Ultrasound Treatment on Stability of Total and Individual Anthocyanin Extraction from Blueberry Pomace: Optimization and Comparison
Abstract
:1. Introduction
2. Results
2.1. Anthocyanin Composition of Blueberry Pomace
2.2. Time-Dependent Anthocyanin Contents by Conventional Solvent Extraction
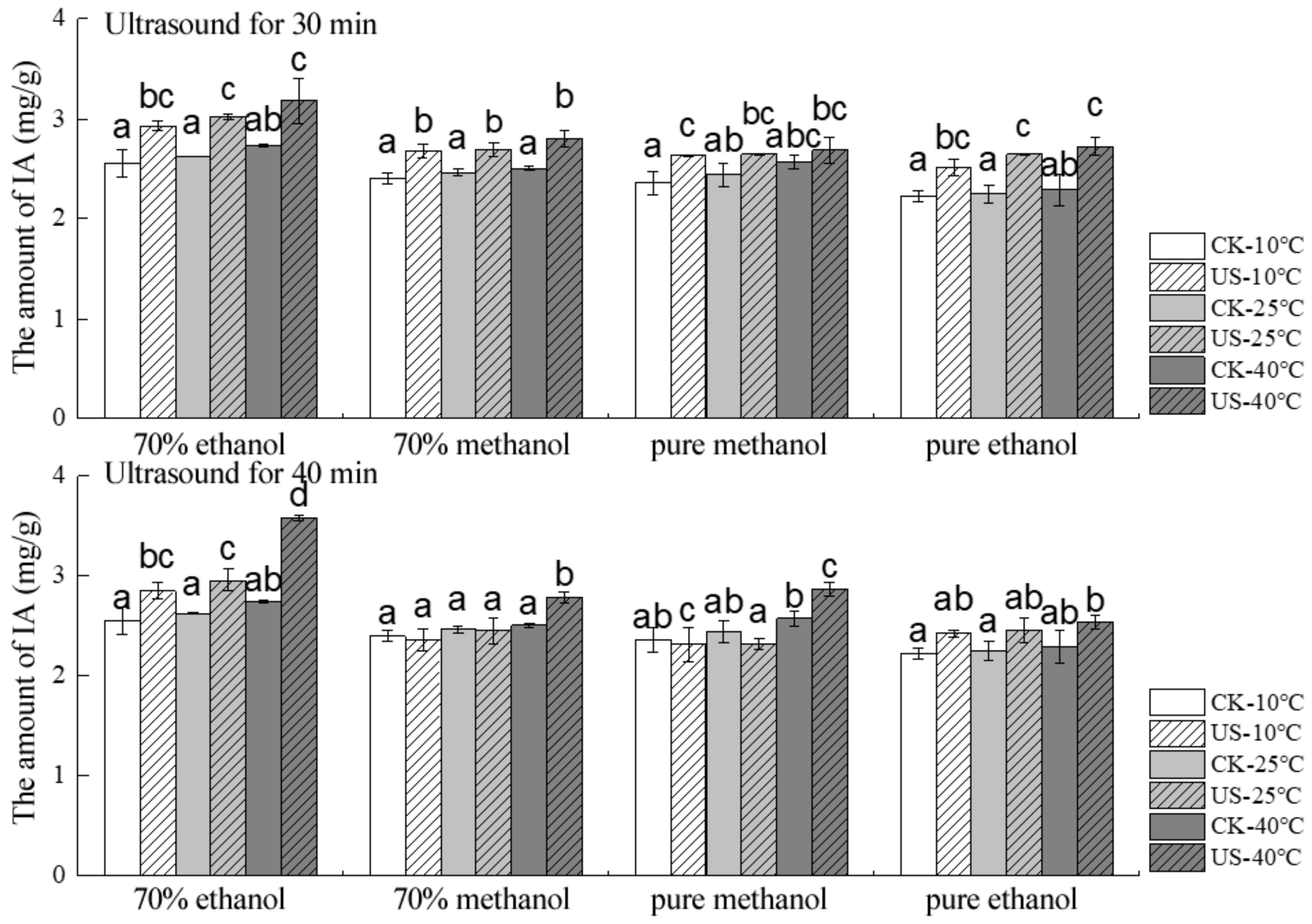
2.3. Effect of Ultrasound Time on the Stability of Total and Individual Anthocyanins
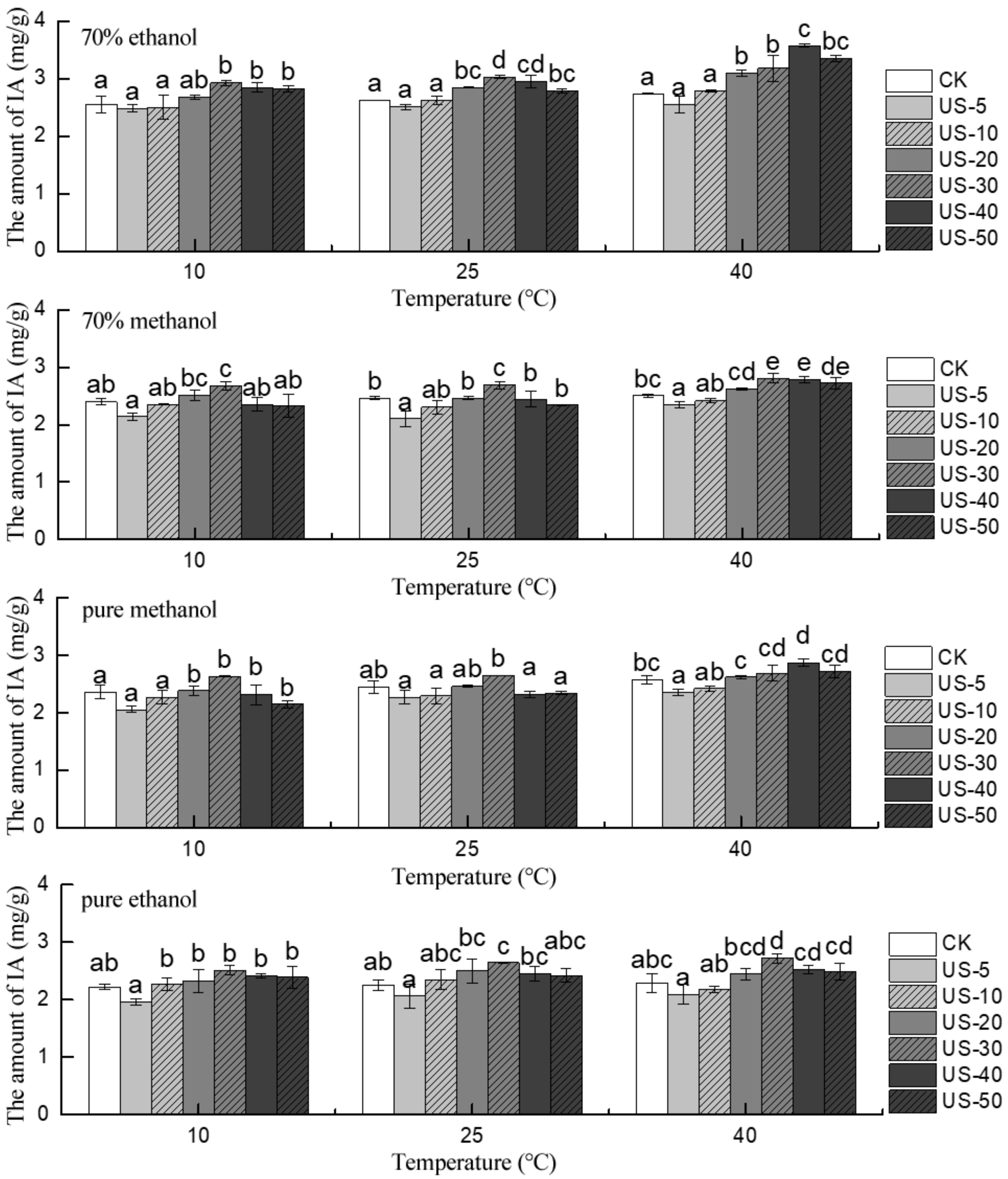
2.4. Effect of Ultrasound Temperature on the Stability of Total and Individual Anthocyanins
2.5. Effect of Ultrasound Solvent on the Stability of Total and Individual Anthocyanins
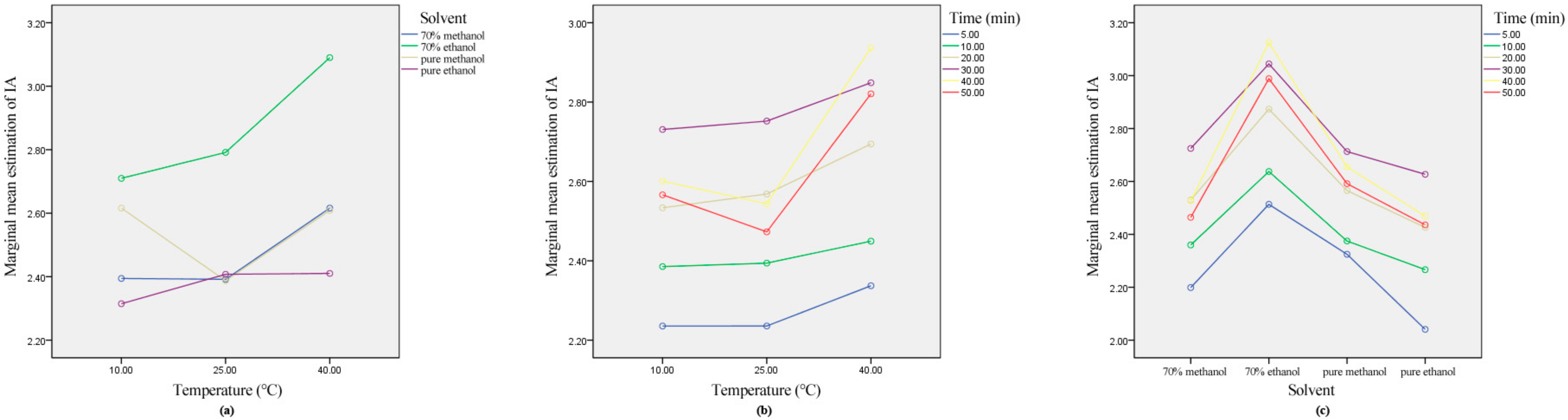
2.6. Ultrasound Interactions on Total and Individual Anthocyanins Extraction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Blueberry Ppomace Samples
4.3. Identification of Anthocyanins in Blueberry Pomace
4.4. Ultrasound Treatment
4.5. Conventional Solvent Extraction Treatment
4.6. Determination of Total and Individual Anthocyanins Contents
4.7. Statistical Analysis and Figure Plotting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chu, W.; Gao, H.; Cao, S.; Fang, X.; Chen, H.; Xiao, S. Composition and morphology of cuticular wax in blueberry (Vaccinium spp.) fruits. Food Chem. 2017, 219, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Q.; Chen, X.Y.; Li, X.; Wang, Y.; Zhang, J.L. Comparison and screening of bioactive phenolic compounds in different blueberry cultivars: Evaluation of anti-oxidation and α-glucosidase inhibition effect. Food Res. Int. 2017, 100, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Istek, N.; Gurbuz, O. Investigation of the impact of blueberries on metabolic factors influencing health. J. Funct. Foods 2017, 38, 298–307. [Google Scholar] [CrossRef]
- Melo, P.S.; Massarioli, A.P.; Denny, C.; dos Santos, L.F.; Franchin, M.; Pereira, G.E.; Vieira, T.M.F.D.S.; Rosalen, P.L.; Alencar, S.M.D. Winery by-products: Extraction optimization, phenolic composition and cytotoxic evaluation to act as a new source of scavenging of reactive oxygen species. Food Chem. 2015, 181, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.T. Influence of extraction conditions on ultrasound-assisted recovery of bioactive phenolics from blueberry pomace and their antioxidant activity. Molecules 2018, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jung, J.; Tomasino, E.; Zhao, Y. Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT Food Sci. Technol. 2016, 72, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Khalid, M.; Saeed ur, R.; Bilal, M.; Huang, D.F. Role of flavonoids in plant interactions with the environment and against human pathogens—A review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Jaeschke, D.P.; Tessaro, I.C.; Marczak, L.D.F. Effects of ohmic and conventional heating on anthocyanin degradation during the processing of blueberry pulp. LWT Food Sci. Technol. 2013, 51, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Du, X.; Cui, D.; Wang, X.; Yang, Z.; Zhu, G. Improvement of stability of blueberry anthocyanins by carboxymethyl starch/xanthan gum combinations microencapsulation. Food Hydrocoll. 2019, 91, 238–245. [Google Scholar] [CrossRef]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef]
- Swer, T.L.; Mukhim, C.; Bashir, K.; Chauhan, K. Optimization of enzyme aided extraction of anthocyanins from Prunus nepalensis L. LWT 2018, 91, 382–390. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Extraction, multi-response analysis, and optimization of biologically active phenolic compounds from the pulp of Indian jamun fruit. Food Sci. Biotechnol. 2014, 23, 9–14. [Google Scholar] [CrossRef]
- Jiao, G.; Kermanshahi pour, A. Extraction of anthocyanins from haskap berry pulp using supercritical carbon dioxide: Influence of co-solvent composition and pretreatment. LWT 2018, 98, 237–244. [Google Scholar] [CrossRef]
- Garcia Mendoza, M.D.P.; Espinosa Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Marostica Junior, M.R.; Rostagno, M.A.; Martinez, J. Extraction of phenolic compounds and anthocyanins from juçara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
- Romero Diez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodriguez Rojo, S.; Cocero, M.J.; Matias, A.A. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Q.; Li, X.; Wang, Y.; Zhu, J.; Ning, C.; Chang, X.; Meng, X. Effects of high hydrostatic pressure on physicochemical properties, enzymes activity, and antioxidant capacities of anthocyanins extracts of wild Lonicera caerulea berry. Innov. Food Sci. Emerg. Technol. 2016, 36, 48–58. [Google Scholar] [CrossRef]
- Putnik, P.; Bursac Kovacevic, D.; Jezek, D.; Sustic, I.; Zoric, Z.; Dragovic Uzelac, V. High-pressure recovery of anthocyanins from grape skin pomace (Vitis vinifera cv. Teran) at moderate temperature. J. Food Process. Preserv. 2018, 42, e13342. [Google Scholar] [CrossRef]
- Wu, Y.; Han, Y.; Tao, Y.; Fan, S.; Chu, D.T.; Ye, X.; Ye, M.; Xie, G. Ultrasound assisted adsorption and desorption of blueberry anthocyanins using macroporous resins. Ultrason. Sonochem. 2018, 48, 311–320. [Google Scholar] [CrossRef]
- Backes, E.; Pereira, C.; Barros, L.; Prieto, M.A.; Genena, A.K.; Barreiro, M.F.; Ferreira, I.C.F.R. Recovery of bioactive anthocyanin pigments from Ficus carica L. peel by heat, microwave, and ultrasound based extraction techniques. Food Res. Int. 2018, 113, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization of heat- and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, Q.; Belwal, T.; Li, L.; Aalim, H.; Wu, Q.; Duan, Z.; Zhang, X.; Luo, Z. Ultrasonic impact on viscosity and extraction efficiency of polyethylene glycol: A greener approach for anthocyanins recovery from purple sweet potato. Food Chem. 2019, 283, 59–67. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of Ultrasound-Assisted Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography–electrospray ionization-ion trap-time of flight-mass spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Han, Y.; Zhou, F.; Zhang, D.; Ji, B.; Hu, J.; Lv, Y.; Cai, S.; Wei, Y.; et al. Identification of anthocyanin components of wild chinese blueberries and amelioration of light-induced retinal damage in pigmented rabbit using whole berries. J. Agric. Food Chem. 2011, 59, 356–363. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Barcia, M.T.; Rebello, L.P.G.; Gomez Alonso, S.; Duarte, R.M.T.; Duarte, M.C.T.; Godoy, H.T.; Hermosin Gutierrez, I. Antimicrobial activity and differentiation of anthocyanin profiles of rabbiteye and highbush blueberries using HPLC–DAD–ESI-MSn and multivariate analysis. J. Funct. Foods 2016, 26, 506–516. [Google Scholar] [CrossRef]
- Li, D.; Meng, X.; Li, B. Profiling of anthocyanins from blueberries produced in China using HPLC-DAD-MS and exploratory analysis by principal component analysis. J. Food Compos. Anal. 2016, 47, 1–7. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Ma, Y.; Sun, X.; Lin, Y.; Meng, X. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. J. Food Compos. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Ekici, L.; Simsek, Z.; Ozturk, I.; Sagdic, O.; Yetim, H. Effects of temperature, time, and pH on the stability of anthocyanin extracts: Prediction of total anthocyanin content using nonlinear models. Food Anal. Methods 2014, 7, 1328–1336. [Google Scholar] [CrossRef]
- Borges, G.D.S.C.; Vieira, F.G.K.; Copetti, C.; Gonzaga, L.V.; Fett, R. Optimization of the extraction of flavanols and anthocyanins from the fruit pulp of Euterpe edulis using the response surface methodology. Food Res. Int. 2011, 44, 708–715. [Google Scholar] [CrossRef]
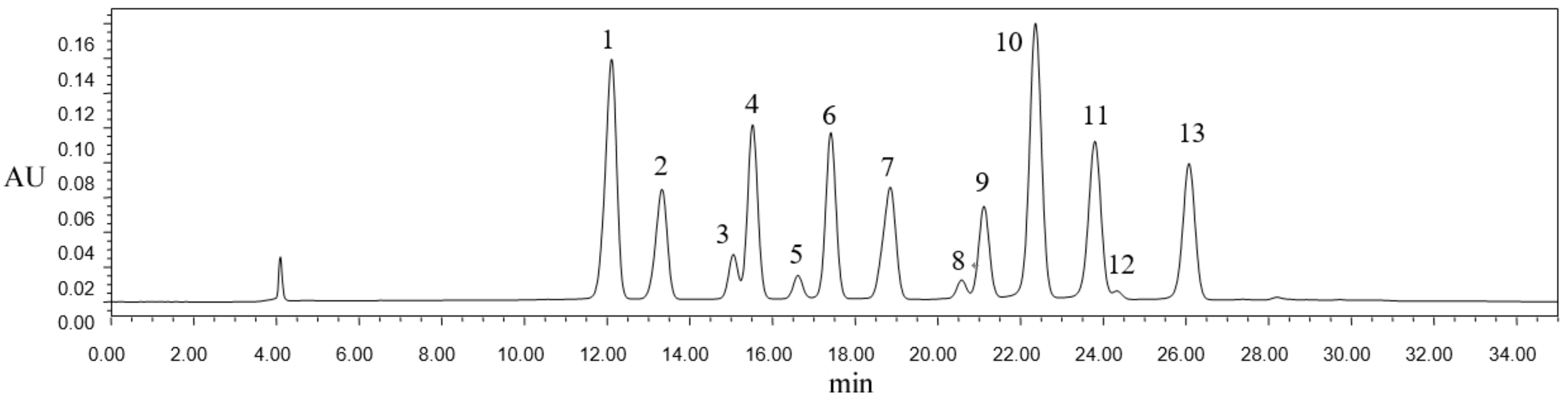

| Peak | Retention Time | +ESIMS (m/z) | Formula | Proportion (%) | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 12.109 | 429 | - | 15.29 | unknown |
| 2 | 13.327 | 465 | C21H21O12 | 7.00 | delphinidin-3-galactoside |
| 34 | 15.055 | 384 | - | 2.27 | unknown |
| 15.521 | 465 | C21H21O12 | 9.84 | delphinidin-3-glucoside | |
| 5 | 16.618 | 464 | - | 1.29 | unknown |
| 6 | 17.412 | 435 | C20H19O11 | 9.46 | delphinidin-3-arabinoside |
| 7 | 18.848 | 449 | C21H21O11 | 8.01 | cyanidin-3- glucoside |
| 8 | 20.576 | 479 | C22H23O12 | 0.99 | petunidin-3-galactoside |
| 9 | 21.116 | 479 | C22H23O12 | 5.50 | petunidin-3-glucoside |
| 10 | 22.362 | 493 | C23H25O12 | 18.56 | malvidin-3-galactoside |
| 11 | 23.799 | 449 | C21H21O11 | 11.11 | petunidin-3-arabinoside |
| 12 | 24.33 | 493 | C23H25O12 | 9.15 | malvidin-3- glucoside |
| 13 | 26.076 | 463 | C22H23O11 | 0.12 | peonidin-3-galactoside |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Gong, H.; Li, L.; Chen, S.; Ye, X. Ultrasound Treatment on Stability of Total and Individual Anthocyanin Extraction from Blueberry Pomace: Optimization and Comparison. Molecules 2019, 24, 2621. https://doi.org/10.3390/molecules24142621
Hu W, Gong H, Li L, Chen S, Ye X. Ultrasound Treatment on Stability of Total and Individual Anthocyanin Extraction from Blueberry Pomace: Optimization and Comparison. Molecules. 2019; 24(14):2621. https://doi.org/10.3390/molecules24142621
Chicago/Turabian StyleHu, Weiwei, Hui Gong, Lanqi Li, Shiguo Chen, and Xingqian Ye. 2019. "Ultrasound Treatment on Stability of Total and Individual Anthocyanin Extraction from Blueberry Pomace: Optimization and Comparison" Molecules 24, no. 14: 2621. https://doi.org/10.3390/molecules24142621
APA StyleHu, W., Gong, H., Li, L., Chen, S., & Ye, X. (2019). Ultrasound Treatment on Stability of Total and Individual Anthocyanin Extraction from Blueberry Pomace: Optimization and Comparison. Molecules, 24(14), 2621. https://doi.org/10.3390/molecules24142621





