Abstract
Forty-three metabolites including several methoxylated flavonoids, tremetones, and ent-clerodane diterpenes were accurately identified for the first time in the ethanolic extract of P. quadrangularis by means of hyphenated UHPLC-quadrupole Orbitrap mass spectrometry, and seven isolated compounds were tested regarding gastroprotective activity using the HCl/EtOH-induced lesion model in mice. A new tremetone (compound 6) is reported based on spectroscopic evidence. The isolated clerodanes and tremetones showed gastroprotective activity in a mouse model, evidenced by compound 7 (p-coumaroyloxytremetone), which showed the highest gastroprotective activity (76%), which was higher than the control drug lansoprazole (72%). Our findings revealed that several constituents of this plant have gastroprotective activity, and particularly, p-coumaroyloxytremetone could be considered as a lead molecule to explore new gastroprotective agents. This plant is a rich source of biologically active tremetones and terpenoids which can support the ethnobotanical use of the plant.
1. Introduction
Parastrephia quadrangularis (Meyen) Cabrera, (Figure 1) commonly known as Tola-Tola (Alpachtola or Burru suputula, Aymara names) since pre-Hispanic times, is a resinous shrub that grows up to 2 m high, typically found in dry semi-arid places in the Cordillera of the Central Andes in the Puna habitats, at altitudes of 3500 to 5000 m above the sea level. This plant is medicinal and used for gastrointestinal ailments, plus the treatment of urinary and respiratory diseases, fever, altitude sickness, and to treat bone dislocations and bruises [1,2,3,4], besides cattle feeding in the Atacama Desert [5]. Furthermore, Parastrephia (tola) is an important highland genus of South American perennial plants in the aster (sunflower) family (Asteraceae) growing in the Altiplano of Chile, Bolivia, Argentina, and Peru. Many interesting bioactivities were reported for plants within this genus. For example, the plant Parastrephia lucida (Meyen) Cabrera is used in traditional medicine as an antiseptic and anti-inflammatory [6]. The plant resin is also used for the healing of wounds and showed inhibition of arachidonic acid metabolism [7]. The related plant P. lepidophylla showed antifungal activity on some phytopatogenic fungi of lemon [8] and inhibition of cell proliferation using Caco-2 cells [9]. In addition, the infusions of P. lepidophylla and P. lucida showed a protective effect agaist oxidative damage on human erythrocytes [10]. From these genera, antioxidant and analgesic tremetone (5-acetyl-2,3-dihydro-2-isopropenyl-benzofuran) derivatives were isolated [11,12]. Moreover, bioactive constituents of snakeroot (Eupatorium rugosum) and several rayless goldenrods (especially Haplopappus heterophyllus) and other species are reported to be tremetones, causing milk sickness in humans and trembles in livestock [13,14].

Figure 1.
Photographs of aerial parts of Parastrephia quadrangularis collected in El tatio, Atacama Desert, in November 2015.
Regarding the phytochemical components of P. quadrangularis, a poly-methylated flavonoid: 5,7- dihydroxy-3,8,3′,4′-tetramethoxyflavone, some common coumarins and tremetones were reported [12,15]. Recently, other tentative molecules (coumaroyloxytremetone-O-hexoside and coumaroyloxytremetone C-hexoside) found in a sample from Argentina were suggested using only low-resolution mass spectrometry [16]. Some extracts of this species showed antifungal properties against Fusarium verticilloides which were attributed to the presence of tremetones in the active fractions of the plant [16]. On the other hand, medicinal tinctures are extracts of the active metabolites of the most usable part of a medicinal plant; For this, an extraction method is used in which the plants are submerged or macerated for days in mixtures of edible ethanol water or edible pure ethanol [17,18]. Moreover, one out of five persons suffer from ulcers associated to diet, stress, and certain drugs, due to an imbalance among aggressive factors (bile and hydrochloride acids, pepsins, hypoxia, drugs, and alcohol) and defensive factors (mucose blood flow, nitric oxide, sulfhydryl, growth factors bicarbonate, prostaglandins and mucus) in the stomach. Medicines used in the treatment of gastric ulcers are mainly H2-receptor antagonists, anti-acids, and proton-pump inhibitors and when the gastric ulcer is produced by Helicobacter pylori, antibiotics are included in the treatment. In this regard, numerous pharmacological agents with known anti-ulcer activity produce severe collateral effects, showing the need for new agents, including natural products which can be valuable as antiulcer agents [19,20,21].
Following our program to analyze and isolate interesting bioactive compounds from the Atacama Desert flora [22,23,24], we report in this study the gastroprotective activity of several compounds isolated from this plant; furthermore, the high resolution UHPLC fingerprinting analysis of the ethanolic extract (medicinal tincture) of this important Aymara plant is reported for the first time.
2. Results and Discussion
2.1. Isolation and Identification of the Compounds in Parastrephia Quadrangularis Extract
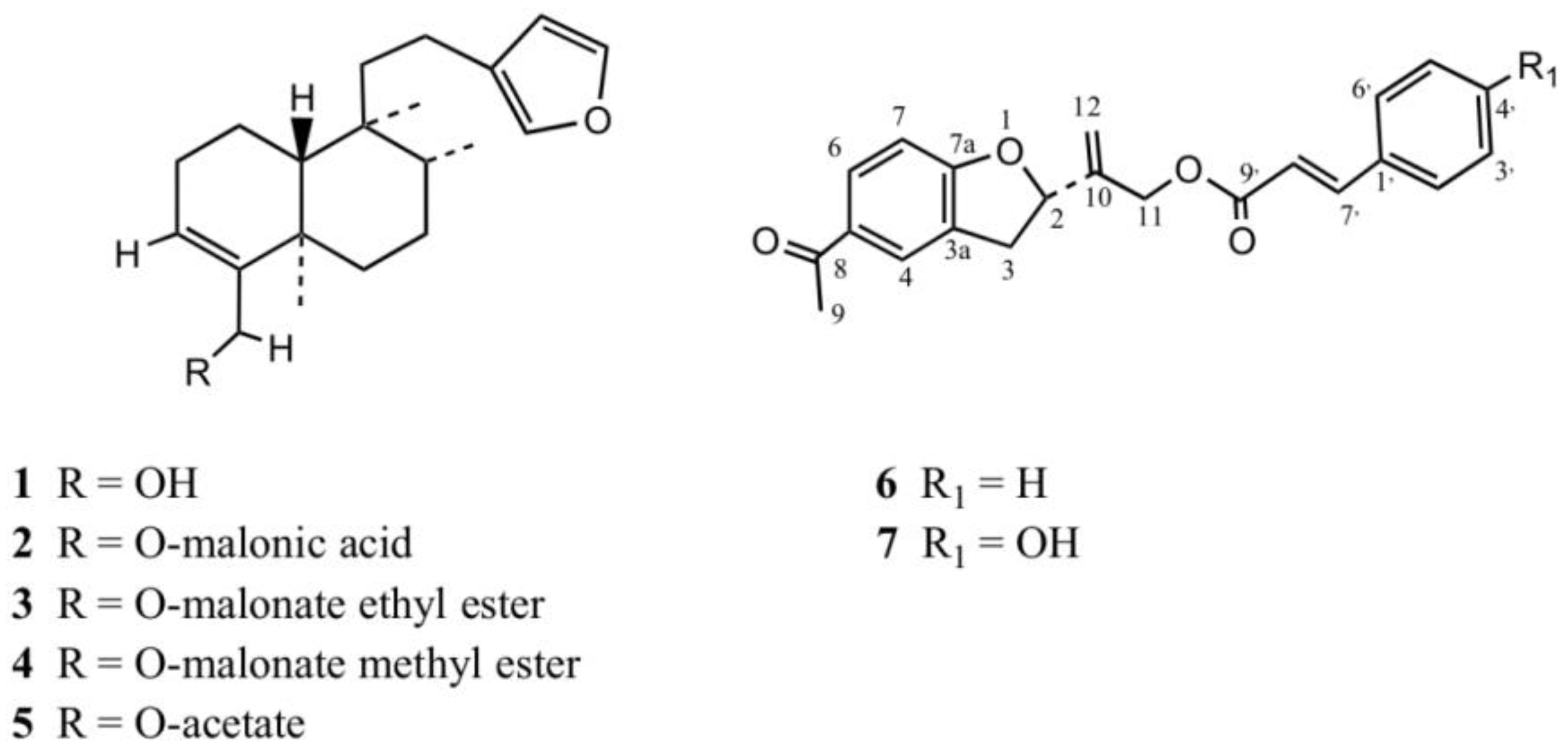
Several isolation steps of the ethanol extract of P. quadrangularis allowed the isolation of the known clerodane diterpenes (Figure 2): 1 (bacchalineol), 2 bacchalineol 18-O-malonic acid) 3 (bacchalineol 18-O-malonate methyl ester), 4 (bacchalineol 18-O-malonate ethyl ester), and 5 (bacchalineol 18-O-acetate) [25]. In addition, the new tremetone 6 plus the known tremetone 7 [26] were isolated, together with the known methylated flavones 5,7-dihydroxy-3,8,3′,4′-tetramethoxyflavone [15], 3′,4′-dimethoxymyricetin [27], 3,7,3′-trimethoxyquercetin, and hesperetin plus the coumarins umbelliferone and scopoletin [28].
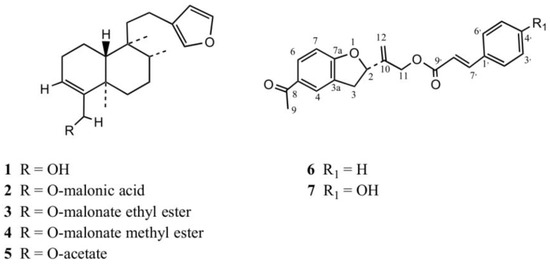
Figure 2.
Structures of clerodanes (1–5) and tremetones (6–7) isolated from P. quadrangularis.
After data comparison with other tremetones [12], and examination of the NMR spectra we realized that compound 6 (Figure 2) showed similar NMR data to that reported for compound 7 [26], particularly the missing of a doublet of doublets in the proton spectra corresponding to the aromatic ring of the coumaroyl moiety (Table S1 and Figure S2, Supplementary Material) plus similar IR bands, but differences in the substitution pattern of only the aromatic protons of the cynnamoyl attached to the tremetone, in the 1H- and 13C-NMR spectra evidenced by 1-D and 2-D NMR analyses (HMBC, HMQC, Tables S1 and S2, Supplementary Material), which led to the identification of 6 as a p-cinnamoyloxyltremetone, a newly reported compound.
p-Cinnamoyloxytremetone (compound 6, Figure 3) IR (CCl4, neat) 1650 cm−1 (CO), 1685 (PhCO), 1610, 1590 (Ph). = −0.4, c = 2.2. Proton NMR (1H Bruker Avance 400 MHz, CDCl3) δ ppm: 7.85 (1H, br s, H-4), 7.83 (1H, br d, J = 8.5 Hz, H-6), 7.65 (1H, d, J = 15.9 Hz, H-7′), 7.53 (2H, d, J = 8.6 Hz, H-3′, H-5′), 7.40 (2H, d, J = 8.6 Hz, H-2′, H-6′), 7.40 (1H, m, H-4′), 6.84 (1H, d, J = 8.3 Hz, H-7), 6.39 (1H, d, J = 15.9 Hz, H-8′), 5.46 (1H, dd, J = 8.8, 8.6 Hz, H-2), 5.38 (2H, d, J = 8.8 Hz, H-12), 4.84 (2H, q, J = 13.4 Hz, H-11a-H-11b), 3.50 (1H, dd, J = 15.9, 9.8 Hz, H-3β), 3.28 (1H, dd, J = 15.9, 7.8 Hz, H-3α), 2.50 (3H, s, COCH3). 13C-NMR (CDCl3) δ ppm: 196.8 (C-8), 166.9 (C-9′), 162.8 (C-7a), 125.5 (C-4), 145.5 (C-7′), 141.8 (C-10), 130.6 (C-6), 134.4 (C-5), 129.0 (C-3′), 129.0 (C-5′), 129.0 (C-3a), 128.5 (C-4′), 127.2 (C-1′), 128.2 (C-2′), 128.2 (C-6′), 114.8 (C-12), 109.4 (C-7), 117.8 (C-8′), 84.6 (C-2), 63.8 (C-11), 35.1 (C-3), 26.4 (C-9). HR-ESI-MS spectra: See Table 1.
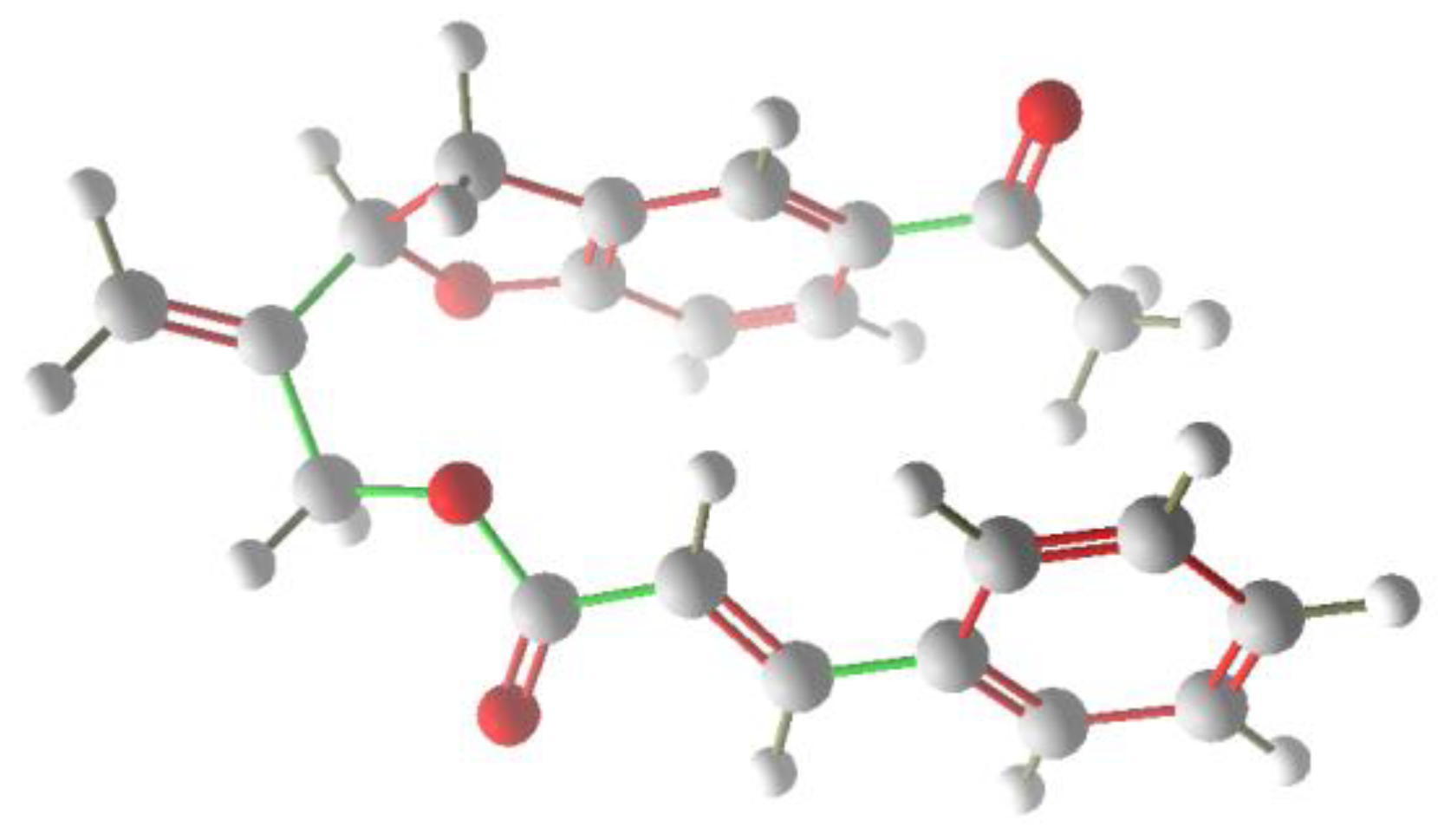
Figure 3.
Minimized molecule of compound 6 (Gaussian 9.0, MM1).

Table 1.
UHPLC PDA and HR-MS analysis of P. quadrangularis ethanol extract.
2.2. Full Metabolome Identification by UHPLC-PDA-MS
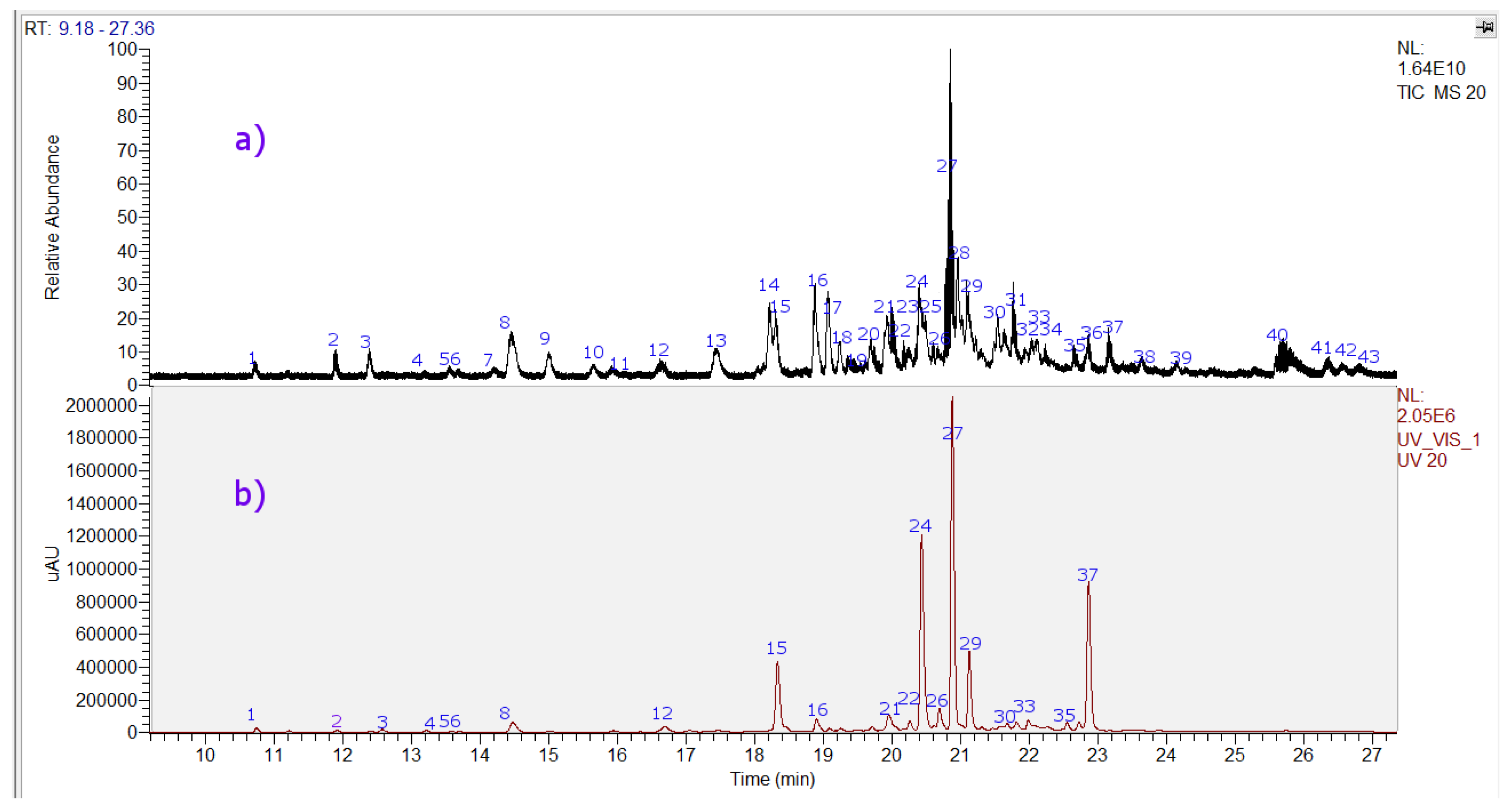
Forty-three compounds were identified including two tremetones (peaks 27 and 37, the last one a new compound), two phenolic acids (peaks 1 and 3), eighteen flavonoids (peaks 4–16, 20, 21, 26, 38, and 41), and twelve diterpenoids, (peaks 17, 18, 22, 23, 25, 28, 29, 31, 32, 34, 36, and 39 in the chromatogram of the ethanol extract of P. quadrangularis. (Figure 4, Table 1). Figure S1a–n (Supplementary Materials) show spectra and structures of compounds detected as examples. The detailed identification is explained below.
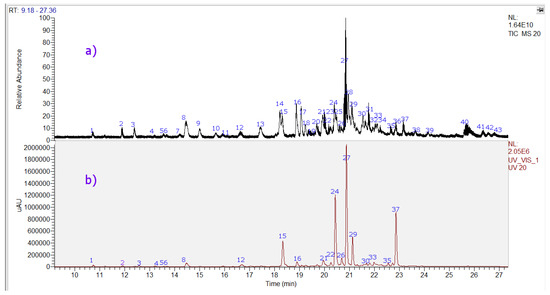
Figure 4.
UHPLC Chromatograms of Parastrephia quadrangularis resin extract. (a) TIC total ion current, negative mode (b) UV at 280 nm.
2.2.1. Phenolic Acids
Peak 1 was identified as caffeoyl-quinic acid (DiCOA, C25H23O12−, Figure S1a) [23] and peak 3 with a [M − H]- ion at m/z: 179.0344 was identified as caffeic acid (C9H7O4−).
2.2.2. Coumarins
Peaks 42 and 43 were identified as umbelliferone and scopoletin by spiking experiments with authentic standards. Peak 2 with a pseudomolecular ion at m/z 353.02919 was identified as the dicoumarin euphorbetin (Figure S1b) [29].
2.2.3. Flavonols
Peak 7 with a pseudomolecular ion at m/z 315.05090 (C16H11O7−) was identified as isorhamnetin, [23] identity confirmed by using co-injection with an authentic standard, and peak 6 as the flavonol kaempferol (C15H9O6−), respectively. The 3,7-methoxilated derivatives of isorhamnetin, peaks 14 and 24 (7-O-methyl-isorhamnetin and 3,7-di-O-methyl-isorhamnetin) were also detected. Peaks 8, 11, and 12 with molecular ions at m/z 375.07202, 345.06152 and 345.06149 were identified as methoxylated myricetin derivatives with molecular formulas C18H15O9−, C17H13O8−, and C17H13O8−, Table 1 [27]. Among those, peaks 11 and 12 were identified as the isomers 3′,5′-dimethoxymyricetin and 3′,4′-dimethoxymyricetin [27], respectively. In the same way, peak 26 was identified as the tetramethyl flavone 5,7-dihydroxy-3,8,3′,4′-tetramethoxyflavone (C19H17O8−) [15]. Peaks 16 and 20 with [M – H]− ions at m/z: 359.07715 (Figure S1d) and 389.08780 (Figure S1g) were identified as 7,3′,5′-trimethoxymyricetin and 6-hydroxy-3,7,3′,5′-tetramethoxymyricetin [27] (C18H15O8−, C19H17O9−), respectively.
2.2.4. Flavanones
Peak 5 was identified as the flavanone eriodictyol (C15H11O6−), peak 4 as Hydroxy-hesperetin (C16H13O7−), peak 9 and 19 as the derivatives hydroxyeriodictyol (C15H11O7−) and methoxyeriodictyol (C16H13O6−). In the same manner, peak 15 was identified as the flavanone hesperetin (C16H13O6−), which was isolated and confirmed by spiking experiments, and peak 4 was identified as hydroxyhesperetin (C16H13O7).
2.2.5. Prenylated Flavonoids
Several interesting isoprenylated derivatives of flavonoids were also detected. Peak 10 with a pseudomolecular anion at m/z: 413.12411 was identified as the dimethoxylated isoprenylated myricetin derivative: 8-isoprenyl-7,4′-dimethoxymyricetin (C22H21O8−, Figure S1c), while peak 13 as the trimethoxylated derivative: 8-isoprenyl-7,3′,4′-trimethoxymyricetin (C23H23O8−), Peak 41 was identified as 8-isoprenyl-7-methoxyquercetin (C21H19O7−) and peak 21 as 8-isoprenyl-7,4′-dimethoxykaempferol (C22H21O6−). Peak 30 was identified as the derivative of the latter, 3-acetyl-8-isoprenyl-7,5,4′-trimethoxykaempferol (C25H25O7−) and peak 35 as the acetylated one, 3-O-acetyl-8-isoprenyl-7,4′-dimethoxykaempferol (C24H23O7−). Peak 38 was identified as 8-isoprenyl-7,4′-dimethoxyapigenin (C22H21O5−, Figure S1m).
2.2.6. Kaurene Terpenoids
Peak 18 with a [M − H]− ion at m/z: 437.21780 (Figure S1f) was identified as the kaurene diterpenoid Adenolin C (C23H33O8−) and peaks 23 and 42 as its derivatives, 11-acetoxy-11,12- dehydrated adenolin C (C25H33O9−) and 11-acetoxy-7-methoxyadenolin C (C26H37O9−), respectively. Peak 32 was identified as the 11,12-dehydrated derivative of Adenolin C (C23H31O7−, Figure S1k).
2.2.7. Clerodane Terpenoids
Bacchalineol was identified with peak 29 (Table 2) while bacchalineol 18-O-malonic acid, bacchalineol 18-O-malonate methyl ester ([M – H]− ion at m/z: 401.23328, C24H33O5−, Figure S1n,e, respectively), and bachalineol 18-O-malonate ethyl ester (Figure S1i) [25] were isolated and identified in the chromatograms with peaks 17, 25, and 28, identity confirmed with spiking experiments with authentic standards. Peak 34 with a [M – H]− ion at m/z: 331.19141 was identified as the furanyl clerodane diterpene hawtriwaic acid (C20H27O4−) [30]. Other compounds detected were related to the antibacterial compound soligagoic acid A (C20H29O2−), [31,32]. Peak 33 with a [M – H]− ion at m/z: 347.18637 was identified as 1,18-dihydroxysolidagoic acid (C20H27O5−), while peak 36 with a pseudomolecular ion at m/z: 359.22278 was identified as the hydroxyl-acetyl derivative of the alcohol, 19-hydroxy-solidagoiol A acetate (C22H31O4−, Figure S1l), and finally peak 31 as the tri-hydroxy-derivative: 1,2,19-trihydroxy-18-acetyl-solidagoiol A (C22H31O6−) [31,32]. Peak 39 with a [M – H]− ion at m/z 401.23328 was identified as the related clerodane compound barticulidiol diacetate (C24H33O5−) [33], Peak 40 with an anion at m/z: 343.22784 was identified as the acetyl derivative of the alcohol, bacchalineol acetate (C22H31O3−, Figure S1n) [25,34].

Table 2.
Gastroprotective effect of compounds isolated from P. quadrangularis at 20 mg/kg on HCl/EtOH-induced gastric lesions in mice.
2.2.8. Tremetones
Peak 27 with a [M – H]− ion at m/z: 363.12385 was identified as p-coumaroyloxyltremetone, identity confirmed by spiking experiments with a standard sample, and peak 37 with a [M – H]− ion at m/z: 347.12855 as a derivative of the latter, which was isolated and used as standard for spiking experiments (see experimental).
2.3. Gastroprotective Capacities of Isolated Compounds (1–7) from Parastrephia Quadrangularis
The results of the gastroprotective effects of compounds 1–7 in the HCl/EtOH-induced gastric lesion model are presented in Table 2. All compounds tested showed gastroprotective activity at a dose of 20 mg/kg (p.o) except compound 2 and 4. The best effect was shown by compound 7 (76%) which was close to that observed with lansoprazole (72%). In addition, the protection displayed by compound 6 (41%) was nearly half of that showed by the positive control, showing that an addition of an OH group in the cinnamoyl moiety for compound 7 is key for the bioactivity. Among the most lipophilics compounds (1–5), the lowest gastroprotective activity was evidenced by compound 5 (bacchalineol 18-O-acetate, 19%), compound 1 (bacchalineol, 12%), and compound 3 (bacchalineol methyl malonate, 11%). Compound 2 (bacchalineol malonic acid) and 4 (bacchalineol 18-O-malonate ethyl ester) did not show any significant difference with the control group.
The gastroprotective activity of several terpenoids have been reported in the literature. Among them, plaunotol, ferruginol and their derivatives, mulinane diterpenoids, dehydroabietic acid derivatives, carnosol and carnosic acid derivatives, labdane diterpenoids, poligodial sesquiterpenoids, oleanolic acid and their derivatives, suaveolol diterpenoid, ent-beyerene derivatives, and lupeol [35,36,37,38,39,40]. The gastroprotective effects of these terpenoids reported in those studies were comparable with our results at the same oral dose. A high amount of compound 7 could explain in part for some of the putative medicinal properties assigned to this species. Further studies are necessary to explain the mechanism of action of compound 7.
3. Materials and Methods
3.1. Chemicals and Plant Material
UHPLC-MS Solvents, LC-MS formic acid, reagent grade lanzoprasole, formalin, ethanol, HCl, deuterated chloroform and deuterated methanol, and reagent grade chloroform were from Merck (Santiago, Chile). Ultrapure water was obtained from a Millipore water purification system (Milli-Q Merck Millipore, Santiago, Chile). UHPLC standards, (kaempferol, caffeic acid, isorhamnetin, hesperetin, eriodictyol, all standards with purity higher than 95% by HPLC) were purchased either from Sigma Aldrich (Saint Louis, Mo, USA), ChromaDex (Santa Ana, CA, USA), or Extrasynthèse (Genay, France).
3.2. Plant Material
Parastrephia quadrangularis (Meyen), Cabrera aerial parts were collected in El Tatio, Atacama Desert, in November 2015 at 4000 m.a.s.l. and were identified by the botanist Alicia Marticorena from the University of Concepción, Chile. Voucher herbarium specimens are kept at the Natural Products Laboratory of the Universidad de Antofagasta under reference number: PQ20151115.
3.3. Extraction
Dried and chopped aerial parts of P. quadrangularis (500 g) collected in Northern Chile were extracted with absolute ethanol (1 L, per 3 times in the dark, 24 h each time) to obtain a medicinal tincture, at room temperature. The tincture was then concentrated in vacuum below 40 °C to yield 36 g of a dark gummy extract.
3.4. Isolation
Thirty-six grams of the crude ethanol extract (concentrated medicinal tincture) was submitted to flash permeation thorough Sephadex LH-20 (700 g) using methanol as eluent and three fractions (PQ-A to PQ-C) were collected after TLC analyses and clear spots (Kieselgel F254 plates, developed with Hexane: EtOAc 8:2 v/v, and spots visualized by spraying with vanillin:sulfuric acid 2% in ethanol and heating) . Fraction PQ-B (7 g) was submitted to open column chromatography (Silica gel 60, 500 g) using hexane ethyl acetate of increasing polarity and 7 fractions were collected (pq-a to pq-f) according to TLC profiles. Fractions pq-d and pq-f were pooled, (2 g) and submitted to a medium pressure column chromatography system composed of an 2.5 cm × 48 cm medium pressure column (Aceglass Inc., Vineland, NY, USA) packed with silicagel (Kieselgel 60 H, Merck, Darmstadt, Germany) using an isocratic solvent system of n-hexane-ethyl acetate (9.5:0.5 v:v) pumped with a medium pressure pump (FMI lab pump, Syosset, NY, USA) with a flow rate of 10 mL-minute. The collected fractions (75) were combined according to TLC analysis and 12 combined fractions (Pq-1 to Pq-12) were obtained. Fraction Pq-3 (376 mg) was re-chromatographed using the same chromatographic system and the known compounds: 1 (32 mg) and 2 (44 mg) were isolated which showed similar NMR spectra [25]. Fraction Pq-5 (543 mg) was rechromatographed on Sephadex L-H 20 to yield diterpenes 3 (35 mg), 4 (43 mg) and 5 (50 mg) whose NMR data corresponded to the data previously reported [25]. Fraction Pq-7 (612 mg) was rechromatographed on Sephadex L-H 20 to yield the new tremetone compound 6 (42 mg) plus the known tremetone 7 (55 mg) [26]. From fraction PQ-C (5 g), after several steps on Sephadex LH-20 permeation (500 g and 200 g, using as mobile phase HPLC grade methanol), the methylated flavones 5,7-dihydroxy-3,8,3′,4′-tetramethoxyflavone (13 mg) 3′,4′-dimethoxymyricetin (23 mg), 3,7,3′-trimethoxyquercetin (17 mg), plus hesperetin (12 mg), showing NMR data as previously reported [15,27,41] plus the coumarins umbelliferone (15 mg) and scopoletin (23 mg) [28], were isolated.
3.5. UHPLC-PDA-MS Instrument
A Thermo Scientific Dionex Ultimate 3000 UHPLC system hyphenated with a Thermo Q exactive focus machine was used [24]. For the analysis, 5 mg of the extract were dissolved in 2 mL of methanol, filtered (thorough PTFE filter) and 10 µL were injected in the instrument, with all specifications set as previously reported [24].
3.6. LC Parameters and MS Parameters
Liquid chromatography was performed using an UHPLC C18 column (Accucore, 150 mm × 4.6 mm internal diameter, 2.5 μm particle size, Thermo Fisher Scientific, Bremen, Germany) operated at 25 °C. The detection wavelengths were 254, 280, 330 and 354 nm, and PDA was recorded from 200 to 800 nm for peak characterization. Mobile phases were 1% formic aqueous solution (A) and 1% formic acid in acetonitrile (B). The gradient program (time (min), % B) was: (0.00, 12); (5.00, 12); (10.00, 20); (15.00, 40); (20.00, 40); (25.00, 70); (35.00, 12) and 15 min for column equilibration before each injection. The flow rate was 1.00 mL min−1, and the injection volume was 10 µL. Standards and the resin extract dissolved in methanol were kept at 10 °C during storage in the autosampler. The HESI II and Orbitrap spectrometer parameters were optimized as previously reported [24,42].
3.7. Animals
Animals were acquired from the Chilean Institute of Health, Chile, Santiago. Swiss albino mice weighing 30 ± 3 g were fasted for 24 h before the experiments. The animals were fed on certified Champion diet with free access to water under standard conditions of 12 h dark-light period, 50% relative humidity and room temperature (22 °C). The protocols were approved by the Animal Use and Care Committee of the Universidad de Chile (07022010) following the recommendations of the Canadian Council on Animal Care as stated previously [40].
3.8. Gastroprotective Effects
The gastroprotective activity of the compounds 1–7 was performed in the HCl/EtOH-induced lesion model as described previously [19]. Briefly, mice were distributed into groups of seven animals each and fasted for 24 h with free access to water prior to the experiments. Fifty min after oral administration of the compounds (20 mg/kg), lansoprazole (20 mg/kg) or 12% Tween 80 (10 mL/kg), all groups were orally treated with 0.2 mL of a solution containing 0.3 M HCl/60% ethanol (HCl/EtOH) for gastric lesion induction. Animals were sacrificed 1 h after the administration of HCl/EtOH, and the stomachs were excised and inflated by injection of saline (1 mL). The ulcerated stomachs were fixed in 5% formalin for 30 min and opened along the greater curvature. Gastric damage visible to the naked eye was observed in the gastric mucosa as elongated black-red lines, parallel to the long axis of the stomach. The length (mm) of each lesion was measured, and the lesion index was expressed as the sum of the length of all lesions.
3.9. Statistical Analysis
The statistical analysis was carried out using the originPro 9.1 software packages (Originlab Corporation, Northampton, MA, USA). The determination was repeated at least three times for each sample solution. Analysis of variance was performed using ANOVA. Significant differences between means were determined by Dunnet comparison test (p values < 0.05 were regarded as significant).
4. Conclusions
The ethanol extract of an endemic species from the Atacama Desert showed several metabolites which were isolated using chromatography and detected using a hybrid UHPLC-PDA-OT-MS instrument. The isolated tremetones and clerodanes showed gastroprotective activity in a mouse model, evidenced by compound 7, which showed better gastroprotective capacity than the control drug lansoprazole (76%). The hyphenated machine equipped with orbitrap-PDA detectors and high-resolution collision cell is an outstanding tool for accurate and fast metabolomics analysis of the Atacama Desert flora, and allowed for the first time the identification of several ent-clerodane and kaurene diterpenes. P. Quadrangularis is rich in phenolic compounds and terpenoids and thus can be useful for the preparation of nutritional supplements. This study might support in part the putative medicinal properties of the plant as a gastroprotective agent.
Supplementary Materials
The following are available online, NMR data and Full mass spectra and structure of several of the compounds identified by UHPLC-ESI-MS-MS.
Author Contributions
M.J.S. and C.A. conceived and designed the experiments; R.B., B.S., and A.A. set up and run the experiments; B.S. and J.B. analyzed the data; C.A. and M.J.S. wrote the manuscript.
Funding
This research was funded by FONDECYT (1180059), (1170871) and (3160414).
Acknowledgments
We thank to the SAG (Servicio Agrícola y Ganadero) and Corporación Nacional Forestal of Chile (CONAF) for allowing us to collect plants and fruits in the national protected areas of Northern Chile belonging to SNASPE (National System of Protected Areas of Chile). We also acknowledge FONDEQUIP EQM140002 for the purchase of the HPLC-MS equipment.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Correction Statement
This article has been republished with a minor correction to the email address and ORCID. This change does not affect the scientific content of the article.
References
- Hilgert, N.I. Plants used in home medicine in the Zenta River basin, Northwest Argentina. J. Ethnopharmacol. 2001, 76, 11–34. [Google Scholar] [CrossRef]
- Villagrán, C.; Castro, V. Ciencia Indígena de los Andes del Norte de Chile, 1st ed.; Editorial Universitaria: Santiago, Chile, 2003. [Google Scholar]
- Giberti, G.C. Herbal folk medicine in northwestern Argentina-compositae. J. Ethnopharmacol. 1983, 7, 321–341. [Google Scholar] [CrossRef]
- Villagrán, C.; Castro, V.; Sánchez, G.; Romo, M.; Latorre, C.; Hinojosa, L.F. La tradición surandina del desierto: Etnobotánica del área del Salar de Atacama (Provincia de El Loa, Región de Antofagasta, Chile). Estud. Atacameños 1998, 16, 7–105. [Google Scholar] [CrossRef]
- Navarro, G.; Arrázola, S.; Atahuachi, M.; De la Barra, N.; Mercado, M.; Ferreira, W.; Moraes, M. Libro Rojo De la Flora Amenazada de Bolivia; Ministerio de Medio Ambiente y Agua Viceministerio de Medio Ambiente, Biodiversidad, Cambios Climaticos y de Gestion y Desarrollo Forestal: Cochabamba, Bolivia, 2012. [Google Scholar]
- D'Almeida, R.E.; Alberto, M.R.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M.I. Antimicrobial phenylpropanoids from the Argentinean highland plant Parastrephia lucida (Meyen) Cabrera. J. Ethnopharmacol. 2012, 142, 407–414. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, R.E.; Isla, M.I.; Vildoza, E.D.L.; Quispe, C.; Schmeda-Hirschmann, G.; Alberto, M.R. Inhibition of arachidonic acid metabolism by the Andean crude drug Parastrephia lucida (Meyen) Cabrera. J. Ethnopharmacol. 2013, 150, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Palavecino Ruiz, M.D.; Ordonez, R.M.; Isla, M.I.; Sayago, J.E. Activity and mode of action of Parastrephia lepidophylla ethanolic extracts on phytopathogenic fungus strains of lemon fruit from Argentine Northwest. Postharvest. Biol. Technol. 2016, 114, 62–68. [Google Scholar] [CrossRef]
- Rodrigo, G.C.; Almanza, G.R.; Akesson, B.; Duan, R.-D. Antiproliferative activity of extracts of some Bolivian medicinal plants. J. Med. Plants Res. 2010, 4, 2204–2210. [Google Scholar]
- Rojo, L.E.; Benites, J.; Lopez, J.; Rojas, M.; Diaz, P.; Ordoñez, J.; Pastene, E. Antioxidant capacity and polyphenolic content of twelve traditionally used herbal medicinal infusions from the South American Andes. Bol. Latinoam. Caribe Plantas Med. Aromát. 2009, 8, 498–508. [Google Scholar]
- Benites, J.; Gutierrez, E.; Lopez, J.; Rojas, M.; Rojo, L.; Costa, M.D.C.; Pilar Vinardell, M.; Calderon, P.B. Evaluation of Analgesic Activities of Tremetone Derivatives Isolated from the Chilean Altiplano Medicine Parastrephia lepidophylla. Nat. Prod. Commun. 2012, 7, 611–614. [Google Scholar] [PubMed]
- Bohlmann, F.; Fritz, U.; King, R.M. Neue tremeton-derivate aus Parastrephia lepidophylla. Phytochemistry 1979, 18, 1403–1405. [Google Scholar] [CrossRef]
- Cheng Lian Ee, G.; Teh, S.S.; Kwong, H.C.; Ibrahim, M.; Tahira, M.; Mah, S.H. Rac-[3-Hydroxy-6,9-dimethyl-6-(4-methylpent-3-en-1-yl)-6a,7,8,9,10,10ahexahydro-6H-1,9-epoxybenzo[c]-chromen-4-yl](phenyl)methanone. Acta Crystallogr. Sect. E 2012, E68, o1091–o1092. [Google Scholar]
- Davis, T.Z.; Lee, S.T.; Collett, M.G.; Stegelmeier, B.L.; Green, B.T.; Buck, S.R.; Pfister, J.A. Toxicity of white snakeroot (Ageratina altissima) and chemical extracts of white snakeroot in goats. J. Agric. Food Chem. 2015, 63, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Loyola, L.A.; Naranjo, J.; Morales, G. 5,7-Dihydroxy-3,8,3′,4′-tetramethoxyflavone from Parastrephia-quadrangularis. Phytochemistry 1985, 24, 1871–1872. [Google Scholar] [CrossRef]
- Di Ciaccio, L.S.; Spotorno, V.G.; Estevez, M.M.C.; Rios, D.J.L.; Fortunato, R.H.; Salvat, A.E. Antifungal activity of Parastrephia quadrangularis (Meyen) Cabrera extracts against Fusarium verticillioides. Lett. Appl. Microbiol. 2018, 66, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.G.; Barreira, L.; Bijttebier, S.; Pieters, L.; Marques, C.; Santos, T.F.; Rodrigues, M.J.; Varela, J.; Custodio, L. Health promoting potential of herbal teas and tinctures from Artemisia campestris subsp maritima: From traditional remedies to prospective products. Sci. Rep. 2018, 8, 4689. [Google Scholar] [CrossRef] [PubMed]
- Varut, R.M.; Gird, C.E.; Rotaru, L.T.; Varut, M.C.; Pisoschi, C.G. Evaluation of polyphenol and flavonoid profiles and the antioxidant effect of carduus acanthoides hydroalcoholic extract compared with vaccinium myrtillus in an animal model of diabetes mellitus. Pharm.Chem. J. 2018, 51, 1088–1095. [Google Scholar] [CrossRef]
- Areche, C.; Rodriguez, J.A.; Razmilic, I.; Yanez, T.; Theoduloz, C.; Schmeda-Hirschmann, G. Gastroprotective and cytotoxic effect of semisynthetic ferruginol derivatives. J. Pharm. Pharmacol. 2007, 59, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Pongpiriyadacha, Y.; Morikawa, T.; Kashima, Y.; Nakano, K.; Yoshikawa, M. Protective effects of polygodial and related compounds on ethanol-induced gastric mucosal lesions in rats: Structural requirements and mode of action. Bioorg. Med. Chem. Lett. 2002, 12, 477–482. [Google Scholar] [CrossRef]
- Boeing, T.; da Silva, L.M.; Somensi, L.B.; Cury, B.J.; Michels Costa, A.P.; Petreanu, M.; Niero, R.; de Andrade, S.F. Antiulcer mechanisms of Vernonia condensata Baker: A medicinal plant used in the treatment of gastritis and gastric ulcer. J. Ethnopharmacol. 2016, 184, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Benites, J.; Areche, C.; Sepúlveda, B. Antioxidant capacities and analysis of phenolic compounds in three endemic Nolana species by HPLC-PDA-ESI-MS. Molecules 2015, 20, 11490–11507. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B. Fast detection of phenolic compounds in extracts of easter pears (Pyrus communis) from the Atacama Desert by ultrahigh-performance liquid chromatography and mass spectrometry (UHPLC-Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Schmeda-Hirschmann, G.; Avendaño, M.; Sepúlveda, B.; Winterhalter, P. Fast high resolution Orbitrap MS fingerprinting of the resin of Heliotropium taltalense Phil. from the Atacama Desert. Ind. Crops Prod. 2016, 85, 159–166. [Google Scholar] [CrossRef]
- Labbe, C.; Castillo, M.; Hernandez, M. Diterpenoids from Baccharis lejía. Phytochemistry 1991, 30, 1607–1611. [Google Scholar] [CrossRef]
- Echiburu-Chau, C.; Pastén, L.; Parra, C.; Bórquez, J.; Mocan, A.; Simirgiotis, M.J. High resolution UHPLC-MS characterization and isolation of main compounds from the antioxidant medicinal plant Parastrephia lucida (Meyen). Saudi Pharm. J. 2017, 25, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Kumari, G.N.K.; Rao, L.J.M.; Rao, N.S.P. Myricetin methyl ethers from Solanum pubescens. Phytochemistry 1984, 23, 2701–2702. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Ramirez, J.E.; Schmeda Hirschmann, G.; Kennelly, E.J. Bioactive coumarins and HPLC-PDA-ESI-ToF-MS metabolic profiling of edible queule fruits (Gomortega keule), an endangered endemic Chilean species. Food Res. Int. 2013, 54, 532–543. [Google Scholar] [CrossRef]
- Dutta, P.K.; Banerjee, D.; Dutta, N.L. Euphorbetin: A new bicoumarin from Euphorbia lathyris L. Tetrahedron Lett. 1972, 13, 601–604. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Favier, L.S.; Rossomando, P.C.; Giordano, O.S.; Tonn, C.E.; Padrón, J.I.; Vázquez, J.T. Diterpenes from Laennecia sophiifolia. Phytochemistry 2000, 55, 721–726. [Google Scholar] [CrossRef]
- Nogueira, R.T.; Shepherd, G.J.; Laverde, A., Jr.; Marsaioli, A.J.; Imamura, P.M. Clerodane-type diterpenes from the seed pods of Hymenaea courbaril var. stilbocarpa. Phytochemistry 2001, 58, 1153–1157. [Google Scholar] [CrossRef]
- Starks, C.M.; Williams, R.B.; Goering, M.G.; O’Neil-Johnson, M.; Norman, V.L.; Hu, J.-F.; Garo, E.; Hough, G.W.; Rice, S.M.; Eldridge, G.R. Antibacterial clerodane diterpenes from Goldenrod (Solidago virgaurea). Phytochemistry 2010, 71, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Tonn, C.E.; Giordano, O.S.; Bessalle, R.; Frolow, F.; Lavie, D. The structure of bartemidiolide, a clerodane-type diterpene from Baccharis artemisioides. Phytochemistry 1988, 27, 489–491. [Google Scholar] [CrossRef]
- Anthonsen, T.; Henderson, M.S.; Martin, A.; Murray, R.D.H.; McCrindle, R.; McCaster, D. Constituents of solidago species. Part IV. Solidagoic acids A and B, diterpenoids from solidago gigantea var. serotina. Can. J. Chem. 1973, 51, 1333–1345. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.; Bonesi, M.; Menichini, F.; Conforti, F.; Statti, G. Natural products as gastroprotective and antiulcer agents: Recent developments. Nat. Prod. Commun. 2008, 3, 2129–2144. [Google Scholar]
- Awaad, A.S.; El-Meligy, R.M.; Soliman, G.A. Natural products in treatment of ulcerative colitis and peptic ulcer. J. Saudi Chem. Soc. 2013, 17, 101–124. [Google Scholar] [CrossRef]
- Lewis, D.A.; Hanson, P.J. 4 Anti-ulcer drugs of plant origin. In Progress in Medicinal Chemistry; Ellis, G.P., West, G.B., Eds.; Elsevier Science: Amsterdam, Netherlands, 1991; pp. 201–231. [Google Scholar]
- Vera-Arzave, C.; Antonio, L.C.; Arrieta, J.; Cruz-Hernandez, G.; Velasquez-Mendez, A.M.; Reyes-Ramirez, A.; Sanchez-Mendoza, M.E. Gastroprotection of suaveolol, isolated from Hyptis suaveolens, against ethanol-induced gastric lesions in Wistar rats: role of prostaglandins, nitric oxide and sulfhydryls. Molecules 2012, 17, 8917–8927. [Google Scholar] [CrossRef] [PubMed]
- Parra, T.; Benites, J.; Ruiz, L.M.; Sepulveda, B.; Simirgiotis, M.; Areche, C. Gastroprotective activity of ent-beyerene derivatives in mice: Effects on gastric secretion, endogenous prostaglandins and non-protein sulfhydryls. Bioorg. Med. Chem. Lett. 2015, 25, 2813–2817. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.A.; Khundmiri, S.U.K.; Khundmiri, S.R.; Al-Sanea, M.M.; Mok, P.L. Fruit-derived polysaccharides and terpenoids: Recent update on the gastroprotective effects and mechanisms. Front. Pharmacol. 2018, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-P.; Cheng, F.-Q.; Ji, L.; Yu, H.-Y. Chemical constituents of physalis pubescens. Zhongguo Zhong Yao Za Zhi 2015, 40, 4424–4427. [Google Scholar] [PubMed]
- Garneau, F.-X.; Collin, G.J.; Jean, F.-I.; Gagnon, H.; Lopez Arze, J.B. Essential oils from Bolivia. XII. Asteraceae: Ophryosporus piquerioides (DC) Benth. ex Baker. J. Essent. Oil Res. 2013, 25, 388–393. [Google Scholar] [CrossRef]
Sample Availability: Samples of the plant and pure compounds are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).