Discovery of (5-Phenylfuran-2-yl)methanamine Derivatives as New Human Sirtuin 2 Inhibitors
Abstract
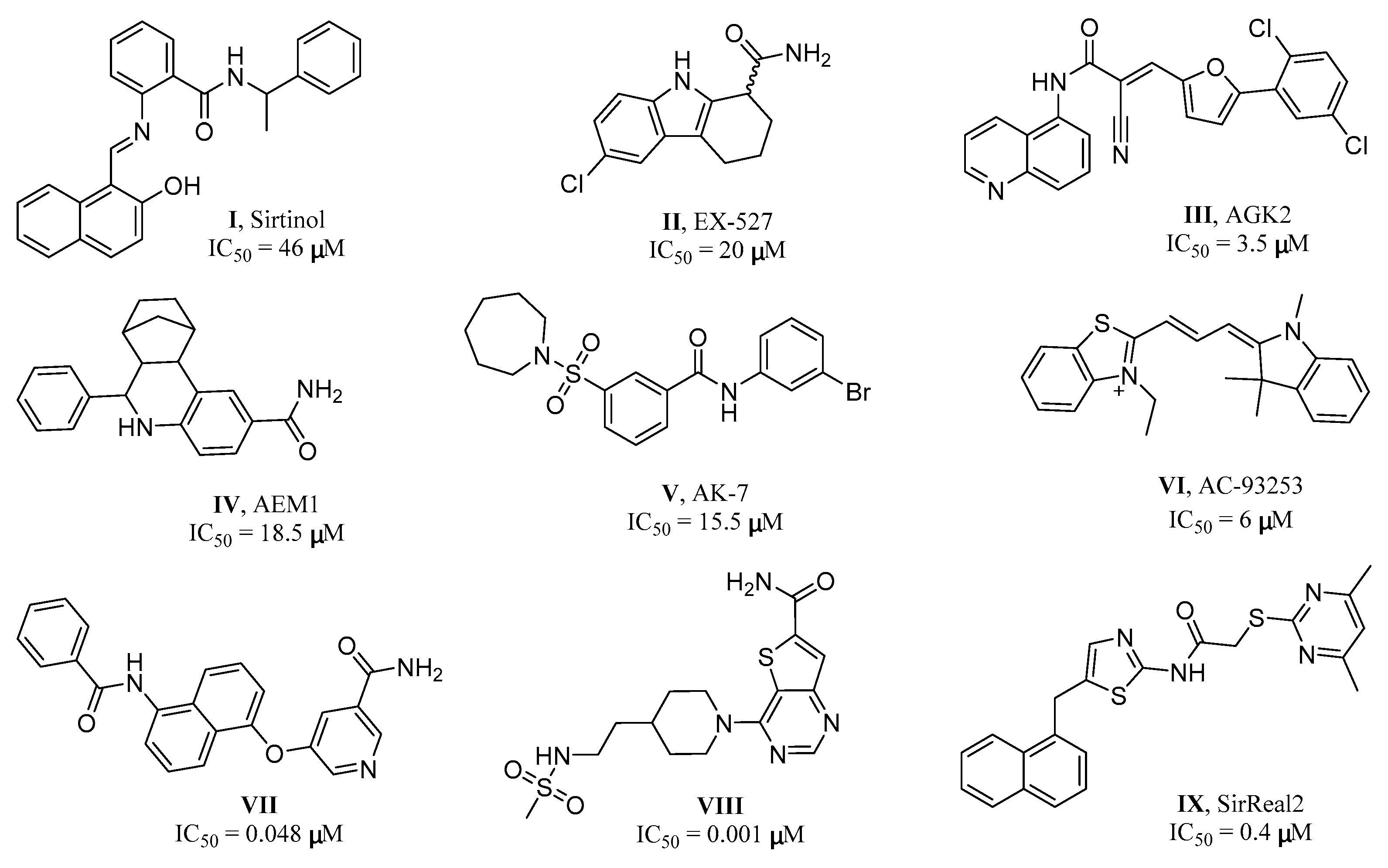
1. Introduction
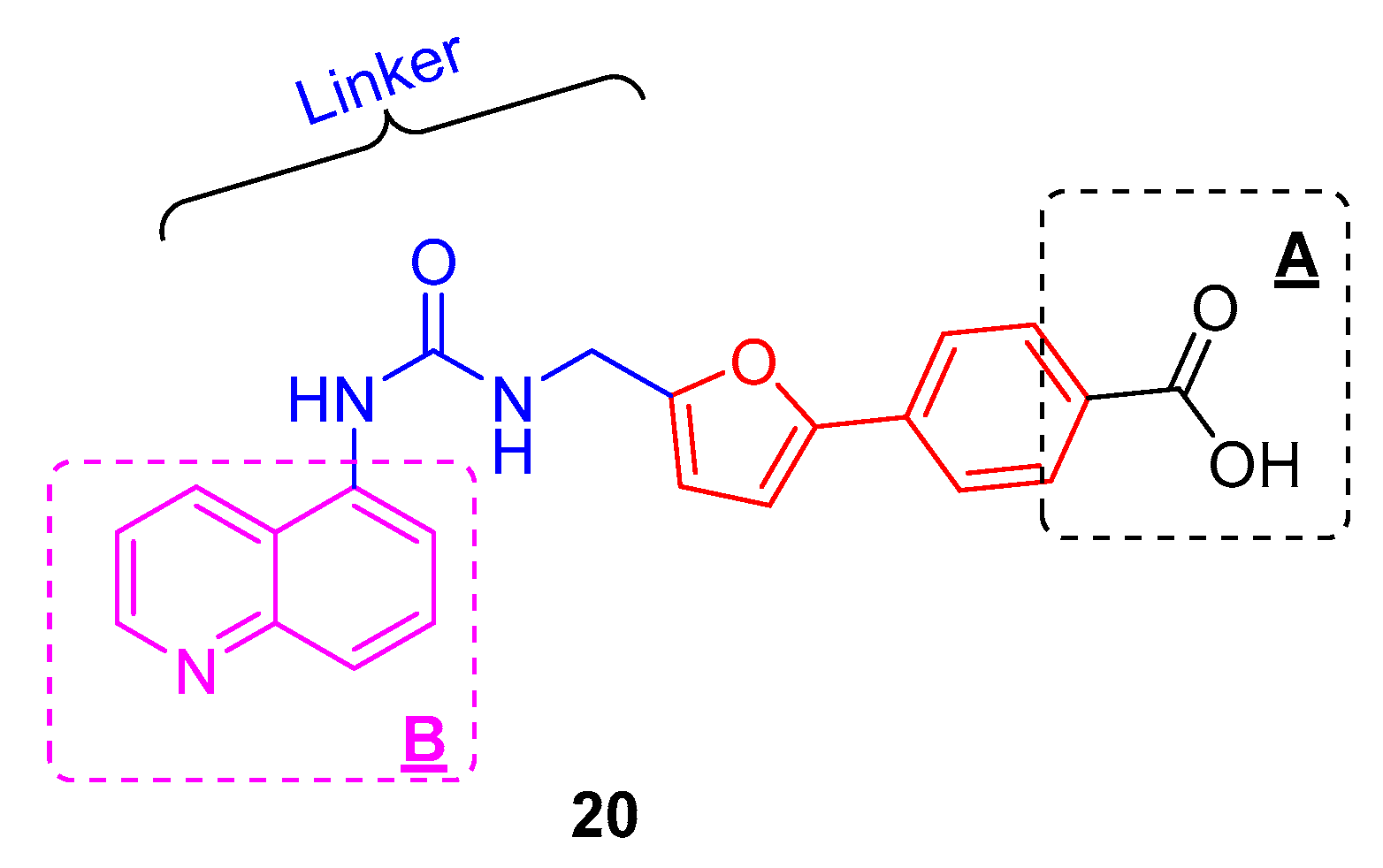
2. Results and Discussion
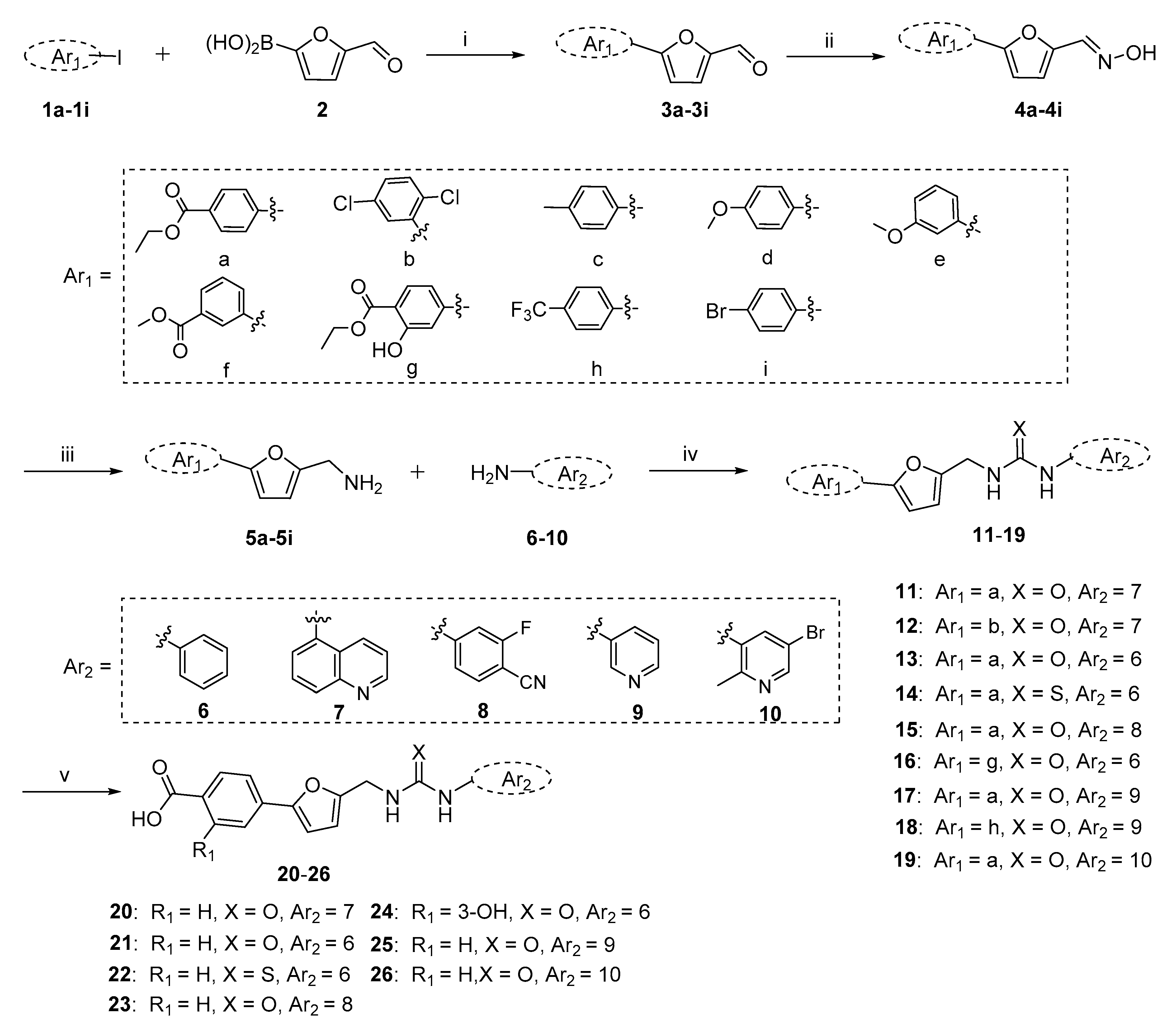
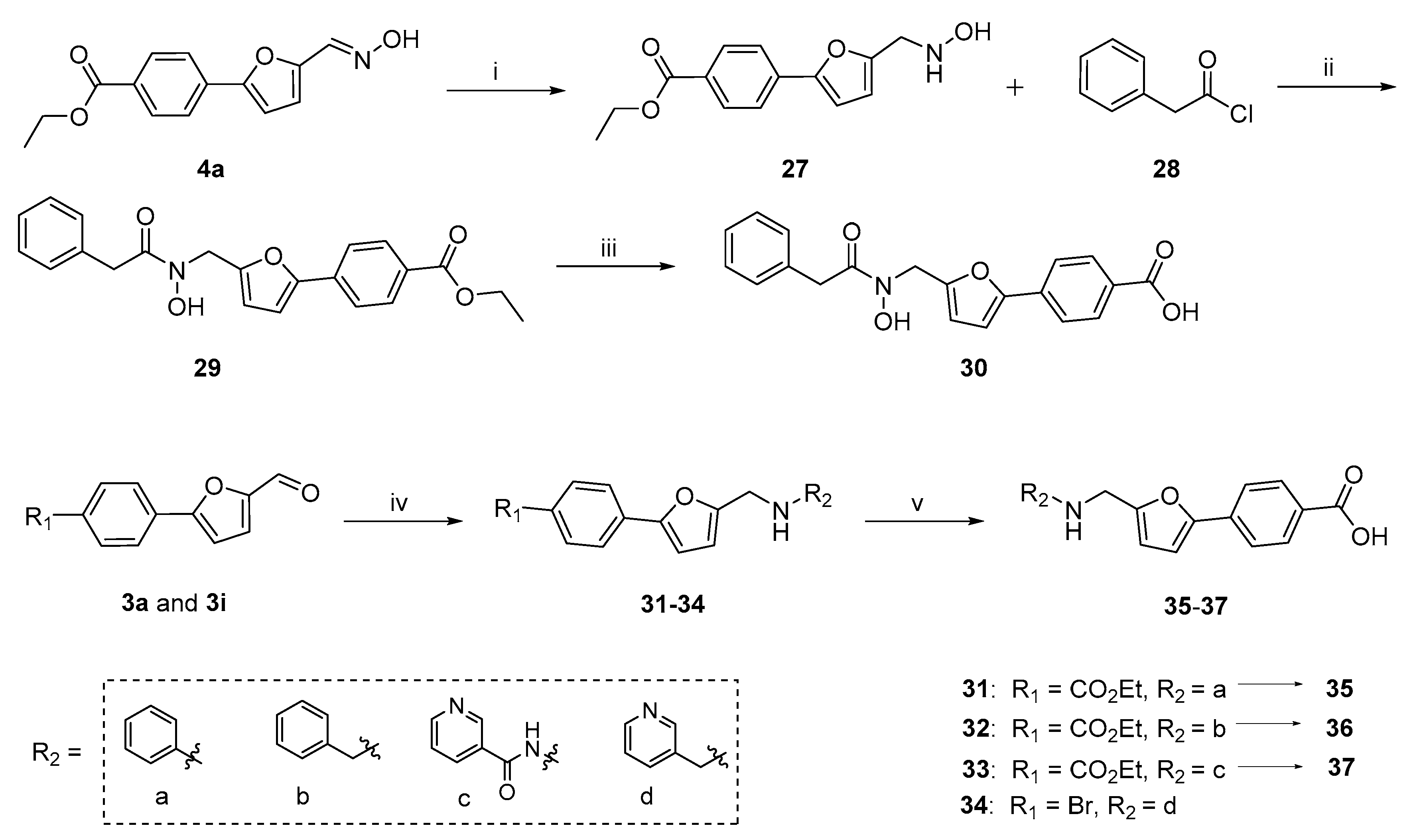
2.1. Chemistry
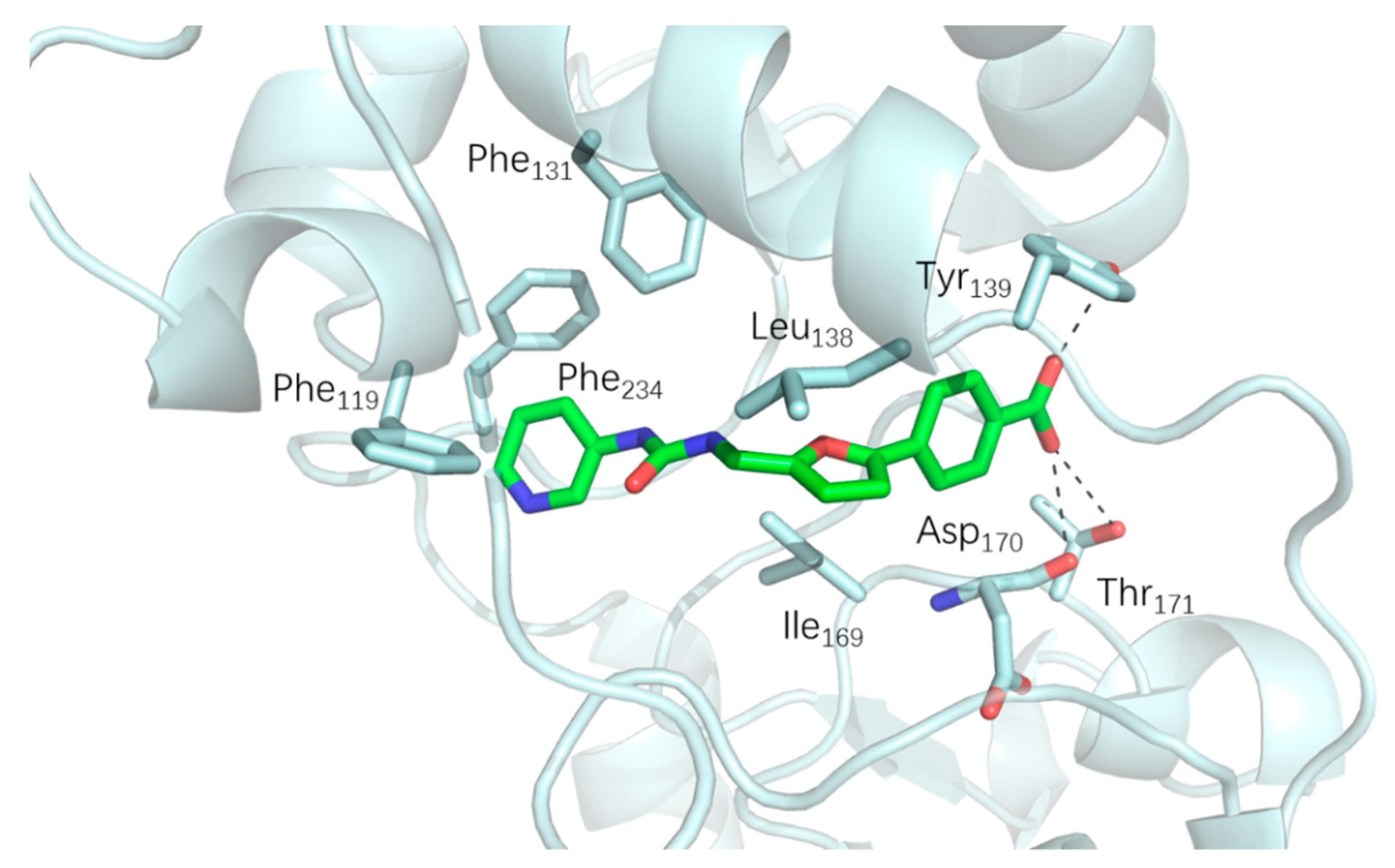
2.2. SAR Studies with SIRT2
3. Materials and Methods
3.1. Synthesis
3.1.1. General Procedure for the Preparation of Key Intermediates 5a–5i
3.1.2. Hantzsch-Involved Reductive Amination Used for Compounds 31–34
3.2. Inhibition Assays
3.3. Molecular Docking Assays
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhalla, K.N. Epigenetic and Chromatin Modifiers as Targeted Therapy of Hematologic Malignancies. J. Clin. Oncol. 2005, 23, 3971–3993. [Google Scholar] [CrossRef] [PubMed]
- Blander, G.; Guarente, L. The Sir2 Family of Protein Deacetylases. Annu. Rev. Biochem. 2004, 73, 417–435. [Google Scholar] [CrossRef]
- Liu, S.; Ji, S.; Yu, Z.-J.; Wang, H.-L.; Cheng, X.; Li, W.-J.; Jing, L.; Yu, Y.; Chen, Q.; Yang, L.-L.; et al. Structure-based discovery of new selective small-molecule sirtuin 5 inhibitors. Chem. Biol. Drug Des. 2018, 91, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Hirschey, M.D. Old Enzymes, New Tricks: Sirtuins Are NAD+-Dependent De-acylases. Cell Metab. 2011, 14, 718–719. [Google Scholar] [CrossRef]
- Jing, H.; Lin, H. Sirtuins in Epigenetic Regulation. Chem. Rev. 2015, 115, 2350–2375. [Google Scholar] [CrossRef]
- Yang, L.; Ma, X.; He, Y.; Yuan, C.; Chen, Q.; Li, G.; Chen, X. Sirtuin 5: A review of structure, known inhibitors and clues for developing new inhibitors. Sci. China Life Sci. 2016, 60, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Goto, Y.; Nishimasu, H.; Morimoto, J.; Ishitani, R.; Dohmae, N.; Takeda, N.; Nagai, R.; Komuro, I.; Suga, H.; et al. Structural basis for potent inhibition of SIRT2 deacetylase by a macrocyclic peptide inducing dynamic structural change. Structure 2014, 22, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, J.; Liao, M.; Hu, M.; Li, W.; Ouyang, H.; Wang, X.; Ye, T.; Zhang, Y.; Ouyang, L. An overview of Sirtuins as potential therapeutic target: Structure, function and modulators. Eur. J. Med. Chem. 2019, 161, 48–77. [Google Scholar] [CrossRef]
- Das, C.; Lucia, M.S.; Hansen, K.C.; Tyler, J.K. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature 2009, 459, 113–117. [Google Scholar] [CrossRef]
- Vaquero, A.; Scher, M.B.; Lee, D.H.; Sutton, A.; Cheng, H.L.; Alt, F.W.; Serrano, L.; Sternglanz, R.; Reinberg, D. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006, 20, 1256–1261. [Google Scholar] [CrossRef]
- North, B.J.; Marshall, B.L.; Borra, M.T.; Denu, J.M.; Verdin, E. The Human Sir2 Ortholog, SIRT2, Is an NAD+-Dependent Tubulin Deacetylase. Mol. Cell 2003, 11, 437–444. [Google Scholar] [CrossRef]
- Peck, B.; Chen, C.-Y.; Ho, K.-K.; Di Fruscia, P.; Myatt, S.S.; Coombes, R.C.; Fuchter, M.J.; Hsiao, C.-D.; Lam, E.W.-F. SIRT Inhibitors Induce Cell Death and p53 Acetylation through Targeting Both SIRT1 and SIRT2. Mol. Cancer Ther. 2010, 9, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Jing, E.; Gesta, S.; Kahn, C.R. SIRT2 Regulates Adipocyte Differentiation through FoxO1 Acetylation/Deacetylation. Cell Metab. 2007, 6, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Matsumori, H.; Nakayama, Y.; Osaki, M.; Kojima, H.; Kurimasa, A.; Ito, H.; Mori, S.; Katoh, M.; Oshimura, M.; et al. SIRT2 down-regulation in HeLa can induce p53 accumulation via p38 MAPK activation-dependent p300 decrease, eventually leading to apoptosis. Genes Cells 2011, 16, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Rothgiesser, K.M.; Erener, S.; Waibel, S.; Luscher, B.; Hottiger, M.O. Correction: SIRT2 regulates NF-kappaB-dependent gene expression through deacetylation of p65 Lys310. J. Cell Sci. 2019, 132, 4251–4258. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Song, C.; Wang, X.; Zhang, G.; Wang, Y.; Jiang, X.; Sun, Q.; Huang, L.; Xiang, R.; Hu, Y.; et al. Discovery of New SIRT2 Inhibitors by Utilizing a Consensus Docking/Scoring Strategy and Structure–Activity Relationship Analysis. J. Chem. Inf. Model. 2017, 57, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Dryden, S.C.; Nahhas, F.A.; Nowak, J.E.; Goustin, A.-S.; Tainsky, M.A. Role for Human SIRT2 NAD-Dependent Deacetylase Activity in Control of Mitotic Exit in the Cell Cycle. Mol. Cell. Biol. 2003, 23, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Hiratsuka, M.; Osaki, M.; Oshimura, M. The Molecular Biology of Mammalian SIRT Proteins: SIRT2 Functions on Cell Cycle Regulation. Cell Cycle 2007, 6, 1011–1018. [Google Scholar] [CrossRef]
- Machado de Oliveira, R.; Sarkander, J.; Kazantsev, A.; Outeiro, T. SIRT2 as a Therapeutic Target for Age-Related Disorders. Front. Pharmacol. 2012, 3, 1–9. [Google Scholar] [CrossRef]
- Beirowski, B.; Gustin, J.; Armour, S.M.; Yamamoto, H.; Viader, A.; North, B.J.; Michán, S.; Baloh, R.H.; Golden, J.P.; Schmidt, R.E.; et al. Sir-two-homolog 2 (Sirt2) modulates peripheral myelination through polarity protein Par-3/atypical protein kinase C (aPKC) signaling. Proc. Natl. Acad. Sci. USA 2011, 108, E952–E961. [Google Scholar] [CrossRef]
- Eskandarian, H.A.; Impens, F.; Nahori, M.-A.; Soubigou, G.; Coppée, J.-Y.; Cossart, P.; Hamon, M.A. A Role for SIRT2-Dependent Histone H3K18 Deacetylation in Bacterial Infection. Science 2013, 341, 1238858. [Google Scholar] [CrossRef] [PubMed]
- Pais, T.F.; Szegő, É.M.; Marques, O.; Miller-Fleming, L.; Antas, P.; Guerreiro, P.; de Oliveira, R.M.; Kasapoglu, B.; Outeiro, T.F. The NAD-dependent deacetylase sirtuin 2 is a suppressor of microglial activation and brain inflammation. EMBO J. 2013, 32, 2603–2616. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Alam, H.B.; Liu, B.; Bronson, R.T.; Nikolian, V.C.; Wu, E.; Chong, W.; Li, Y. Selective Inhibition of SIRT2 Improves Outcomes in a Lethal Septic Model. Curr. Mol. Med. 2015, 15, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Vassilopoulos, A.; Wang, R.-H.; Lahusen, T.; Xiao, Z.; Xu, X.; Li, C.; Veenstra, T.D.; Li, B.; Yu, H.; et al. SIRT2 Maintains Genome Integrity and Suppresses Tumorigenesis through Regulating APC/C Activity. Cancer Cell 2011, 20, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Donmez, G.; Outeiro, T.F. SIRT1 and SIRT2: Emerging targets in neurodegeneration. EMBO Mol. Med. 2013, 5, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Luthi-Carter, R.; Taylor, D.M.; Pallos, J.; Lambert, E.; Amore, A.; Parker, A.; Moffitt, H.; Smith, D.L.; Runne, H.; Gokce, O.; et al. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 7927–7932. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Zhu, Y.M.; Ozden, O.; Kim, H.S.; Jiang, H.Y.; Deng, C.X.; Gius, D.; Vassilopoulos, A. SIRT2 is a tumor suppressor that connects aging, acetylome, cell cycle signaling, and carcinogenesis. Transl. Cancer Res. 2012, 1, 15–21. [Google Scholar] [PubMed]
- Grozinger, C.M.; Chao, E.D.; Blackwell, H.E.; Moazed, D.; Schreiber, S.L. Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J. Biol. Chem. 2001, 276, 38837–38843. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.; Fischer, F.; Nguyen, G.T.; Lakshminarasimhan, M.; Schutkowski, M.; Weyand, M.; Steegborn, C. Ex-527 inhibits Sirtuins by exploiting their unique NAD+-dependent deacetylation mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, E2772–E2781. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, H.; Yu, X.; Peng, T.; Wang, G.; Wen, X.; Sun, Y.; Liu, S.; Zhang, S.; Hu, L.; et al. Synthesis and Evaluation of Novel Benzofuran Derivatives as Selective SIRT2 Inhibitors. Molecules 2017, 22, 1348. [Google Scholar] [CrossRef]
- Outeiro, T.F.; Kontopoulos, E.; Altmann, S.M.; Kufareva, I.; Strathearn, K.E.; Amore, A.M.; Volk, C.B.; Maxwell, M.M.; Rochet, J.C.; McLean, P.J.; et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 2007, 317, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Pirrie, L.; McCarthy, A.R.; Major, L.L.; Morkūnaitė, V.; Zubrienė, A.; Matulis, D.; Lain, S.; Lebl, T.; Westwood, N.J. Discovery and Validation of SIRT2 Inhibitors Based on Tenovin-6: Use of a 1H-NMR Method to Assess Deacetylase Activity. Molecules 2012, 17, 12206–12224. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.; Breitenbucher, F.; Schuler, M.; Ehrenhofer-Murray, A.E. A Novel Sirtuin 2 (SIRT2) Inhibitor with p53-dependent Pro-apoptotic Activity in Non-small Cell Lung Cancer. J. Biol. Chem. 2014, 289, 5208–5216. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M.; Balabadra, U.; Xiang, Z.; Woodman, B.; Meade, S.; Amore, A.; Maxwell, M.M.; Reeves, S.; Bates, G.P.; Luthi-Carter, R.; et al. A Brain-Permeable Small Molecule Reduces Neuronal Cholesterol by Inhibiting Activity of Sirtuin 2 Deacetylase. ACS Chem. Biol. 2011, 6, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Xu, N.; Malyukova, A.; Scarlett, C.J.; Sun, Y.T.; Zhang, X.D.; Ling, D.; Su, S.P.; Nelson, C.; Chang, D.K.; et al. The histone deacetylase SIRT2 stabilizes Myc oncoproteins. Cell Death Differ. 2012, 20, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Kamal, Z.; Ai, T.; Xu, Y.; More, S.S.; Wilson, D.J.; Chen, L. Discovery of Potent and Selective Sirtuin 2 (SIRT2) Inhibitors Using a Fragment-Based Approach. J. Med. Chem. 2014, 57, 8340–8357. [Google Scholar] [CrossRef] [PubMed]
- Schiedel, M.; Rumpf, T.; Karaman, B.; Lehotzky, A.; Gerhardt, S.; Ovádi, J.; Sippl, W.; Einsle, O.; Jung, M. Structure-Based Development of an Affinity Probe for Sirtuin2. Angew. Chem. Int. Ed. 2016, 55, 2252–2256. [Google Scholar] [CrossRef]
- Yang, L.-L.; Wang, H.-L.; Zhong, L.; Yuan, C.; Liu, S.-Y.; Yu, Z.-J.; Liu, S.; Yan, Y.-H.; Wu, C.; Wang, Y.; et al. X-ray crystal structure guided discovery of new selective, substrate-mimicking sirtuin 2 inhibitors that exhibit activities against non-small cell lung cancer cells. Eur. J. Med. Chem. 2018, 155, 806–823. [Google Scholar] [CrossRef]
- Yang, L.-L.; Xu, W.; Yan, J.; Su, H.-L.; Yuan, C.; Li, C.; Zhang, X.; Yu, Z.-J.; Yan, Y.-H.; Yu, Y.; et al. Crystallographic and SAR analyses reveal the high requirements needed to selectively and potently inhibit SIRT2 deacetylase and decanoylase. MedChemComm 2019, 10, 164–168. [Google Scholar] [CrossRef]
- Galleano, I.; Schiedel, M.; Jung, M.; Madsen, A.; Olsen, C. A Continuous, Fluorogenic Sirtuin 2 Deacylase Assay: Substrate Screening and Inhibitor Evaluation. J. Med. Chem. 2016, 59, 1021–1031. [Google Scholar] [CrossRef]
- Yang, L.; Ma, X.; Yuan, C.; He, Y.; Li, L.; Fang, S.; Xia, W.; He, T.; Qian, S.; Xu, Z.; et al. Discovery of 2-((4,6-dimethylpyrimidin-2-yl)thio)-N-phenylacetamide derivatives as new potent and selective human sirtuin 2 inhibitors. Eur. J. Med. Chem. 2017, 134, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Robinson, E.; Hom, K.; Yu, W.; Nguyen, N.; Li, Y.; Zong, Q.; Wilks, A.; Xue, F. Structure-based design and biological evaluation of inhibitors of the pseudomonas aeruginosa heme oxygenase (pa-HemO). Bioorg. Med. Chem. Lett. 2018, 28, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.K.; Denton, T.T.; Cerny, M.A.; Zhang, X.; Johnson, E.F.; Cashman, J.R. Synthetic Inhibitors of Cytochrome P-450 2A6: Inhibitory Activity, Difference Spectra, Mechanism of Inhibition, and Protein Cocrystallization. Eur. J. Med. Chem. 2006, 49, 6987–7001. [Google Scholar] [CrossRef] [PubMed]
- Finnin, M.S.; Donigian, J.R.; Pavletich, N.P. Structure of the histone deacetylase SIRT2. Nat. Struct. Biol. 2001, 8, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, T.; Schiedel, M.; Karaman, B.; Roessler, C.; North, B.J.; Lehotzky, A.; Oláh, J.; Ladwein, K.I.; Schmidtkunz, K.; Gajer, M.; et al. Selective Sirt2 inhibition by ligand-induced rearrangement of the active site. Nat. Commun. 2015, 6, 6263. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all the compounds are available from the authors. |


| ID | Structure | Inhibition% 1/SIRT2-p2270 | cLogP | cLogS | |
|---|---|---|---|---|---|
| @ 100 μM | @ 10 μM | ||||
| 20 |  | 63 ± 5 | 35 ± 3 | 3.05 | −4.04 |
| 12 | 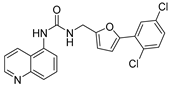 | 60 ± 3 | 33 ± 3 | 5.14 | −4.43 |
| 21 | 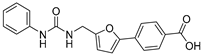 | 46 ± 4 | 33 ± 3 | 2.85 | −3.98 |
| 22 | 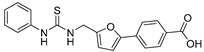 | 44 ± 5 | 23 ± 3 | 3.60 | −4.22 |
| 30 | 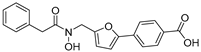 | 13 ± 2 | −2 ± 3 | 2.99 | −4.05 |
| 35 |  | 24 ± 3 | 1 ± 2 | 4.27 | −4.13 |
| 39 | 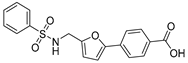 | 38 ± 3 | 11 ± 2 | 1.85 | −4.13 |
| 49 | 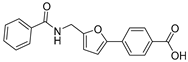 | 32 ± 3 | 8 ± 1 | 3.12 | −4.14 |
| 50 | 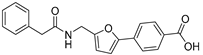 | 30 ± 2 | 5 ± 2 | 3.34 | −4.25 |
| 32 | 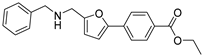 | 9 ± 2 | −2 ± 2 | 4.17 | −4.54 |
| 36 | 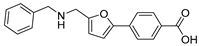 | 15 ± 2 | 3 ± 2 | 2.48 | −4.29 |
| 43 | 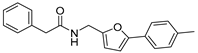 | 3 ± 2 | 2 ± 3 | 4.18 | −4.46 |
| 44 | 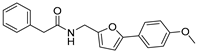 | 20 ± 3 | 8 ± 2 | 3.79 | −4.36 |
| 45 |  | 19 ± 2 | 5 ± 2 | 3.76 | −4.36 |
| 46 | 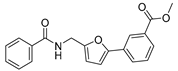 | 15 ± 2 | 3 ± 3 | 3.46 | −4.3 |
| 51 | 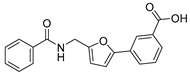 | 18 ± 3 | 13 ± 3 | 3.09 | −4.12 |
| 47 | 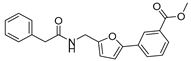 | 16 ± 3 | 5 ± 3 | 3.67 | −4.4 |
| 52 |  | 18 ± 2 | 2 ± 3 | 3.31 | −4.24 |
| AGK2 | 80 ± 6 | 30 ± 5 | 5.65 | −4.25 | |
| ID | Structure | Inhibition% 1/SIRT2-p2270 | cLogP | cLogS | |
|---|---|---|---|---|---|
| @ 100 μM | @ 10 μM | ||||
| 23 | 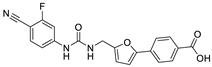 | 35 ± 4 | 10 ± 3 | 3.15 | −3.58 |
| 24 | 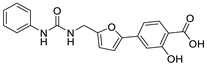 | 43 ± 2 | 13 ± 2 | 3.21 | −3.82 |
| 25 |  | 99 ± 2 | 90 ± 3 | 1.63 | −3.63 |
| 26 | 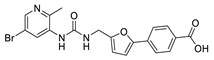 | 9 ± 2 | −5 ± 3 | 3.05 | −3.83 |
| 17 | 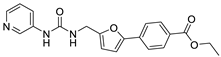 | 50 ± 4 | 37 ± 3 | 2.85 | −3.83 |
| 18 | 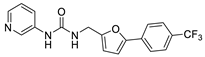 | 40 ± 5 | 23 ± 2 | 3.54 | −4.12 |
| 33 | 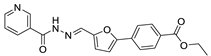 | 20 ± 3 | 3 ± 2 | 3.28 | −4.08 |
| 37 |  | 30 ± 5 | 5 ± 2 | 2.56 | −3.83 |
| 48 |  | 18 ± 2 | 0 ± 2 | 2.82 | −4.11 |
| 34 | 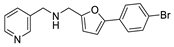 | 3 ± 2 | −2 ± 3 | 3.19 | −4.12 |
| AGK2 | 80 ± 6 | 30 ± 5 | 5.65 | −4.25 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, C.; Chen, W.; Song, C.; Zhang, X.; Yang, F.; Wang, C.; Zhang, Y.; Qian, S.; Wang, Z.; et al. Discovery of (5-Phenylfuran-2-yl)methanamine Derivatives as New Human Sirtuin 2 Inhibitors. Molecules 2019, 24, 2724. https://doi.org/10.3390/molecules24152724
Wang L, Li C, Chen W, Song C, Zhang X, Yang F, Wang C, Zhang Y, Qian S, Wang Z, et al. Discovery of (5-Phenylfuran-2-yl)methanamine Derivatives as New Human Sirtuin 2 Inhibitors. Molecules. 2019; 24(15):2724. https://doi.org/10.3390/molecules24152724
Chicago/Turabian StyleWang, Lijiao, Chao Li, Wei Chen, Chen Song, Xing Zhang, Fan Yang, Chen Wang, Yuanyuan Zhang, Shan Qian, Zhouyu Wang, and et al. 2019. "Discovery of (5-Phenylfuran-2-yl)methanamine Derivatives as New Human Sirtuin 2 Inhibitors" Molecules 24, no. 15: 2724. https://doi.org/10.3390/molecules24152724
APA StyleWang, L., Li, C., Chen, W., Song, C., Zhang, X., Yang, F., Wang, C., Zhang, Y., Qian, S., Wang, Z., & Yang, L. (2019). Discovery of (5-Phenylfuran-2-yl)methanamine Derivatives as New Human Sirtuin 2 Inhibitors. Molecules, 24(15), 2724. https://doi.org/10.3390/molecules24152724




