RETRACTED: Protective Effect of Glycyrrhizic Acid on Alcoholic Liver Injury in Rats by Modulating Lipid Metabolism
Abstract
1. Introduction
2. Results
2.1. Effect of GA on Body Weight, Liver Index, and Fat Index
2.2. Effect of GA on ALT, AST, ALP and GGT
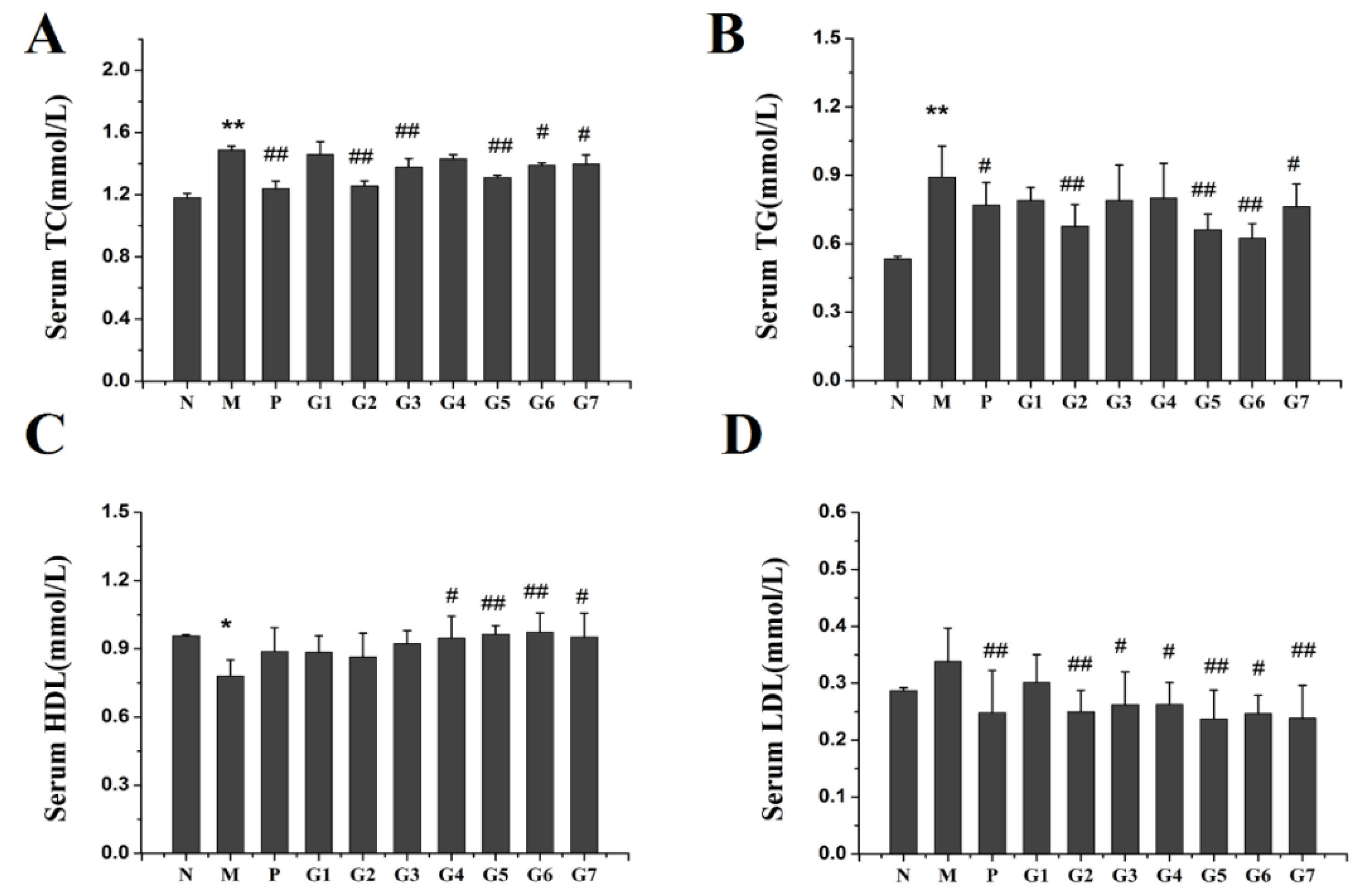
2.3. Effect of GA on Lipid Accumulation
2.4. Effect of GA on Oxidation Stress
2.5. Histopathological Observations
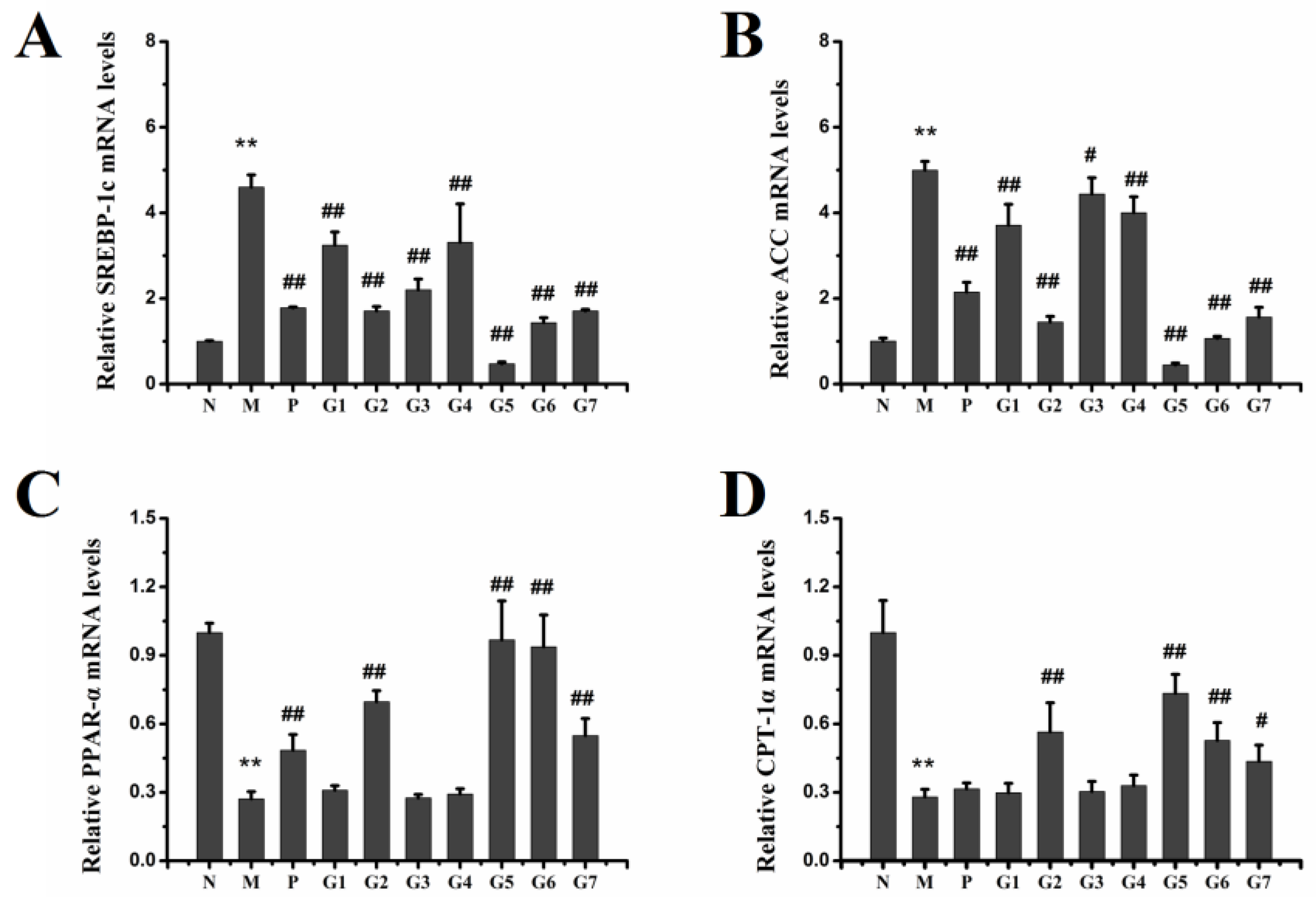
2.6. Effect of GA on mRNA and Protein Expression Associated with Lipid Metabolism
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animal Treatments
4.3. Biochemical Analysis
4.4. Hematoxylin & Eosin (H & E) and Oil-Red O Staining
4.5. Real Time RT-PCR
4.6. Western Blot Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Schwartz, J.M.; Reinus, J.F. Prevalence and natural history of alcoholic liver disease. Clin. Liver Dis. 2012, 16, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Cederbaum, A.I. Alcohol and oxidative liver injury. Hepatology 2006, 43, S63–S74. [Google Scholar] [CrossRef] [PubMed]
- Gramenzi, A.; Caputo, F.; Biselli, M.; Kuria, F.; Loggi, E.; Andreone, P.; Bernardi, M. Review article: Alcoholic liver disease—Pathophysiological aspects and risk factors. Aliment. Pharmacol. Ther. 2006, 24, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Arteel, G.E. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 2003, 124, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Beier, J.I.; Arteel, G.E.; McClain, C.J. Advances in alcoholic liver disease. Curr. Gastroenterol. Rep. 2011, 13, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Orman, E.S.; Odena, G.; Bataller, R. Alcoholic liver disease: Pathogenesis, management, and novel targets for therapy. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S1), 77–84. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, J.; Bataller, R. Alcoholic liver disease: Pathogenesis and new targets for therapy. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.J.; Feng, K.; He, C.; Li, P.; Hu, Y.J.; Su, H.; Wan, J.B. Dietary alpha-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice. Sci. Rep. 2016, 6, 26826. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.B.; Tian, K.; Huang, L.L.; He, C.W.; Jiang, Y.; Wang, Y.T.; Wan, J.B. Herbal medicines for the prevention of alcoholic liver disease: A review. J. Ethnopharmacol. 2012, 144, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiu, P.; Wang, J.; Niu, C.; Pan, S. Effects of compound ginkgo biloba on intestinal permeability in rats with alcohol-induced liver injury. Food Funct. 2015, 6, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, L.; Shan, L.; Fan, G.; Gao, X. Liquorice, a unique “guide drug” of traditional chinese medicine: A review of its role in drug interactions. J. Ethnopharmacol. 2013, 150, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, Z.; Shen, H.; Jin, S.; Zhang, S. Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney injury via suppressing inflammation, apoptosis and oxidative stress. Eur. J. Pharmacol. 2016, 781, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, S.; Wu, X.; Zhang, J.; Chen, R.; Chen, M.; Wang, Y. Chinese herbal medicine-derived compounds for cancer therapy: A focus on hepatocellular carcinoma. J. Ethnopharmacol. 2013, 149, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Duan, X.; Wang, C.; Liu, Z.; Sun, P.; Huo, X.; Ma, X.; Sun, H.; Liu, K.; Meng, Q. Protective effects of glycyrrhizic acid against non-alcoholic fatty liver disease in mice. Eur. J. Pharmacol. 2017, 806, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.L.; Viswanathan, P.; Anuradha, C.V. Taurine enhances the metabolism and detoxification of ethanol and prevents hepatic fibrosis in rats treated with iron and alcohol. Environ. Toxicol. Pharmacol. 2009, 27, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.W.; Jiang, Y.; Zhang, D.Y.; Zhang, X.J.; Hu, Y.J.; Li, P.; Su, H.; Wan, J.B. The hepatoprotective effect of aqueous extracts of penthorum chinense pursh against acute alcohol-induced liver injury is associated with ameliorating hepatic steatosis and reducing oxidative stress. Food Funct. 2015, 6, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.C.; Lee, Y.H.; Kim, S.H.; Kim, K.J.; Kim, K.M.; Oh, S.; Jung, Y.S. Hepatoprotective effect of licorice, the root of glycyrrhiza uralensis fischer, in alcohol-induced fatty liver disease. BMC Complement. Altern. Med. 2016, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Yang, Y.; Yao, Y.L.; Sun, P.; Lian, L.H.; Wu, Y.L.; Nan, J.X. Betulin alleviated ethanol-induced alcoholic liver injury via sirt1/ampk signaling pathway. Pharmacol. Res. 2016, 105, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Le, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Crabb, D.W. Recent advances in alcoholic liver disease ii. Minireview: Molecular mechanisms of alcoholic fatty liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1–G6. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, E.V.; Chi, Y. Mangiferin modulation of metabolism and metabolic syndrome. Biofactors 2016, 42, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Derdak, Z.; Villegas, K.A.; Harb, R.; Wu, A.M.; Sousa, A.; Wands, J.R. Inhibition of p53 attenuates steatosis and liver injury in a mouse model of non-alcoholic fatty liver disease. J. Hepatol. 2013, 58, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, G.; Moustafa, T.; Woelkart, G.; Buttner, S.; Schmidt, A.; van de Weijer, T.; Hesselink, M.; Jaeger, D.; Kienesberger, P.C.; Zierler, K.; et al. Atgl-mediated fat catabolism regulates cardiac mitochondrial function via ppar-alpha and pgc-1. Nat. Med. 2011, 17, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. Ppargamma signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Zollner, G.; Trauner, M. Nuclear receptors in liver disease. Hepatology 2011, 53, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, C.; Terroni, P.L.; Cagampang, F.R.; Hanson, M.; Byrne, C.D. High-unsaturated-fat, high-protein, and low-carbohydrate diet during pregnancy and lactation modulates hepatic lipid metabolism in female adult offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R112–R118. [Google Scholar] [CrossRef] [PubMed]
- Pucci, S.; Zonetti, M.J.; Fisco, T.; Polidoro, C.; Bocchinfuso, G.; Palleschi, A.; Novelli, G.; Spagnoli, L.G.; Mazzarelli, P. Carnitine palmitoyl transferase-1A (CPT1A): A new tumor specific target in human breast cancer. Oncotarget 2016, 7, 19982–19996. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Pinaire, J.; Fischer, M.; Dorris, R.; Crabb, D.W. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J. Biol. Chem. 2001, 276, 68–75. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of 18α-GA and 18β-GA are available from the authors. |








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, X.; Yang, S.; Sun, X.; Meng, X.; Zhao, Y. RETRACTED: Protective Effect of Glycyrrhizic Acid on Alcoholic Liver Injury in Rats by Modulating Lipid Metabolism. Molecules 2018, 23, 1623. https://doi.org/10.3390/molecules23071623
Huo X, Yang S, Sun X, Meng X, Zhao Y. RETRACTED: Protective Effect of Glycyrrhizic Acid on Alcoholic Liver Injury in Rats by Modulating Lipid Metabolism. Molecules. 2018; 23(7):1623. https://doi.org/10.3390/molecules23071623
Chicago/Turabian StyleHuo, Xiaowei, Sa Yang, Xiaoke Sun, Xiangbo Meng, and Yanyan Zhao. 2018. "RETRACTED: Protective Effect of Glycyrrhizic Acid on Alcoholic Liver Injury in Rats by Modulating Lipid Metabolism" Molecules 23, no. 7: 1623. https://doi.org/10.3390/molecules23071623
APA StyleHuo, X., Yang, S., Sun, X., Meng, X., & Zhao, Y. (2018). RETRACTED: Protective Effect of Glycyrrhizic Acid on Alcoholic Liver Injury in Rats by Modulating Lipid Metabolism. Molecules, 23(7), 1623. https://doi.org/10.3390/molecules23071623




