913–1919. [Google Scholar] [CrossRef]
- Hofmann, R.W.; Swinny, E.E.; Bloor, S.J.; Markham, K.R.; Ryan, K.G.; Campbell, B.D.; Jordan, B.R.; Fountain, D.W. Responses of nine Trifolium repens L. populations to ultraviolet-B radiation: Differential flavonol glycoside accumulation and biomass production. Ann. Bot. 2000, 86, 527–537. [Google Scholar] [CrossRef]
- Umek, A.; Kreft, S.; Kartnig, T.; Heydel, B. Quantitative phytochemical analyses of six Hypericum species growing in Slovenia. Planta Med. 1999, 65, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2007: Synthesis Report. IPCC Fourth Assessment Report, Valencia. Available online: http://www.ipcc.ch/pdf/assessment-report/ar4/syr/ar4_syr.pdf (accessed on 12–17 November 2007).
- Jylhä, K.; Ruosteenoja, K.; Räisänen, J.; Venäläinen, A.; Tuomenvirta, H.; Ruokolainen, L.; Saku, S.; Seitola, T. The changing climate in Finland: Estimates for adaptation studies. ACCLIM Project Rep. 2009, 4, 102. [Google Scholar]
- Zheng, J.; Yang, B.; Ruusunen, V.; Laaksonen, O.; Tahvonen, R.; Hellsten, J.; Kallio, H. Compositional differences of phenolic compounds between black currant (Ribes nigrum L.) cultivars and their response to latitude and weather conditions. J. Agric. Food Chem. 2012, 60, 6581–6593. [Google Scholar] [CrossRef] [PubMed]
- Bernáth, J.; Tétényi, P. The Effect of environmental factors on growth. Development and alkaloid production of Poppy (Papaver somniferum L.): I. Responses to day-length and light intensity. Biochem. Physiol. Pflanzen 1979, 174, 468–478. [Google Scholar] [CrossRef]
- Janas, K.M.; Cvikrová, M.; Pałagiewicz, A.; Szafranska, K.; Posmyk, M.M. Constitutive elevated accumulation of phenylpropanoids in soybean roots at low temperature. Plant Sci. 2002, 163, 369–373. [Google Scholar] [CrossRef]
- Zu, Y.G.; Tang, Z.H.; Yu, J.H.; Liu, S.G.; Wang, W.; Guo, X.R. Different responses of camptothecin and 10-hydroxycamptothecin to heat shock in Camptotheca acuminata seedlings. Acta Bot. Sin. 2003, 45, 809–814. [Google Scholar]
- Jansen, G.; Jürgens, H.U.; Ordon, F. Effects of temperature on the alkaloid content of seeds of Lupinus angustifolius cultivars. J. Agric. Crop Sci. 2009, 195, 172–177. [Google Scholar] [CrossRef]
- Guo, X.R.; Yang, L.; Yu, J.H.; Tang, Z.H.; Zu, Y.G. Alkaloid variations in Catharanthus roseus seedlings treated by different temperatures in short term and long term. J. For. Res. 2007, 18, 313–315. [Google Scholar] [CrossRef]
- Dutta, A.; Sen, J.; Deswal, R. Downregulation of terpenoid indole alkaloid biosynthetic pathway by low temperature and cloning of a AP2 type C-repeat binding factor (CBF) from Catharanthus roseus (L). G. Don. Plant Cell Rep. 2007, 26, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Virjamo, V.; Sutinen, S.; Julkunen-Tiitto, R. Combined effect of elevated UVB, elevated temperature and fertilization on growth, needle structure and phytochemistry of young Norway spruce (Picea abies) seedlings. Glob. Chang. Biol. 2014, 20, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Hanson, D.T.; Sharkey, T.D. Effect of growth conditions on isoprene emission and other thermotolerance-enhancing compounds. Plant Cell Environ. 2001, 24, 929–936. [Google Scholar] [CrossRef]
- Rosenfeld, H.J.; Aaby, K.; Lea, P. Influence of temperature and plant density on sensory quality and volatile terpenoids of carrot (Daucus carota L.) root. J. Sci. Food Agric. 2002, 82, 1384–1390. [Google Scholar] [CrossRef]
- Helmig, D.; Ortega, J.; Duhl, T.; Tanner, D.; Guenther, A.; Harley, P.; Wiedinmyer, C.; Milford, J.; Sakulyanontvittaya, T. Sesquiterpene emissions from pine trees-identifications, emission rates and flux estimates for the contiguous United States. Environ. Sci. Technol. 2007, 41, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Mäenpää, M.; Hassinen, V.; Kontunen-Soppela, S.; Malec, L.; Rousi, M.; Pietikainen, L.; Tervahauta, A.; Karenlampi, S.; Holopainen, J.K.; et al. Elevation of night-time temperature increases terpenoid emissions from Betula pendula and Populus tremula. J. Exp. Bot. 2010, 61, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Filella, I.; Wilkinson, M.J.; Llusia, J.; Hewitt, C.N.; Peñuelas, J. Volatile organic compounds emissions in Norway spruce (Picea abies) in response to temperature changes. Physiol. Plant. 2007, 130, 58–66. [Google Scholar] [CrossRef]
- Kivimaenpaa, M.; Riikonen, J.; Ahonen, V.; Tervahauta, A.; Holopainen, T. Sensitivity of Norway spruce physiology and terpenoid emission dynamics to elevated ozone and elevated temperature under open-field exposure. Environ. Exp. Bot. 2013, 90, 32–42. [Google Scholar] [CrossRef]
- Christie, P.J.; Alfenito, M.R.; Walbot, V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 1994, 194, 541–549. [Google Scholar] [CrossRef]
- Leyva, A.; Jarillo, J.A.; Salinas, J.; Martinez-Zapater, J.M. Low temperature induces the accumulation of phenylalanine ammonia-lyase and chalcone synthase mRNAs of Arabidopsis thaliana in a light-dependent manner. Plant Physiol. 1995, 108, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Yamamoto, N.G.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Shvarts, M.; Borochov, A.; Weiss, D. Low temperature enhances petunia flower pigmentation and induces chalcone synthase gene expression. Physiol. Plant. 1997, 99, 67–72. [Google Scholar] [CrossRef]
- Lo Piero, A.R.; Puglisi, I.; Rapisarda, P.; Petrone, G. Anthocyanins accumulation and related gene expression in red orange fruit induced by low temperature storage. J. Agric. Food Chem. 2005, 53, 9083–9088. [Google Scholar] [CrossRef] [PubMed]
- Dela, G.; Or, E.; Ovadia, R.; Nissim-Levi, A.; Weiss, D.; Oren-Shamir, M. Changes in anthocyanin concentration and composition in ‘Jaguar’ rose flowers due to transient high-temperature conditions. Plant Sci. 2003, 164, 333–340. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Phytochemical and physiological changes in the leaves of St. John’s wort plants under a water stress condition. Environ. Exp. Bot. 2007, 59, 109–116. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, Z.; Han, R.; Wang, X. Impact of fertilization on drought response in the medicinal herb Bupleurum chinense DC: Growth and saikosaponin production. Ind. Crops Prod. 2009, 29, 629–633. [Google Scholar] [CrossRef]
- Nogués, S.; Allen, D.J.; Morison, J.I.; Baker, N.R. Ultraviolet-B radiation effects on water relations, leaf development, and photosynthesis in droughted pea plants. Plant Physiol. 1998, 117, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Wang, Y.; Yan, X. Effect of soil moisture on growth and root-salidroside content in Rhodiola sachalinensis. Plant Physiol. Commun. 2002, 39, 335–336. [Google Scholar]
- Kirakosyan, A.; Kaufman, P.; Warber, S.; Zick, S.; Aaronson, K.; Bolling, S.; Chul Chang, S. Applied environmental stresses to enhance the levels of polyphenolics in leaves of hawthorn plants. Physiol. Plant. 2004, 121, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, F.; Yu, L.; Chen, X.; Lei, J.; Ji, J. Influence of drought on oxidative stress and flavonoid production in cell suspension culture of Glycyrrhiza inflata Batal. Z. Naturforsch. C 2007, 62, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, X.; Wang, D.; Zou, Z.; Liang, Z. Effect of drought stress on growth and ccumulation of active constituents in Salvia miltiorrhiza Bunge. Ind. Crops Prod. 2011, 33, 84–88. [Google Scholar] [CrossRef]
- Oh, M.M.; Trick, H.N.; Rajashekar, C.B. Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. J. Plant Physiol. 2009, 166, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, Y.; Wu, C.; Chen, S.; Wang, Z.; Yang, Z.; Huang, L. Water deficit affected flavonoid accumulation by regulating hormone metabolism in Scutellaria baicalensis Georgi roots. PLoS ONE 2012, 7, e42946. [Google Scholar] [CrossRef] [PubMed]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Matros, A.; Peterek, S.; Martin, C. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, H.; Kong, W.; Peng, R.; Liu, Q.; Yao, Q. The Antirrhinum AmDEL gene enhances flavonoids accumulation and salt and drought tolerance in transgenic Arabidopsis. Planta 2016, 244, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z. Drought-induced in vivo synthesis of camptothecin in Camptotheca acuminata seedlings. Physiol. Plant. 2000, 110, 483–488. [Google Scholar] [CrossRef]
- Szabó, B.; Tyihák, E.; Szabó, G.; Botz, L. Mycotoxin and drought stress induced change of alkaloid content of Papaver somniferum plantlets. Acta Bot. Hung. 2003, 45, 409–417. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Panneerselvam, R. Calcium chloride effects on salinity-induced oxidative stress, proline metabolism and indole alkaloid accumulation in Catharanthus roseus. C. R. Biol. 2007, 330, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Turtola, S.; Manninen, A.M.; Rikala, R.; Kainulainen, P. Drought stress alters the concentration of wood terpenoids in Scots pine and Norway spruce seedlings. J. Chem. Ecol. 2003, 29, 1981–1995. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, I.; Zakhama, N.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci. Hortic. 2009, 20, 271–275. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Mueller, M.; Schwarz, K.; Alegre, L. Diterpenes and antioxidative protection in drought-stressed Salvia officinalis plants. J. Plant Physiol. 2001, 158, 1431–1437. [Google Scholar] [CrossRef]
- Nowak, M.; Kleinwächter, M.; Manderscheid, R.; Weigel, H.J.; Selmar, D. Drought stress increases the accumulation of monoterpenes in sage (Salvia officinalis), an effect that is compensated by elevated carbon dioxide concentration. J. Appl. Bot. Food Qual. 2010, 83, 133–136. [Google Scholar]
- Vaughan, M.M.; Christensen, S.; Schmelz, E.A.; Huffaker, A.; Mcauslane, H.J.; Alborn, H.T.; Romero, M.; Allen, H.L.; Teal, P.E. Accumulation of terpenoid phytoalexins in maize roots is associated with drought tolerance. Plant Cell Environ. 2015, 38, 2195–2207. [Google Scholar] [CrossRef] [PubMed]
- Randriamampionona, D.; Diallo, B.; Rakotoniriana, F.; Rabemanantsoa, C.; Cheuk, K.; Corbisier, A.M.; Jaziri, M. Comparative analysis of active constituents in Centella asiatica samples from Madagascar: Application for ex situ conservation and clonal propagation. Fitoterapia 2007, 78, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Rahajanirina, V.; Raoseta, R.; Roger, E.; Razafindrazaka, H.; Pirotais, S.; Boucher, M.; Danthu, P. The influence of certain taxonomic and environmental parameters on biomass production and triterpenoid content in the leaves of Centella asiatica (L.) Urb. from Madagascar. Chem. Biodivers. 2012, 9, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Benavente, L.; Kernodle, S.P.; Margis-Pinheiro, M.; Scandalios, J.G. Salt-induced antioxidant metabolism defenses in maize (Zea mays L.) seedlings. Redox Rep. 2004, 9, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, J.; Bolarin, M.C.; Asins, M.J.; Moreno, V. Increasing salt tolerance in the tomato. J. Exp. Bot. 2006, 57, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Minh, L.T.; Khang, D.T.; Ha, P.T.T.; Tuyen, P.T.; Minh, T.N.; Quan, N.V.; Xuan, T.D. Effects of salinity stress on growth and phenolics of rice (Oryza sativa L.). Int. Lett. Nat. Sci. 2016, 57, 1–10. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Sanada, Y.; Mohanty, P. Effects of salinity on biochemical components of the mangrove, Aegiceras corniculatum. Aquat. Bot. 2004, 80, 77–87. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Hanen, F.; Ksouri, R.; Megdiche, W.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Effect of salinity on growth, leaf-phenolic content and antioxidant scavenging activity in Cynara cardunculus L. In Biosaline Agriculture and High Salinity Tolerance; Abdelly, C., Öztürk, M., Ashraf, M., Grignon, C., Eds.; Birkhäuser: Basel, Switzerland, 2008; pp. 335–343. [Google Scholar]
- Lim, J.H.; Park, K.J.; Kim, B.K.; Jeong, J.W.; Kim, H.J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012, 135, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Petridis, A.; Therios, I.; Samouris, G.; Tananaki, C. Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ. Exp. Bot. 2012, 79, 37–43. [Google Scholar] [CrossRef]
- Borgognone, D.; Cardarelli, M.; Rea, E.; Lucini, L.; Colla, G. Salinity source-induced changes in yield, mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon grown in floating system. J. Sci. Food Agric. 2014, 94, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, B.; Sileoni, V.; Marconi, O.; Perretti, G.; Quinet, M.; Lutts, S.; Benincasa, P. Germination under moderate salinity increases phenolic content and antioxidant activity in rapeseed (Brassica napus var oleifera Del.) sprouts. Molecules 2017, 22, 1377. [Google Scholar] [CrossRef] [PubMed]
- López-Berenguer, C.; Martínez-Ballesta, M.D.C.; Moreno, D.A.; Carvajal, M.; García-Viguera, C. Growing hardier crops for better health: Salinity tolerance and the nutritional value of broccoli. J. Agric. Food Chem. 2009, 57, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B. Salinity effects on phenolic content and antioxidant activity of Salvia macrosiphon. Iran. J. Sci. Technol. A 2017, 41, 295–300. [Google Scholar] [CrossRef]
- Imen, T.; Cristina, S.; Riccardo, I.; Flavia, N.I.; Zeineb, O. Phenolic acids and total antioxidant activity in Ocimum basilicum L. grown under Na2SO4 medium. J. Med. Plants Res. 2012, 6, 5868–5875. [Google Scholar]
- Osman, M.E.; Elfeky, S.S.; El-Soud, K.A.; Hasan, A.M. Response of Catharanthus roseus shoots to salinity and drought in relation to vincristine alkaloid content. Asian J. Plant Sci. 2007, 6, 1223–1228. [Google Scholar]
- Wang, J.Y.; Liu, Z.P. Alkaloid accumulation in Catharanthus roseus increases with addition of seawater salts to the nutrient solution. Pedosphere 2010, 20, 718–724. [Google Scholar] [CrossRef]
- Neelam, M.; Rahul, M.; Ajiboye, M.; Kafayat, Y.; Lateefat, Y. Salicylic acid alters antioxidant and phenolics metabolism in Catharanthus roseus grown under salinity stress. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 118–125. [Google Scholar] [CrossRef]
- Tounekti, T.; Vadel, A.M.; Ennajeh, M.; Khemira, H.; Munné-Bosch, S. Ionic interactions and salinity affect monoterpene and phenolic diterpene composition in rosemary (Rosmarinus officinalis). J. Plant Nutr. Soil Sci. 2011, 174, 504–514. [Google Scholar] [CrossRef]
- Stewart, A.J.; Chapman, W.; Jenkins, G.I.; Graham, I.; Martin, T.; Crozier, A. The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant Cell Environ. 2001, 24, 1189–1197. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.; Rahmat, A.; Rahman, Z.A. The relationship between phenolics and flavonoids production with total non structural carbohydrate and photosynthetic rate in Labisia pumila Benth. under high CO2 and nitrogen fertilization. Molecules 2010, 16, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Bongue-Bartelsman, M.; Phillips, D.A. Nitrogen stress regulates gene expression of enzymes in the flavonoid biosynthetic pathway of tomato. Plant Physiol. Biochem. 1995, 33, 539–546. [Google Scholar]
- Coria-Cayupán, Y.S.; Sánchez de Pinto, M.I.; Nazareno, M.A. Variations in bioactive substance contents and crop yields of lettuce (Lactuca sativa L.) cultivated in soils with different fertilization treatments. J. Agric. Food Chem. 2009, 57, 10122–10129. [Google Scholar] [CrossRef] [PubMed]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of nutrient deficiency and abiotic environmental stresses on yield, phenolic compounds and antiradical activity in lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- Gremigni, P.; Wong, M.T.F.; Edwards, N.K.; Harris, D.; Hamblin, J. Potassium nutrition effects on seed alkaloid concentrations, yield and mineral content of lupins (Lupinus angustifolius). Plant Soil 2001, 234, 131–142. [Google Scholar] [CrossRef]
- Barlóg, P.K. Effect of magnesium and nitrogenous fertilisers on the growth and alkaloid content in Lupinus angustifolius L. Aust. J. Agric. Res. 2002, 53, 671–676. [Google Scholar] [CrossRef]
- Ciesiołka, D.; Muzquiz, M.; Burbano, C.; Altares, P.; Pedrosa, M.M.; Wysocki, W.; Gulewicz, K. An effect of various nitrogen forms used as fertilizer on Lupinus albus L. yield and protein, alkaloid and α-galactosides content. J. Agric. Crop Sci. 2005, 191, 458–463. [Google Scholar] [CrossRef]
- Al-Humaid, A.I. Effects of compound fertilization on growth and alkaloids of datura plants. J. Plant Nutr. 2005, 27, 2203–2219. [Google Scholar] [CrossRef]
- Lerdau, M.; Matson, P.; Fall, R.; Monson, R. Ecological controls over monoterpene emissions from Douglas-fir (Pseudotsuga menziesii). Ecology 1995, 76, 2640–2647. [Google Scholar] [CrossRef]
- Burney, O.T.; Davis, A.S.; Jacobs, D.F. Phenology of foliar and volatile terpenoid production for Thuja plicata families under differential nutrient availability. Environ. Exp. Bot. 2012, 77, 44–52. [Google Scholar] [CrossRef]
- Blanch, J.S.; Peñuelas, J.; Llusià, J. Sensitivity of terpene emissions to drought and fertilization in terpene-storing Pinus halepensis and non-storing Quercus ilex. Physiol. Plant. 2007, 131, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.F.; Rothauer, A.; Hagels, H.; Kayser, O. Influence of light, temperature, and macronutrients on growth and scopolamine biosynthesis in Duboisia species. Planta Med. 2017, 83, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, Z.; Jiang, S.; Xu, H.; Wang, Y.; Feng, S.; Chen, X. Synergistic effects of light and temperature on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult. 2016, 127, 217–227. [Google Scholar] [CrossRef]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D-Amelia, L.; Dell-Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef] [PubMed]



| Metabolite Class | Metabolite Name | Structural Image | Environment Factor | Concentration Change | Plant Species |
|---|---|---|---|---|---|
| Phenols | Caffeoylquinic acids [38] | 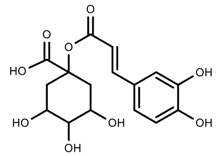 | Short day of light | Decrease | X. pensylvanicuim |
| Phenols | Pelargonidin [39] | 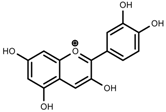 | Short day of light | Decrease | P. contorta |
| Phenols | Catechins [40] |  | Long day of light | Increase | I. batatas |
| Phenols | Hydroxybenzoic acids [40] | 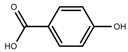 | Long day of light | Increase | I. batatas |
| Phenols | Chlorogenic acid [41] | 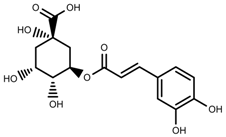 | Long day of light | Increase | V. myrtillus |
| Metabolite Class | Metabolite Name | Structural Image | Environment Factor | Concentration Change | Plant Species |
|---|---|---|---|---|---|
| Alkaloids | Camptothecin [45] | 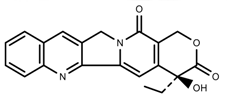 | 27% Full sunlight | Increase | C. acuminate |
| Phenols | Asiatic acid [46] |  | 70% Shade | Increase | C. asiatica |
| Phenols | Asiaticoside [46] | 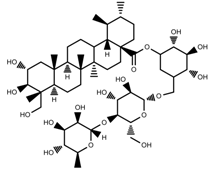 | Full sunlight | Increase | C. asiatica |
| Phenols | Chlorogenic acid [47] |  | Full sunlight | Increase | V. myrtillus |
| Metabolite Class | Metabolite Name | Structural Image | Environment Factor | Concentration Change | Plant Species |
|---|---|---|---|---|---|
| Phenols | Ferulic acid [50] | 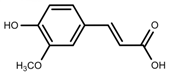 | Increase red light | Decrease | L. sativa |
| Phenols | Kaempferol [50] | 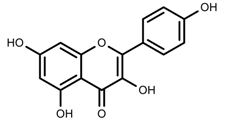 | Increase red light | Decrease | L. sativa |
| Alkaloids | Catharanthine [53] |  | UV-B | Increase | C. roseus |
| Alkaloids | Vindoline [53] | 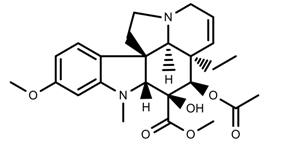 | UV-B | Increase | C. roseus |
| Phenols | Rutin [57] |  | UV | Increase | F. esculentum |
| Phenols | Quercetin [57] | 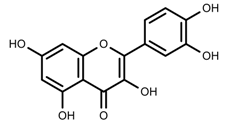 | UV | Increase | F. esculentum |
| Phenols | Catechins [57] | 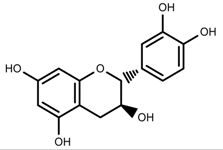 | UV | Increase | F. esculentum |
| Metabolite Class | Metabolite Name | Structural Image | Environment Factor | Concentration Change | Plant Species |
|---|---|---|---|---|---|
| Alkaloids | Morphine [65] |  | Low temperature | Decrease | P. somniferum |
| Phenols | Genistein [66] |  | 10 °C for 24 h | Increase | G. max |
| Phenols | Daidzein [66] | 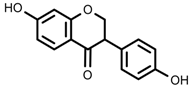 | 10 °C for 24 h | Increase | G. max |
| Alkaloids | 10-hydroxycamptothecin [67] | 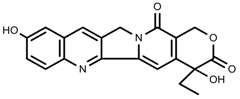 | 40 °C for 2 h | Increase | C. acuminata |
| Alkaloids | Vindoline [69] | 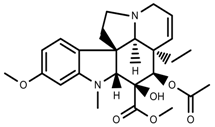 | Short-term heat | Increase | C. roseus |
| Alkaloids | Catharanthine [69] | 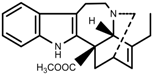 | Long-term heat | Increase | C. roseus |
| Alkaloids | Vindoline [70] | 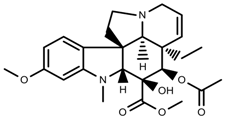 | low temperature | Decrease | C. roseus |
| Terpenes | Isoprene [72] | 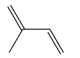 | High temperature | Increase | Q. rubra |
| Terpenes | α-terpinolene [73] |  | High temperature | Decrease | D. carota |
| Terpenes | β-caryophyllene [74] | 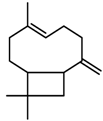 | High temperature | Increase | D. carota |
| Terpenes | α-farnesene [74] | 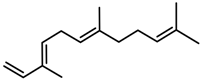 | High temperature | Increase | D. carota |
| Terpenes | DMNT [78] | 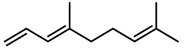 | Night-time warming | Increase | B. pendula |
| Phenols | Pelargonidin [78] |  | Low temperature | Increase | Z. mays |
| Metabolite Class | Metabolite Name | Structural Image | Environment Factor | Concentration Change | Plant Species |
|---|---|---|---|---|---|
| Phenols | Salidroside [87] |  | Soil moisture of 55–75% | Increase | R. sachalinensis |
| Phenols | Chlorogenic acid [88] |  | Deficit | Increase | C. rataegus |
| Phenols | Catechins [88] | 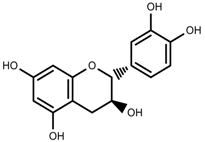 | Deficit | Increase | C. rataegus |
| Phenols | (−)-epicatechins [88] | 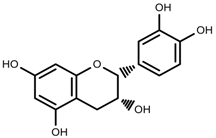 | Deficit | Increase | C. rataegus |
| Phenols | Tanshinone [91] | 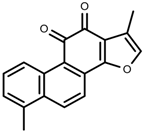 | Severe drought | Increase | S. miltiorrhiza |
| Phenols | Cryptotanshinone [91] | 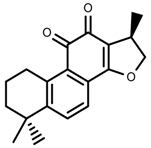 | Severe drought | Increase | S. miltiorrhiza |
| Alkaloids | Camptothecin [96] | 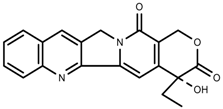 | Drought | Increase | C. acuminate |
| Alkaloids | Morphine [97] | 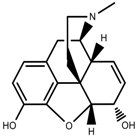 | Drought | Increase | P. somniferum |
| Alkaloids | Codeine [97] | 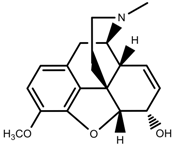 | Drought | Increase | P. somniferum |
| Alkaloids | Glycine betaine [98] | 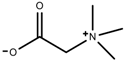 | Drought | Increase | C. roseus |
| Phenols | Abietic acid [99] | 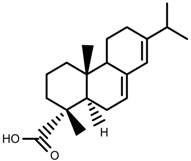 | Severe drought | Increase | P. sylvestris |
| Phenols | Asiaticoside [104] | 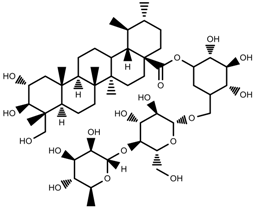 | Humidity increase | Increase | C. asiatica |
| Metabolite Class | Metabolite Name | Structural Image | Environment Factor | Concentration Change | Plant Species |
|---|---|---|---|---|---|
| Phenols | Isoorientin [113] | 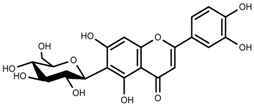 | NaCl (10–100 mM) | Increase | F. esculentum |
| Phenols | Rutin [113] | 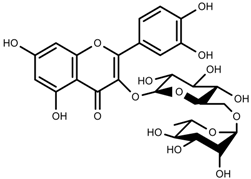 | NaCl (10-100 mM) | Increase | F. esculentum |
| Phenols | Vitexin [113] |  | NaCl (10–100 mM) | Increase | F. esculentum |
| Terpenes | Oleuropein [114] | 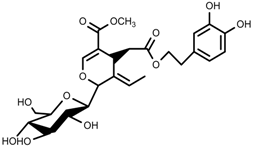 | NaCl (125 mM) | Increase | O. europaea |
| Alkaloids | Catharanthine [121] |  | 5% Seawater | Increase | C. roseus |
| Phenolic | Chlorogenic acid [117] | 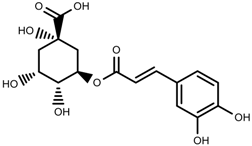 | Salinity | Decrease | B. oleracea var. italica cv. Marathon |
| Phenolic | Vanillin [118] |  | Salinity | Increase | S. macrosiphon |
| Phenolic | Sinapic acid [117] | 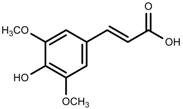 | Salinity | Decrease | B. oleracea var. italica cv. Marathon |
| Phenolic | Protocatechuic acid [118] | 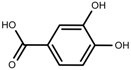 | Salinity | Increase | S. macrosiphon |
| Terpenes | Borneol [123] | 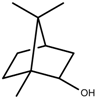 | NaCl (100 mM) | Decrease | R. officinalis |
| Terpenes | Cineole [123] |  | NaCl (100 mM) | Increase | R. officinalis |
| Terpenes | Camphene [123] | 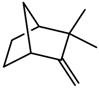 | NaCl (100 mM) | Decrease | R. officinalis |
| Terpenes | Camphor [123] | 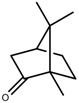 | NaCl (100 mM) | Increase | R. officinalis |
| Terpenes | α-terpineol [123] |  | NaCl (100 mM) | Decrease | R. officinalis |
| Terpenes | Hydroxytyrosol [114] |  | NaCl (125 mM) | Decrease | O. europaea |
| Metabolite Class | Metabolite Name | Structural Image | Environment Factor | Concentration Change | Plant Species |
|---|---|---|---|---|---|
| Phenols | Quercetin [124] | 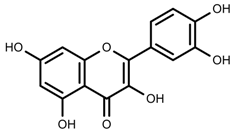 | Nitrogen and phosphate | Increase | L. esculentum cv. Chaser |
| Phenols | Kaempferol [124] | 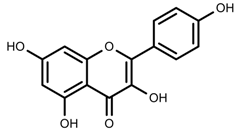 | Nitrogen and phosphate | Increase | L. esculentum cv. Chaser |
| Phenols | Isorhamnetin [124] | 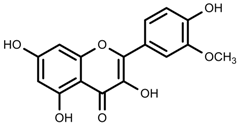 | Nitrogen and phosphate | Increase | L. esculentum cv. Chaser |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. https://doi.org/10.3390/molecules23040762
Yang L, Wen K-S, Ruan X, Zhao Y-X, Wei F, Wang Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules. 2018; 23(4):762. https://doi.org/10.3390/molecules23040762
Chicago/Turabian StyleYang, Li, Kui-Shan Wen, Xiao Ruan, Ying-Xian Zhao, Feng Wei, and Qiang Wang. 2018. "Response of Plant Secondary Metabolites to Environmental Factors" Molecules 23, no. 4: 762. https://doi.org/10.3390/molecules23040762
APA StyleYang, L., Wen, K.-S., Ruan, X., Zhao, Y.-X., Wei, F., & Wang, Q. (2018). Response of Plant Secondary Metabolites to Environmental Factors. Molecules, 23(4), 762. https://doi.org/10.3390/molecules23040762






