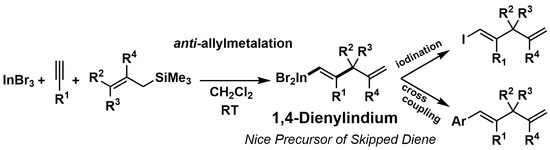
Regio- and Stereoselective Allylindation of Alkynes Using InBr3 and Allylic Silanes: Synthesis, Characterization, and Application of 1,4-Dienylindiums toward Skipped Dienes
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Analysis
3.2. Typical Procedure













4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Normant, J.F.; Alexakis, A. Carbometallation (C-metallation) of alkynes: Stereospecific synthesis of Alkenyl derivatives. Synthesis 1981, 841–870, 841–870. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Asao, N. Selective reactions using allylic metals. Chem. Rev. 1993, 93, 2207–2293. [Google Scholar] [CrossRef]
- Asao, N.; Yamamoto, Y. Lewis acid-catalyzed hydrometalation and carbometalation of unactivated alkynes. Bull. Chem. Soc. Jpn. 2000, 73, 1071–1087. [Google Scholar] [CrossRef]
- Fallis, A.G.; Forgione, P. Metal mediated carbometallation of alkynes and alkenes containing adjacent heteroatoms. Tetrahedron 2001, 57, 5899–5913. [Google Scholar] [CrossRef]
- Shirakawa, E.; Hiyama, T. Transition metal-catalyzed carbostannylation of alkynes and dienes. Bull. Chem. Soc. Jpn. 2002, 75, 1435–1450. [Google Scholar] [CrossRef]
- Knochel, P. Comprehensive Organometallic Chemistry III; Mingos, D.M.P., Crabtree, R.H., Eds.; Elsevier: Oxford, UK, 2007; Volume 9. [Google Scholar]
- Ojima, I. Comprehensive Organometallic Chemistry III; Mingos, D.M.P., Crabtree, R.H., Eds.; Elsevier: Oxford, UK, 2007; Volume 10. [Google Scholar]
- Singleton, D.A.; Waller, S.C.; Zang, Z.; Frantz, D.E.; Leung, S.-W. Allylboration of alkenes with allyldihaloboranes. J. Am. Chem. Soc. 1996, 118, 9986–9987. [Google Scholar] [CrossRef]
- Negishi, E.; Miller, J.A. Selective carbon-carbon bond formation via transition metal catalysis. 37. Controlled carbometalation. 16. Novel syntheses of α,β-unsaturated cyclopentenones via allylzincation of alkynes. J. Am. Chem. Soc. 1983, 105, 6761–6763. [Google Scholar] [CrossRef]
- Eishi, J.J.; Bokslawski, M.P. Effects of Lewis acids and bases on the carbotitanation of unsaturated hydrocarbons and ketones with η3-allyl(di-η5-cyclopentadienyl)titanium(III). J. Organomet. Chem. 1987, 334, C1–C4. [Google Scholar]
- Yeon, S.H.; Han, J.S.; Hong, E.; Do, Y.; Jung, I.N. Aluminum chloride catalyzed stereo- and regiospecific allylsilylation of alkynes: A convenient route to silyldienes. J. Organomet. Chem. 1995, 499, 159–165. [Google Scholar] [CrossRef]
- Chatani, N.; Amishiro, N.; Morri, T.; Yamashita, T.; Murai, S. Pd-catalyzed coupling reaction of acetylenes, iodotrimethylsilane, and organozinc reagents for the stereoselective synthesis of vinylsilanes. J. Org. Chem. 1995, 60, 1834–1840. [Google Scholar] [CrossRef]
- Fujiwara, N.; Yamamoto, Y. Allylation of Unactivated and/or Functionalized Alkynes with Allylindiums. J. Org. Chem. 1997, 62, 2318–2319. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, E.; Yoshida, H.; Nakao, Y.; Hiyama, T. Mechanistic aspects of palladium-catalyzed allylstannylation of alkynes. Org. Lett. 2000, 2, 2209–2211. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, Y.; Asao, A.; Kitahara, H.; Yamamoto, Y. Lewis acid catalyzed allylstannylation of unactivated alkynes. Tetrahedron 1999, 55, 3779–3790. [Google Scholar] [CrossRef]
- Yoshikawa, E.; Gevorgyan, V.; Asao, N.; Yamamoto, Y. Lewis acid Catalyzed trans-Allylsilylation of Unactivated Alkynes. J. Am. Chem. Soc. 1997, 119, 6781–6786. [Google Scholar] [CrossRef]
- Shin, S.; RajanBabu, T.V. Regio- and Stereochemical Control in Bis-functionalization−Cyclization: Use of Alleneyne Precursors for Carbocyclic and Heterocyclic Synthesis. J. Am. Chem. Soc. 2001, 123, 8416–8417. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Fujisawa, N.; Hosomi, A. Indium (III) Chloride-Promoted Intramolecular Addition of Allylstannanes to Alkynes. J. Org. Chem. 2004, 69, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Jie, M.; Pasha, M.K.; Syed-Rahmatullah, M.S.K. Fatty acids, fatty acid analogues and their derivatives. Nat. Prod. Rep. 1997, 14, 163–189. [Google Scholar] [CrossRef]
- Shinohara, Y.; Kudo, F.; Eguchit, T. A Natural Protecting Group Strategy to Carry an Amino Acid Starter Unit in the Biosynthesis of Macrolactam Polyketide Antibiotics. J. Am. Chem. Soc. 2011, 133, 18134–18137. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.; Hiller, W.; Christmann, M. Access to Skipped Polyene Macrolides through Ring-Closing Metathesis: Total Synthesis of the RNA Polymerase Inhibitor Ripostatin B. Angew. Chem. Int. Ed. 2012, 51, 3396–3400. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Prusov, E.V. Total Synthesis of RNA-Polymerase Inhibitor Ripostatin B and 15-Deoxyripostatin A. Angew. Chem. Int. Ed. 2012, 51, 3401–3404. [Google Scholar] [CrossRef] [PubMed]
- Eisch, J.J.; Merkley, J.H. Intramolecular coordinative assistance in the addition of Grignard reagents to unconjugated carbon-carbon unsaturation. J. Organomet. Chem. 1969, 20, P27–P31. [Google Scholar] [CrossRef]
- Eisch, J.J.; Merkley, J.H.; Galle, J.E. Rearrangements of organometallic compounds. 16. Stereochemical factors in the carbomagnesiation of unsaturated alcohols. J. Org. Chem. 1979, 44, 587–593. [Google Scholar] [CrossRef]
- Richey, H.G.; von Rein, F.W. Promotion by hydroxyl functions of additions of Grignard reagents to alkynes. J. Organomet. Chem. 1969, 20, P32–P35. [Google Scholar] [CrossRef]
- Richey, H.G.; von Rein, F.W. The stereochemistry of additions of grignard reagents to alkynols. Tetrahedron Lett. 1971, 12, 3777–3780. [Google Scholar] [CrossRef]
- Miller, R.B.; Reichenbach, T. Grignard Addition to Alkynols. Synth. Commun. 1976, 6, 319–323. [Google Scholar] [CrossRef]
- Richey, H.G.; Erickson, W.E.; Heyn, A.S. Promotion by primary and tertiary amine functions of additions of grignard reagents to alkenes and alkynes. Tetrahedron Lett. 1971, 12, 2183–2186. [Google Scholar] [CrossRef]
- Mauzé, B.; Nivert, C.; Miginiac, L. Étude de l’addition des organozinciques α-éthyléniques aux amines éthyléniques, acétyléniques et alléniques. J. Organomet. Chem. 1972, 44, 69–96. [Google Scholar] [CrossRef]
- Imamura, K.; Yoshikawa, E.; Gevorgyan, V.; Yamamoto, Y. First Exclusive Endo-dig Carbocyclization: HfCl4-Catalyzed Intramolecular Allylsilylation of Alkynes. J. Am. Chem. Soc. 1998, 120, 5339–5340. [Google Scholar] [CrossRef]
- Asao, N.; Yoshikawa, E.; Yamamoto, Y. Lewis Acid-Catalyzed trans-Carbosilylation of Simple Alkynes. J. Org. Chem. 1996, 61, 4874–4875. [Google Scholar] [CrossRef]
- Jung, I.N.; Yoo, B.R. Lewis Acid-Catalyzed Regio- and Stereoselective Allylsilation of Simple Unsaturated Hydrocarbons. Synlett 1999, 519–528, 519–528. [Google Scholar] [CrossRef]
- Ranu, B.C.; Majee, A. Indium-mediated regioselective Markovnikov allylation ofunactivated terminal alkynes. Chem. Commun. 1997, 13, 1225–1226. [Google Scholar] [CrossRef]
- Araki, S.; Imai, A.; Shimizu, K.; Yamada, M.; Mori, A.; Butsugan, Y. Carboindation of Alkynes. Regio- and Stereoselective Allylation of Carbon-Carbon Triple Bonds of Alkynols by Allylic Indium Reagents. J. Org. Chem. 1995, 60, 1841–1847. [Google Scholar] [CrossRef]
- Fujiwara, N.; Yamamoto, Y. Allyl- and Benzylindium Reagents. Carboindation of Carbon–Carbon and Carbon–Nitrogen Triple Bonds. J. Org. Chem. 1999, 64, 4095–4101. [Google Scholar] [CrossRef]
- Klaps, E.; Schmid, W. Carboindation of Carbon–Carbon Triple Bonds: Regioselective Indium-Mediated Allylation of Functionalized Alkynes and Transformation into Halogen-Substituted 1,4-Dienes. J. Org. Chem. 1999, 64, 7537–7546. [Google Scholar] [CrossRef]
- Goeta, A.; Salterb, M.M.; Shah, H. New indium-mediated cyclisation reactions of tethered haloenynes in aqueous solvent systems. Tetrahedron 2006, 62, 3582–3599. [Google Scholar] [CrossRef]
- Salter, M.M.; Sardo-Inffiri, S. Novel Intramolecular Allylindination of Terminal Alkynes in Aqueous Media. Synlett 2002, 2068–2070. [Google Scholar] [CrossRef]
- Araki, S.; Imai, A.; Shimizu, K.; Butsugan, Y. Carboindation of alkynols. A facile synthesis of yomogi alcohol. Tetrahedron Lett. 1992, 33, 2581–2582. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Moritoh, R.; Yasuda, M.; Baba, A. Regio- and Stereoselective Generation of Alkenylindium Compounds from Indium Tribromide, Alkynes, and Ketene Silyl Acetals. Angew. Chem. Int. Ed. 2009, 48, 4577–4580. [Google Scholar]
- Nishimoto, Y.; Ueda, H.; Yasuda, M.; Baba, A. Carbogallation of Alkynes Using Gallium Tribromide and Silyl Ketene Acetals and Synthetic Application to Cross-Coupling with Aryl Iodides. Chem. Eur. J. 2011, 17, 11135–11138. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Takeuchi, M.; Yasuda, M.; Baba, A. Regio- and Stereoselective Carbobismuthination of Alkynes. Angew. Chem. Int. Ed. 2012, 51, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Kang, K.; Yasuda, M. Regio- and Stereoselective Anti-Carbozincation of Alkynyl Ethers Using ZnBr2 toward (Z)-β-Zincated Enol Ether Synthesis. Org. Lett. 2017, 19, 3927–3930. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Hirase, R.; Yasuda, M. Anti-Carboalumination of Alkynes Using Aluminum Trihalide and Silyl Ketene Imines: Stereo- and Regioselective Synthesis of Alkenylaluminum Compounds Bearing a Cyano Group. Org. Lett. 2018, 20, 3651–3655. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Hirokawa, H.; Shibata, I.; Yasuda, M.; Baba, A. Hydroindation of allenes and its application to radical cyclization. Org. Biomol. Chem. 2008, 6, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Kim, S.; Lee, K.; Seomoon, D.; Kim, H.; Lee, S.; Kim, M.; Han, M.; Noh, K.; Livinghouse, T. Cyclization of 1-Bromo-2,7- and 1-Bromo-2,8-Enynes Mediated by Indium. Org. Lett. 2004, 6, 4825–4828. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Entry | InX3 | Solvent | Yield/% |
|---|---|---|---|
| 1 | InBr3 | CH2Cl2 | 89 |
| 2 b | InCl3 | CH2Cl2 | 42 |
| 3 | InF3 | CH2Cl2 | 0 |
| 4 | InI3 | CH2Cl2 | 0 |
| 5 | In(OTf)3 | CH2Cl2 | 0 |
| 6 | InBr3 | toluene | 0 |
| 7 | InBr3 | Et2O | 0 |
| 8 | InBr3 | CH3CN | 0 |
| 9 | InBr3 | THF | 0 |

| Entry | Alkyne 1 | Product 4 | Yield/% |
|---|---|---|---|
| 1 |  1b |  4ba | 64 |
| 2 |  1c |  4ca | 41 |
| 3 |  1d |  4da | 40 |
| 4 |  1e |  4ea | 0 |
| 5 |  1f |  4fa | 59 |
| 6 |  1g |  4ga | 80 |
| 7 |  1h |  4ha | 65 |
| 8 | 78 b |

| Entry | Allylic Silane 2 | Product 3 | Yield/% |
|---|---|---|---|
| 1 |  2b |  4hb | 48 |
| 2 |  2c |  4hc | 76 |
| 3 |  2d |  4hd | 72 |
| 4 |  2e |  4he | 39 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimoto, Y.; Yi, J.; Takata, T.; Baba, A.; Yasuda, M. Regio- and Stereoselective Allylindation of Alkynes Using InBr3 and Allylic Silanes: Synthesis, Characterization, and Application of 1,4-Dienylindiums toward Skipped Dienes. Molecules 2018, 23, 1884. https://doi.org/10.3390/molecules23081884
Nishimoto Y, Yi J, Takata T, Baba A, Yasuda M. Regio- and Stereoselective Allylindation of Alkynes Using InBr3 and Allylic Silanes: Synthesis, Characterization, and Application of 1,4-Dienylindiums toward Skipped Dienes. Molecules. 2018; 23(8):1884. https://doi.org/10.3390/molecules23081884
Chicago/Turabian StyleNishimoto, Yoshihiro, Junyi Yi, Tatsuaki Takata, Akio Baba, and Makoto Yasuda. 2018. "Regio- and Stereoselective Allylindation of Alkynes Using InBr3 and Allylic Silanes: Synthesis, Characterization, and Application of 1,4-Dienylindiums toward Skipped Dienes" Molecules 23, no. 8: 1884. https://doi.org/10.3390/molecules23081884
APA StyleNishimoto, Y., Yi, J., Takata, T., Baba, A., & Yasuda, M. (2018). Regio- and Stereoselective Allylindation of Alkynes Using InBr3 and Allylic Silanes: Synthesis, Characterization, and Application of 1,4-Dienylindiums toward Skipped Dienes. Molecules, 23(8), 1884. https://doi.org/10.3390/molecules23081884