A Convenient Synthesis of Pentaporphyrins and Supramolecular Complexes with a Fulleropyrrolidine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
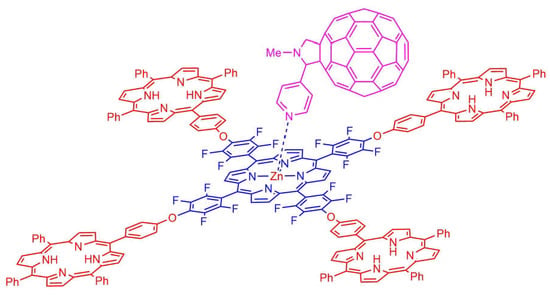
2.2. Formation of Porphyrin–Fullerene Complexes
3. Materials and Methods
3.1. Chemicals and Instrumentation
3.2. Synthesis
3.2.1. 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrinatozinc(II) (Zn2)
3.2.2. Diporphyrin 3
3.2.3. Diporphyrin 4
3.2.4. Pentaporphyrin 5
3.2.5. Pentaporphyrin Zn5
3.2.6. Pentaporphyrin 6
3.3. Absorption and Fluorescence Titrations
3.4. Single-Crystal X-Ray Diffraction Studies of Zn5·Solvent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aratani, N.; Takagi, A.; Yanagawa, Y.; Matsumoto, T.; Kawai, T.; Yoon, Z.S.; Kim, D.; Osuka, A. Giant meso–meso-linked porphyrin arrays of micrometer molecular length and their fabrication. Chem. Eur. J. 2005, 11, 3389–3404. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.; Stern, C. Supramolecular chemistry of metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713. [Google Scholar] [CrossRef] [PubMed]
- Drain, C.M.; Varotto, A.; Radivojevic, I. Self-organized porphyrinic materials. Chem. Rev. 2009, 109, 1630–1658. [Google Scholar] [CrossRef] [PubMed]
- Balaban, T.S. Self-assembling porphyrins and chlorins as synthetic mimics of the chlorosomal bacteriochlorophylls. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2010; Volume 1, Chapter 3; pp. 221–306. [Google Scholar]
- Morisue, M.; Kobuke, Y. Supramolecular organization of porphyrins and phthalocyanines by use of biomimetic coordination methodology. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2014; Volume 32, Chapter 166; pp. 1–126. [Google Scholar]
- Bessmertnykh-Lemeune, A.G.; Guilard, R.; Stern, C.; Enakieva, Y.Y.; Gorbunova, Y.G.; Tsivadze, A.Y.; Nefedov, S.E. Biomimetic studies of porphyrin self-assembled systems. In Supramolecular Systems: Chemistry, Types and Applications; Pena, C., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2017; Chapter 4; pp. 213–278. [Google Scholar]
- Jeong, Y.-H.; Son, M.; Yoon, H.; Kim, P.; Lee, D.-H.; Kim, D.; Jang, W.-D. Guest-induced photophysical property switching of artificial light-harvesting dendrimers. Angew. Chem. Int. Ed. 2014, 53, 6925–6928. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeong, Y.-H.; Kim, J.-H.; Kim, I.; Lee, E.; Jang, W.-D. Supramolecular coordination polymer formed from artificial light-harvesting dendrimer. J. Am. Chem. Soc. 2015, 137, 12394–12399. [Google Scholar] [CrossRef] [PubMed]
- Hasobe, T.; Ida, K.; Sakai, H.; Ohkubo, K.; Fukuzumi, S. Coronenetetraimide-centered cruciform pentamers containing multiporphyrin units: Synthesis and sequential photoinduced energy- and electron-transfer dynamics. Chem. Eur. J. 2015, 21, 11196–11205. [Google Scholar] [CrossRef] [PubMed]
- Yim, D.; Sung, J.; Kim, S.; Oh, J.; Yoon, H.; Sung, Y.M.; Kim, D.; Jang, W.-D. Guest-induced modulation of the energy transfer process in porphyrin-based artificial light harvesting dendrimers. J. Am. Chem. Soc. 2017, 139, 993–1002. [Google Scholar] [CrossRef]
- Terazono, Y.; Kodis, G.; Chachisvilis, M.; Cherry, B.R.; Fournier, M.; Moore, A.; Moore, T.A.; Gust, D. Multiporphyrin arrays with π-π interchromophore interactions. J. Am. Chem. Soc. 2015, 137, 245–258. [Google Scholar] [CrossRef]
- Delavaux-Nicot, B.; Aziza, H.B.; Nierengarten, I.; Trinh, T.M.N.; Meichsner, E.; Chessé, M.; Holler, M.; Abidi, R.; Maisonhaute, E.; Nierengarten, J.-F. A rotaxane scaffold for the construction of multiporphyrinic light-harvesting devices. Chem. Eur. J. 2018, 24, 133–144. [Google Scholar] [CrossRef]
- Amati, A.; Cavigli, P.; Demitri, N.; Natali, M.; Indelli, M.T.; Iengo, E. Sn(IV) multiporphyrin arrays as tunable photoactive systems. Inorg. Chem. 2019, 58, 4399–4411. [Google Scholar] [CrossRef]
- Tanaka, T.; Osuka, A. Conjugated porphyrin arrays: Synthesis, properties and applications for functional materials. Chem. Soc. Rev. 2015, 44, 943–969. [Google Scholar] [CrossRef] [PubMed]
- Iengo, E.; Cavigli, P.; Milano, D.; Tecilla, P. Metal mediated self-assembled porphyrin metallacycles: Synthesis and multipurpose applications. Inorg. Chim. Acta 2014, 417, 59–78. [Google Scholar] [CrossRef]
- Wytko, J.A.; Ruppert, R.; Jeandon, C.; Weiss, J. Metal-mediated linear self-assembly of porphyrins. Chem. Commun. 2018, 54, 1550–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, L.; Gao, Y.; Yang, W.; Li, Y.; Guo, C. Porous metalloporphyrinic nanospheres constructed from metal 5,10,15,20-tetraksi(4’-ethynylphenyl)porphyrin for efficient catalytic degradation of organic dyes. RSC Adv. 2018, 8, 7330–7339. [Google Scholar] [CrossRef]
- Mongin, O.; Sankar, M.; Charlot, M.; Mir, Y.; Blanchard-Desce, M. Strong enhancement of two-photon absorption properties in synergic ‘semi-disconnected’ multiporphyrin assemblies designed for combined imaging and photodynamic therapy. Tetrahedron Lett. 2013, 54, 6474–6478. [Google Scholar] [CrossRef]
- Aratani, N.; Osuka, A. Exploration of giant functional porphyrin arrays. Bull. Chem. Soc. Jpn. 2015, 88, 1–27. [Google Scholar] [CrossRef]
- Lacerda, P.S.S.; Silva, A.M.G.; Tomé, A.C.; Neves, M.G.P.M.S.; Silva, A.M.S.; Cavaleiro, J.A.S.; Llamas-Saiz, A.L. [1,2,3]Triazolo[4,5-b]porphyrins: New building blocks for porphyrinic materials. Angew. Chem. Int. Ed. 2006, 45, 5487–5491. [Google Scholar] [CrossRef]
- Pedro-Hernández, L.D.; Cortez-Maya, S.; Calderón-Pardo, J.; Hernández-Ortega, S.; Martínez-García, M. Nanostructured multiporphyrin dendrimers: Synthesis, characterization and their spectroscopic properties. Curr. Org. Chem. 2018, 22, 2308–2314. [Google Scholar] [CrossRef]
- Martin, M.M.; Dill, M.; Langer, J.; Jux, N. Porphyrin−hexaphenylbenzene conjugates via mixed cyclotrimerization reactions. J. Org. Chem. 2019, 84, 1489–1499. [Google Scholar] [CrossRef]
- Yang, J.; Lee, J.-E.; Lee, C.Y.; Aratani, N.; Osuka, A.; Hupp, J.T.; Kim, D. The role of electronic coupling in linear porphyrin arrays probed by single-molecule fluorescence spectroscopy. Chem. Eur. J. 2011, 17, 9219–9225. [Google Scholar] [CrossRef]
- Morisue, M.; Hoshino, Y.; Shimizu, M.; Uemura, S.; Sakurai, S. A tightly stretched ultralong supramolecular multiporphyrin array propagated by double-strand formation. Chem. Eur. J. 2016, 22, 13019–13022. [Google Scholar] [CrossRef] [PubMed]
- Kamonsutthipaijit, N.; Anderson, H.L. Template-directed synthesis of linear porphyrin oligomers: Classical, Vernier and mutual Vernier. Chem. Sci. 2017, 8, 2729–2740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kim, J.O.; Li, Y.; Wen, B.; Zhou, M.; Liu, S.; Aratani, N.; Xu, L.; Kim, D.; Song, J. Meso-to-meso 2,5-Pyrrolylene bridged zig-zag porphyrin arrays. Chem. Commun. 2017, 53, 11488–11491. [Google Scholar] [CrossRef]
- He, Z.; Ishizuka, T.; Jiang, D. Dendritic architectures for design of photo- and spin-functional nanomaterials. Polymer J. 2007, 39, 889–922. [Google Scholar] [CrossRef]
- Li, W.-S.; Aida, T. Dendrimer porphyrins and phthalocyanines. Chem. Rev. 2009, 109, 6047–6076. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Jang, S.Y.; Tanaka, T.; Aratani, N.; Lim, J.M.; Kim, K.S.; Kim, D.; Osuka, A. Two-dimensionally extended porphyrin tapes: Synthesis and shape-dependent two-photon absorption properties. Chem. Eur. J. 2008, 14, 8279–8289. [Google Scholar] [CrossRef]
- Tanaka, T.; Lee, B.S.; Aratani, N.; Yoon, M.-C.; Kim, D.; Osuka, A. Synthesis and properties of hybrid porphyrin tapes. Chem. Eur. J. 2011, 17, 14400–14412. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Tanaka, T.; Lee, S.; Lim, J.M.; Kim, D.; Osuka, A. Meso-meso Linked porphyrin−[26]hexaphyrin−porphyrin hybrid arrays and their triply linked tapes exhibiting strong absorption bands in the NIR region. J. Am. Chem. Soc. 2015, 137, 2097–2106. [Google Scholar] [CrossRef]
- Xue, S.; Kuzuhara, D.; Aratani, N.; Yamada, H. Synthesis of a porphyrin(2.1.2.1) nanobelt and its ability to bind fullerene. Org. Lett. 2019, 21, 2069–2072. [Google Scholar] [CrossRef]
- Song, J.; Aratani, N.; Shinokubo, H.; Osuka, A. A porphyrin nanobarrel that encapsulates C60. J. Am. Chem. Soc. 2010, 132, 16356–16357. [Google Scholar] [CrossRef]
- Aratani, N.; Kim, D.; Osuka, A. Discrete cyclic porphyrin arrays as artificial light-harvesting antenna. Acc. Chem. Res. 2009, 42, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.C.; Sprafke, J.K.; Kondratuk, D.V.; Rinfray, C.; Claridge, T.D.W.; Saywell, A.; Blunt, M.O.; O’Shea, J.N.; Beton, P.H.; Malfois, M.; et al. Vernier templating and synthesis of a 12-porphyrin nano-ring. Nature 2011, 469, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-W.; Ham, S.; Aratani, N.; Kim, D.; Osuka, A. A 1,3-phenylene-bridged hexameric porphyrin wheel and efficient excitation energy transfer along the wheel. Chem. Eur. J. 2013, 19, 13328–13336. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, S.A.L.; Gong, J.Q.; Haver, R.; Odell, B.; Claridge, T.D.W.; Herz, L.M.; Anderson, H.L. Self-assembly of russian doll concentric porphyrin nanorings. J. Am. Chem. Soc. 2015, 137, 12713–12718. [Google Scholar] [CrossRef] [PubMed]
- Kondratuk, D.V.; Perdigão, L.M.A.; Esmail, A.M.S.; O’Shea, J.N.; Beton, P.H.; Anderson, H.L. Supramolecular nesting of cyclic polymers. Nat. Chem. 2015, 7, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Favereau, L.; Cnossen, A.; Kelber, J.B.; Gong, J.Q.; Oetterli, R.M.; Cremers, J.; Herz, L.M.; Anderson, H.L. Six-coordinate zinc porphyrins for template-directed synthesis of spiro-fused nanorings. J. Am. Chem. Soc. 2015, 137, 14256–14259. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-P.; Shen, Y.-F.; Zhu, B.-Y.; Wu, J.; Li, S. Recent advances in the template-directed synthesis of porphyrin nanorings. Chem. Commun. 2016, 52, 10205–10216. [Google Scholar] [CrossRef]
- Liu, P.; Hisamune, Y.; Peeks, M.D.; Odell, B.; Gong, J.Q.; Herz, L.M.; Anderson, H.L. Synthesis of five-porphyrin nanorings by using ferrocene and corannulene templates. Angew. Chem. Int. Ed. 2016, 55, 8358–8362. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Kawakami, Y.; Satake, A. Synthesis and photophysical properties of porphyrin macrorings composed of free-base porphyrins and slipped-cofacial zinc porphyrin dimers. Inorg. Chem. 2017, 56, 11008–11018. [Google Scholar] [CrossRef]
- Morley, D.O.; Malfois, M.; Kamonsutthipaijit, N.; Kondratuk, D.V.; Anderson, H.L.; Wilson, M. A coarse-grained model for free and template-bound porphyrin nanorings. J. Phys. Chem. A 2017, 121, 5907–5920. [Google Scholar] [CrossRef]
- Bols, P.S.; Anderson, H.L. Shadow mask templates for site-selective metal exchange in magnesium porphyrin nanorings. Angew. Chem. Int. Ed. 2018, 57, 7874–7877. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.D.; Kim, Y.; Koo, J.; Kim, K. Porphyrin boxes. Acc. Chem. Res. 2018, 51, 2730–2738. [Google Scholar] [CrossRef] [PubMed]
- Cremers, J.; Haver, R.; Rickhaus, M.; Gong, J.Q.; Favereau, L.; Peeks, M.D.; Claridge, T.D.W.; Herz, L.M.; Anderson, H.L. Template-directed synthesis of a conjugated zinc porphyrin nanoball. J. Am. Chem. Soc. 2018, 140, 5352–5355. [Google Scholar] [CrossRef] [PubMed]
- Guldi, D.M. Fullerenes: Three dimensional electron acceptor materials. Chem. Commun. 2000, 321–327. [Google Scholar] [CrossRef]
- Guldi, D.M. Fullerene–porphyrin architectures; photosynthetic antenna and reaction center models. Chem. Soc. Rev. 2002, 31, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.F. Synthesis, properties, and potential applications of porphyrin–fullerenes. Macroheterocycles 2011, 4, 186–208. [Google Scholar] [CrossRef]
- Santos, J.; Illescas, B.M.; Wielopolski, M.; Silva, A.M.G.; Tomé, A.C.; Guldi, D.M.; Martín, N. Efficient electron transfer in β-substituted porphyrin-C60 dyads connected through a p-phenylenevinylene dimer. Tetrahedron 2008, 64, 11404–11408. [Google Scholar] [CrossRef]
- Enes, R.F.; Cid, J.-J.; Hausmann, A.; Trukhina, O.; Gouloumis, A.; Vázquez, P.; Cavaleiro, J.A.S.; Tomé, A.C.; Guldi, D.M.; Torres, T. Synthesis and photophysical properties of fullerene–phthalocyanine–porphyrin triads and pentads. Chem. Eur. J. 2012, 18, 1727–1736. [Google Scholar] [CrossRef]
- Hasobe, T. Porphyrin-based supramolecular nanoarchitectures for solar energy conversion. J. Phys. Chem. Lett. 2013, 4, 1771–1780. [Google Scholar] [CrossRef]
- Schlundt, S.; Bauer, W.; Hirsch, A. Synthesis and atropisomerism of cascaded tetraphenylporphyrin–[60]fullerene hybrids. Chem. Eur. J. 2015, 21, 12421–12430. [Google Scholar] [CrossRef]
- Gao, D.; Aly, S.M.; Karsenti, P.-L.; Brisard, G.; Harvey, P.D. Increasing the lifetimes of charge separated states in porphyrin–fullerene polyads. Phys. Chem. Chem. Phys. 2017, 19, 24018–24028. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, K.; Aida, T. Metalloporphyrin hosts for supramolecular chemistry of fullerenes. Chem. Soc. Rev. 2007, 36, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Trabolsi, A.; Urbani, M.; Delgado, J.L.; Ajamaa, F.; Elhabiri, M.; Solladié, N.; Nierengarten, J.-F.; Albrecht-Gary, A.-M. Large photoactive supramolecular ensembles prepared from C60–pyridine substrates and multi-Zn(II)–porphyrin receptors. New J. Chem. 2008, 32, 159–165. [Google Scholar] [CrossRef]
- Tong, L.H.; Wietor, J.-L.; Clegg, W.; Raithby, P.R.; Pascu, S.I.; Sanders, J.K.M. Supramolecular assemblies of tripodal porphyrin hosts and C60. Chem. Eur. J. 2008, 14, 3035–3044. [Google Scholar] [CrossRef] [PubMed]
- Nobukuni, H.; Shimazaki, Y.; Uno, H.; Naruta, Y.; Ohkubo, K.; Kojima, T.; Fukuzumi, S.; Seki, S.; Sakai, H.; Hasobe, T.; et al. Supramolecular structures and photoelectronic properties of the inclusion complex of a cyclic free-base porphyrin dimer and C60. Chem. Eur. J. 2010, 16, 11611–11623. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ramírez, G.; Karlen, S.D.; Shundo, A.; Porfyrakis, K.; Ito, Y.; Briggs, G.A.D.; Morton, J.J.L.; Anderson, H.L. A cyclic porphyrin trimer as a receptor for fullerenes. Org. Lett. 2010, 12, 3544–3547. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Aratani, N.; Shinokubo, H.; Osuka, A. A β-to-β 2,5-thienylene-bridged cyclic porphyrin tetramer: Its rational synthesis and 1:2 binding mode with C60. Chem. Sci. 2011, 2, 748–751. [Google Scholar] [CrossRef]
- Hernández-Eguía, L.P.; Escudero-Adán, E.C.; Pintre, I.C.; Ventura, B.; Flamigni, L.; Ballester, P. Supramolecular inclusion complexes of two cyclic zinc bisporphyrins with C60 and C70: Structural, thermodynamic, and photophysical characterization. Chem. Eur. J. 2011, 17, 14564–14577. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Saito, K.; Ohkubo, K.; Troiani, V.; Qiu, H.; Gadde, S.; D’Souza, F.; Solladié, N. Multiple photosynthetic reaction centres using zinc porphyrinic oligopeptide–fulleropyrrolidine supramolecular complexes. Phys. Chem. Chem. Phys. 2011, 13, 17019–17022. [Google Scholar] [CrossRef]
- Nair, V.S.; Pareek, Y.; Karunakaran, V.; Ravikanth, M.; Ajayaghosh, A. Cyclotriphosphazene appended porphyrins and fulleropyrrolidine complexes as supramolecular multiple photosynthetic reaction centers: Steady and excited states photophysical investigation. Phys. Chem. Chem. Phys. 2014, 16, 10149–10156. [Google Scholar] [CrossRef]
- Calderon, R.M.K.; Valero, J.; Grimm, B.; Mendoza, J.; Guldi, D.M. Enhancing molecular recognition in electron donor-acceptor hybrids via cooperativity. J. Am. Chem. Soc. 2014, 136, 11436–11443. [Google Scholar] [CrossRef] [PubMed]
- Amati, A.; Cavigli, P.; Kahnt, A.; Indelli, M.T.; Iengo, E. Self-assembled ruthenium(II)porphyrin-aluminium(III)porphyrin-fullerene triad for long-lived photoinduced charge separation. J. Phys. Chem. A 2017, 121, 4242–4252. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.I.T.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin: A versatile platform to novel porphyrinic materials. J. Porphyrins Phthalocyanines 2011, 15, 1116–1133. [Google Scholar] [CrossRef]
- Costa, J.I.T.C.; Oliveira, E.; Santos, H.M.; Tomé, A.C.; Neves, M.G.P.M.S.; Lodeiro, C. Study of multiporphyrin compounds as colorimetric sitting-atop metal complexes: Synthesis and photophysical studies. ChemPlusChem 2016, 81, 143–153. [Google Scholar] [CrossRef]
- Costa, J.I.T.; Farinha, A.S.F.; Neves, M.G.P.M.S.; Tomé, A.C. An easy access to porphyrin triads and their supramolecular interaction with a pyridyl [60]fulleropyrrolidine. Dyes Pigments 2016, 135, 163–168. [Google Scholar] [CrossRef]
- D’Souza, F.; Deviprasad, G.R.; Zandler, M.E.; Hoang, V.T.; Klykov, A.; VanStipdonk, M.; Perera, A.; El-Khouly, M.E.; Fujitsuka, M.; Ito, O. Spectroscopic, electrochemical, and photochemical studies of self-assembled via axial coordination zinc porphyrin–fulleropyrrolidine dyads. J. Phys. Chem. A 2002, 106, 3243–3252. [Google Scholar] [CrossRef]
- Tat, F.T.; Zhou, Z.; MacMahon, S.; Song, F.; Rheingold, A.L.; Echegoyen, L.; Schuster, D.I.; Wilson, S.R. A new fullerene complexation ligand: N-pyridylfulleropyrrolidine. J. Org. Chem. 2004, 69, 4602–4606. [Google Scholar] [CrossRef]
- Takai, A.; Chkounda, M.; Eggenspiller, A.; Gros, C.P.; Lachkar, M.; Barbe, J.M.; Fukuzumi, S. Efficient photoinduced electron transfer in a porphyrin tripod-fullerene supramolecular complex via π-π interactions in nonpolar media. J. Am. Chem. Soc. 2010, 132, 4477–4489. [Google Scholar] [CrossRef]
- Jain, K.; Duvva, N.; Badgurjar, D.; Giribabu, L.; Chitta, R. Synthesis and spectroscopic studies of axially bound tetra(phenothiazinyl)/tetra(bis(4′-tert-butylbiphenyl-4-yl)aniline)-zinc(II)porphyrin-fullero[C60 & C70]pyrrolidine donor–acceptor triads. Inorg. Chem. Commun. 2016, 66, 5–10. [Google Scholar] [CrossRef]
- Das, S.K.; Song, B.; Mahler, A.; Nesterov, V.N.; Wilson, A.K.; Ito, O.; D’Souza, F. Electron transfer studies of high potential zinc porphyrin−fullerene supramolecular dyads. J. Phys. Chem. C 2014, 118, 3994–4006. [Google Scholar] [CrossRef]
- Denis, P.A.; Yanney, M. Porphyrins bearing corannulene pincers: Outstanding fullerene receptors. RSC Adv. 2016, 6, 50978–50984. [Google Scholar] [CrossRef]
- Álvarez, C.M.; Barbero, H.; Ferrero, S.; Miguel, D. Synergistic effect of tetraaryl porphyrins containing corannulene and other polycyclic aromatic fragments as hosts for fullerenes. Impact of C60 in a statistically distributed mixture of atropisomers. J. Org. Chem. 2016, 81, 6081–6086. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Barbero, H.; Miguel, D.; García-Rodríguez, R.; Álvarez, C.M. Dual-tweezer behavior of an octapodal pyrene porphyrin-based system as a host for fullerenes. J. Org. Chem. 2019, 84, 6183–6190. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996. [Google Scholar]
- Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Mendonça, A.F.; Pegado, I.N.; Duarte, R.; Valdeira, M.L. Synthesis of glycoporphyrin derivatives and their antiviral activity against herpes simplex virus types 1 and 2. Bioorg. Med. Chem. 2005, 13, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Prato, M.; Maggini, M.; Giacometti, C.; Scorrano, G.; Sandonà, G.; Farnia, G. Synthesis and electrochemical properties of substituted fulleropyrrolidines. Tetrahedron 1996, 52, 5221–5234. [Google Scholar] [CrossRef]
- Connors, K.A. Binding Constants: The Measurement of Molecular Complex Stability; Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Kottke, T.; Stalke, D. Crystal handling at low temperature. J. Appl. Cryst. 1993, 26, 615–619. [Google Scholar] [CrossRef]
- SAINT+ Data Integration Engine v. 8.27b© 1997–2012; Bruker AXS: Madison, WI, USA.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT-2014, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Huebschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON, an integrated tool for the analysis of the results of a single crystal structure determination. Acta Cryst. A 1990, 46, C34. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Van der Sluis, P.; Spek, A.L. Bypass—An effective method for the refinement of the crystal structures containing disordered solvent regions. Acta Cryst. A 1990, 46, 194–201. [Google Scholar] [CrossRef]
- Brandenburg, K. DIAMOND, Version 3.2f; Crystal Impact GbR: Bonn, Germany, 1997–2010.
Sample Availability: Samples of the compounds 3, 4, 5 and 6 are available from the authors. |








| Complex | K (M−1) | Kav (M−1) a | |
|---|---|---|---|
| Absorption | Fluorescence | ||
| Zn2–PyC60 | 1.75 × 104 | 3.57 × 104 | 2.66 × 104 |
| 4–PyC60 | 5.04 × 104 | 4.59 × 104 | 4.82 × 104 |
| 6–PyC60 | 1.77 × 105 | 1.28 × 105 | 1.53 × 105 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.I.T.; Farinha, A.S.F.; Paz, F.A.A.; Tomé, A.C. A Convenient Synthesis of Pentaporphyrins and Supramolecular Complexes with a Fulleropyrrolidine. Molecules 2019, 24, 3177. https://doi.org/10.3390/molecules24173177
Costa JIT, Farinha ASF, Paz FAA, Tomé AC. A Convenient Synthesis of Pentaporphyrins and Supramolecular Complexes with a Fulleropyrrolidine. Molecules. 2019; 24(17):3177. https://doi.org/10.3390/molecules24173177
Chicago/Turabian StyleCosta, Joana I. T., Andreia S. F. Farinha, Filipe A. Almeida Paz, and Augusto C. Tomé. 2019. "A Convenient Synthesis of Pentaporphyrins and Supramolecular Complexes with a Fulleropyrrolidine" Molecules 24, no. 17: 3177. https://doi.org/10.3390/molecules24173177
APA StyleCosta, J. I. T., Farinha, A. S. F., Paz, F. A. A., & Tomé, A. C. (2019). A Convenient Synthesis of Pentaporphyrins and Supramolecular Complexes with a Fulleropyrrolidine. Molecules, 24(17), 3177. https://doi.org/10.3390/molecules24173177