Abstract
Metal tetrapyrrole macrocycles such as porphyrins and chlorins are ubiquitous in nature. Synthetic analogs, including phthalocyanines, have found applications in medicine, particularly as photosensitizers for photodynamic therapy and as fluorescent imaging probes. Tripyrrolic macrocycles, called subphthalocyanines (SPcs) with a smaller boron atom at their core, have similar potential as optical agents. We have recently reported a series of mixed fluorinated SPcs with varying aromaticity, showing that electronic absorption and emission are synthetically tunable across the far visible region, and that the inclusion of 4–12 peripheral fluorine atoms results in strong fluorescence within MDA-MB-231 breast tumor cells. Further probing this system, we report herein the synthesis and characterization of boron trifluorosubphthalocyanine chloride (F3SPc). The constitutional isomers F3SPc(C3) and F3SPc(C1) are readily separable by chromatography, and their identity and purity have been confirmed by 1H NMR, 19F NMR, HR APCI-MS, and HPLC. Unsurprisingly, these structurally similar F3SPcs have identical electronic absorption (λmax = 557 nm; tetrahydrofuran (THF)) and emission (λem = 574 nm; Φf = 0.27–0.28; THF). Strong fluorescence from MDA-MB-231 breast tumor cells was observed following treatment with F3SPc(C3) and F3SPc(C1) (50 µM F3SPc, 15 min), further highlighting the importance of even a limited number of peripheral fluorine atoms for this type of application.
1. Introduction
Subphthalocyanines (SPcs), contracted tripyrrolic cousins of tetrapyrrole phthalocyanines (Pcs), were first prepared in 1972 as a templated cyclization of phthalonitrile derivatives around a central boron atom [1]. There is a rich chemistry surrounding these optically active boron macrocycles, and their structural diversity and applicability have been reviewed extensively [2,3]. More recently, beryllium subphthalocyanines have emerged as equally promising molecules [4]; however, toxicity issues have thus far precluded the widespread attention that boron subphthalocyanines have garnered. Notably relevant to this communication, halogenated SPcs have been used in photovoltaic and organic light–emitting diode technologies [5,6,7,8], and other SPcs have been applied in biological systems as anti-cancer agents [9], optical imaging agents [10], and as antibacterial agents [11]. We intend to build upon these limited biological studies by creating a library of fluorinated SPcs for application as either photodynamic therapeutics or fluorescent probes. These applications are well developed for the tetrapyrrole porphyrins and phthalocyanines [12,13,14,15], but understudied for the SPc family. Our choice of fluorinated SPcs for these purposes is two-fold: Peripheral electron-withdrawing groups allow for varied post-cyclization reactivity [3], and the C–F bond imparts greater pharmacokinetic and physiochemical stability in 20% of commercial pharmaceuticals [16,17,18,19].
We have recently studied a series of mixed fluorinated SPcs and subnapthalocyanines (SnPcs), made through co-cyclization of tetrafluorophthalonitrile, and phthalonitrile (SPcs) or 2,3-napthalenedicarbonitrile (SnPcs) [20]. These two reactions resulted in a series of macrocycles with a variable number of peripheral fluorine atoms (#F = 0, 4, 8, 12) and varied aromaticity (π e− = 14, 16, 18, 20). These studies showed that electronic absorption and emission profiles are highly tunable across the visible region through variation of peripheral F-atoms and aromaticity. Subsequently, it was shown that further modulation and tunability of photophysical properties could be achieved through inclusion of peripheral electron-donating thioethers [21] or fused thiadazole rings [22]. When applied as fluorescent imaging probes in vitro in MDA-MB-231 breast tumor cells, the presence of peripheral fluorine proved to be imperative; there was a marked increase in intracellular fluorescence from subphthalocyanine (#F = 0) to tetrafluorosubphthalocyanine (#F = 4). Considering that these two molecules have nearly identical fluorescence brightness (ε · Φf), we attributed the increase in fluorescence to enhanced cellular uptake, presumably due to the F-atoms’ modulation of polarity or lipophilicity. Other pyrrolic macrocycles with peripheral C–F bonds are known to undergo SNAr reactivity, thereby replacing peripheral F-atoms with a nucleophile [23], and there is extensive evidence of axial reactivity across the B–Cl bond [24]. The possibility therefore exists that fluorinated SPcs undergo similar reactivity in the biological milieu.
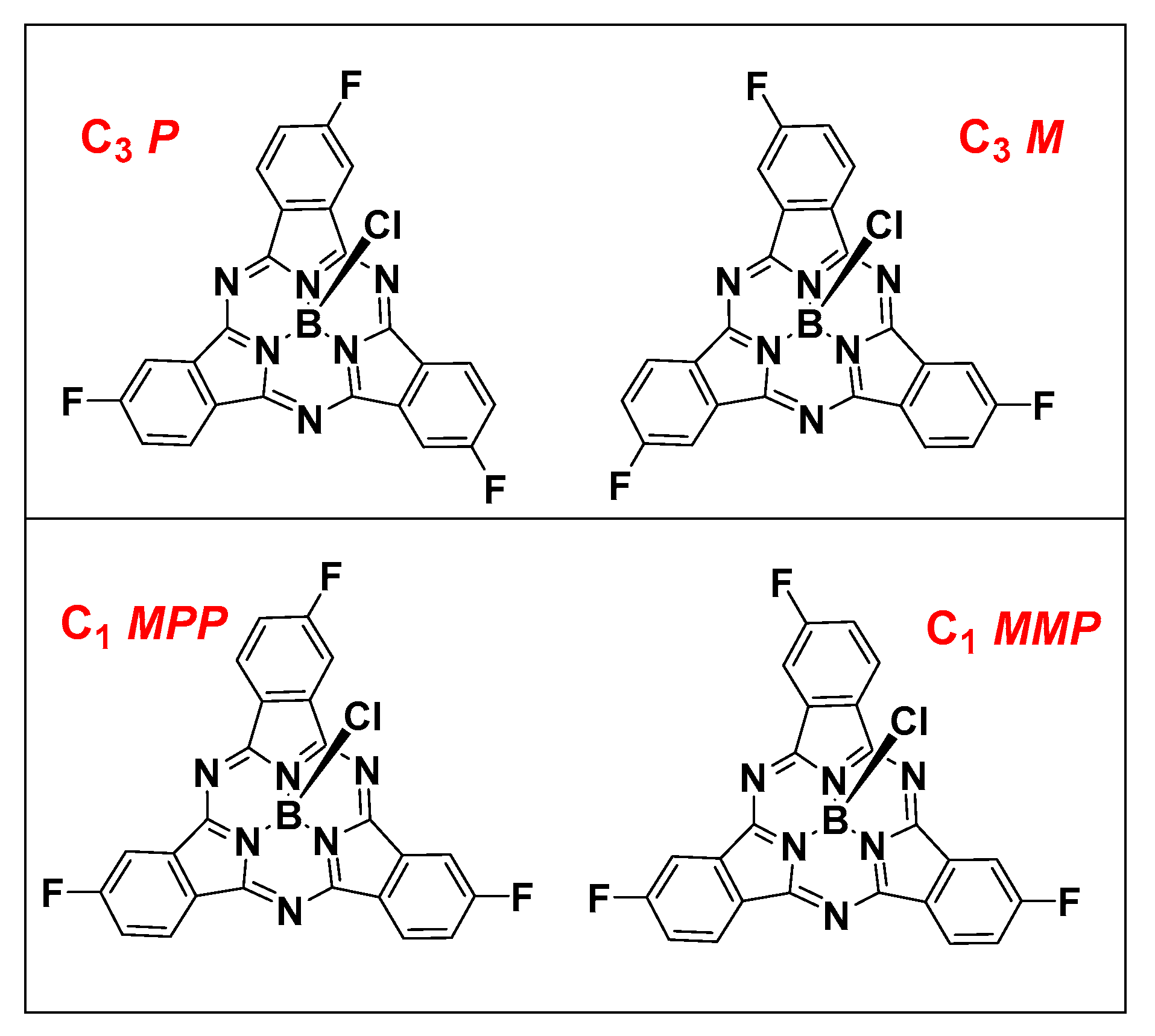
The mono-cyclization of a C2v phthalonitrile with BCl3 results in a single C3v SPc product. However, cyclization with a non-C2v phthalonitrile results in a mixture of two constitutional isomers, statistically forming isomers in a 1:3 C3 to C1 ratio (Figure 1). These constitutional isomers are further subdivided into a racemic mixture of enantiomers due to the inherent axial chirality of non-C3v symmetric SPcs. Despite the similar structure of constitutional isomers, there are examples in the literature where they can be separated chromatographically [25], and enantiomers can be further resolved by chiral HPLC [26].
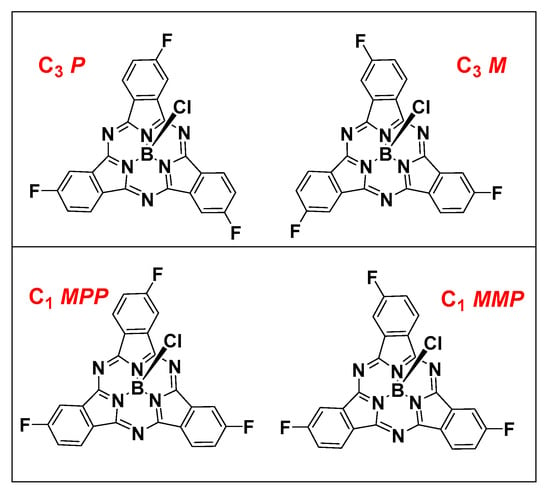
Figure 1.
C3 constitutional isomers (upper) and C1 constitutional isomers (lower) of F3SPcs.
Owing to their nearly identical photophysical properties, constitutional isomers are used in many practical applications as a mixture. Indeed, the trifluorinated SPc discussed herein has been applied in photovoltaics as a mixture of constitutional isomers [8], and considered as a mixture computationally [27]. Use of the mixture with this platform for our future purposes—to study peripheral reactivity of the C–F bond—would be difficult at best. We report herein, the successful isolation of the C3 and C1 isomers of F3SPc, and associated photophysical properties relevant to their application as fluorescent imaging probes in MDA-MB-231 breast tumor cells.
2. Results and Discussion
2.1. Synthesis and Characterization of C3 and C1 Constitutional Isomers of Trifluorosubphthalcyanine
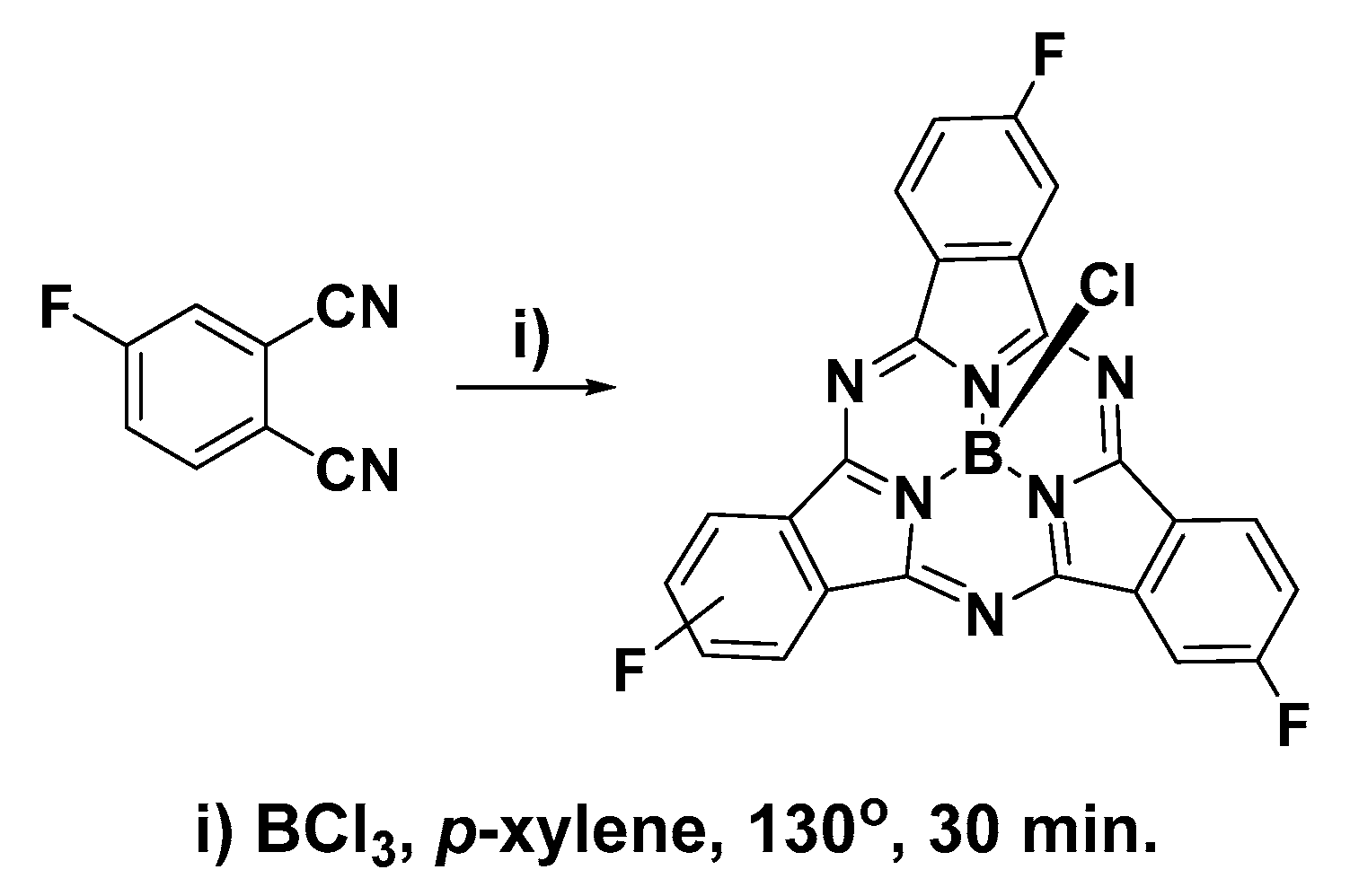
Cyclization of 4-fluorophthalonitrile in the presence of BCl3 resulted in a dark purple solid consisting of a mixture of boron 2,9,16-trifluorosubphthalocyanine chloride (F3SPc(C3)) and boron 2,9,17-trifluorosubphthalocyanine chloride (F3SPc(C1)) (Scheme 1). These two constitutional isomers were resolved by thin-layer chromatography (1:19 ethyl acetate/hexane) and purified by flash chromatography on silica gel using an eluent gradient of 0–5% ethyl acetate in hexane. Isolated yields of 4.7% and 7.4% were achieved for F3SPc(C3) and F3SPc(C1), respectively. Relatively low yields are indicative of the tedious and difficult chromatographic purification; however, the samples collected were highly pure. Purity was assessed and confirmed by HPLC using a diode-array detector (see Supplementary Materials).
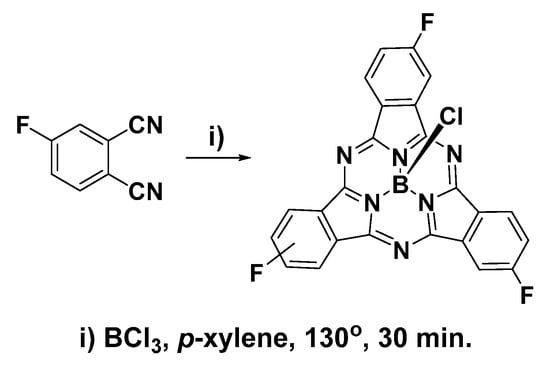
Scheme 1.
Synthesis of trifluorosubphthalocyanines.
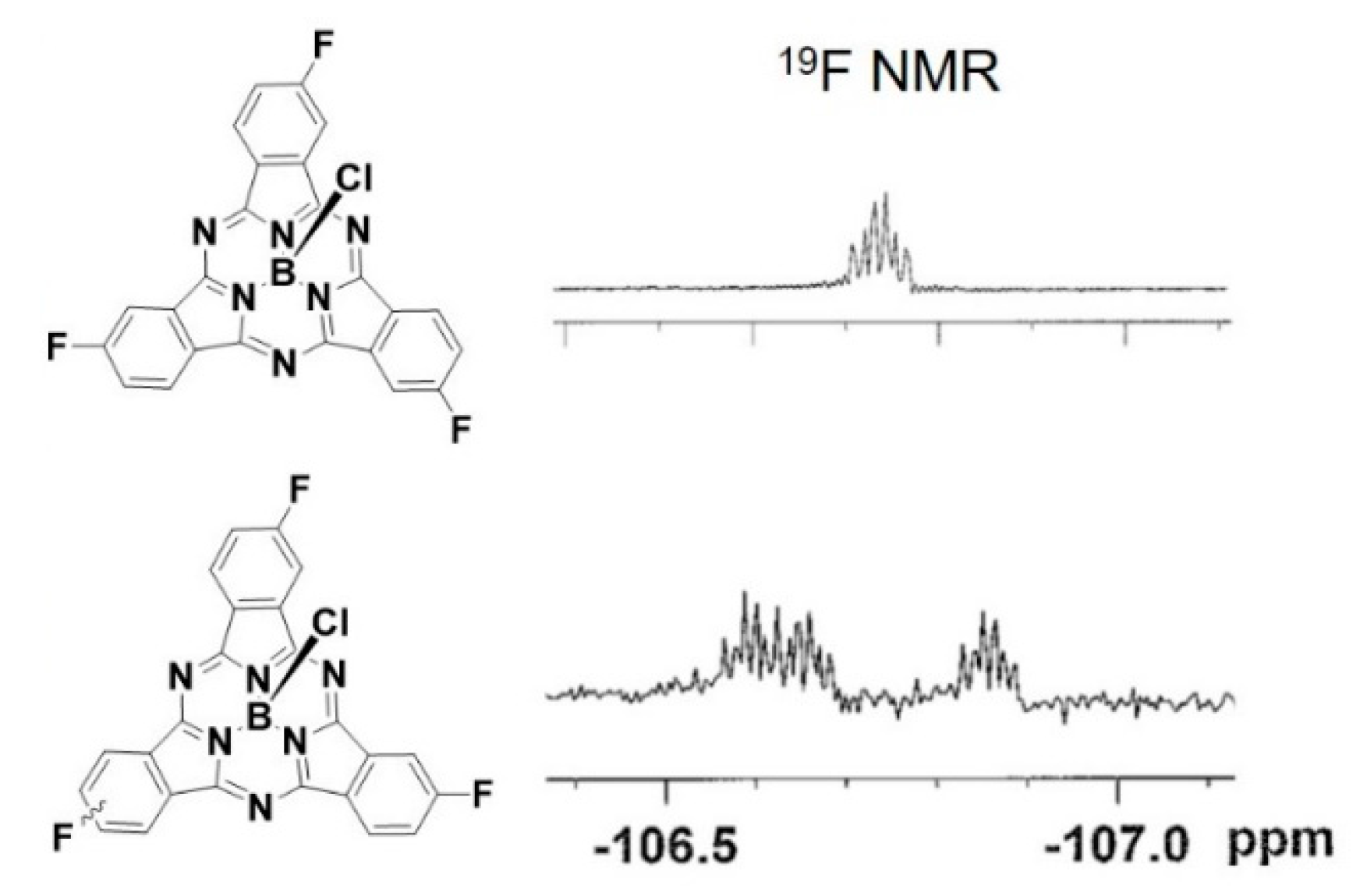
Identification of SPcs of constitutional isomers with very few 1H NMR signals can be difficult. Indeed, F3SPc(C3) and F3SPc(C1) display identical 1H NMR chemical shifts, with the only difference in spectra being the resolution of H–H and H–F coupling constants (see Supplementary Materials). One advantage of studying fluorinated SPcs with disparate symmetry is the ability to use 19F NMR to unequivocally identify isomers. The symmetric F3SPc(C3) shows one 19F NMR signal at δ −106.7 ppm (Figure 2). Breakage of the symmetry in F3SPc(C1) results in three separate signals at δ −106.6, −106.7, and −106.9 ppm. These β–F signals lie ~30 ppm downfield from those of SPcs with F-atoms in both α and β positions [20], in line with the trend that the extent of fluorination alters 19F chemical shifts further upfield.
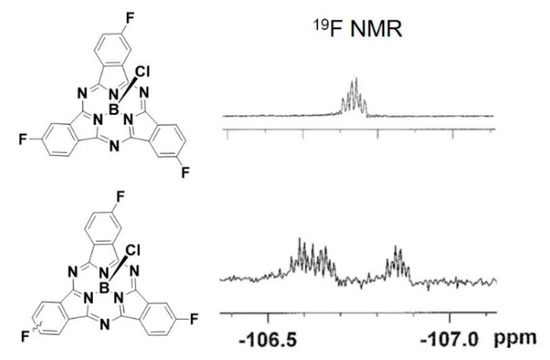
Figure 2.
19F NMR of F3SPc(C3) (upper) and F3SPc(C1) (lower).
2.2. Photophysical Properties of Subphthalocyanines
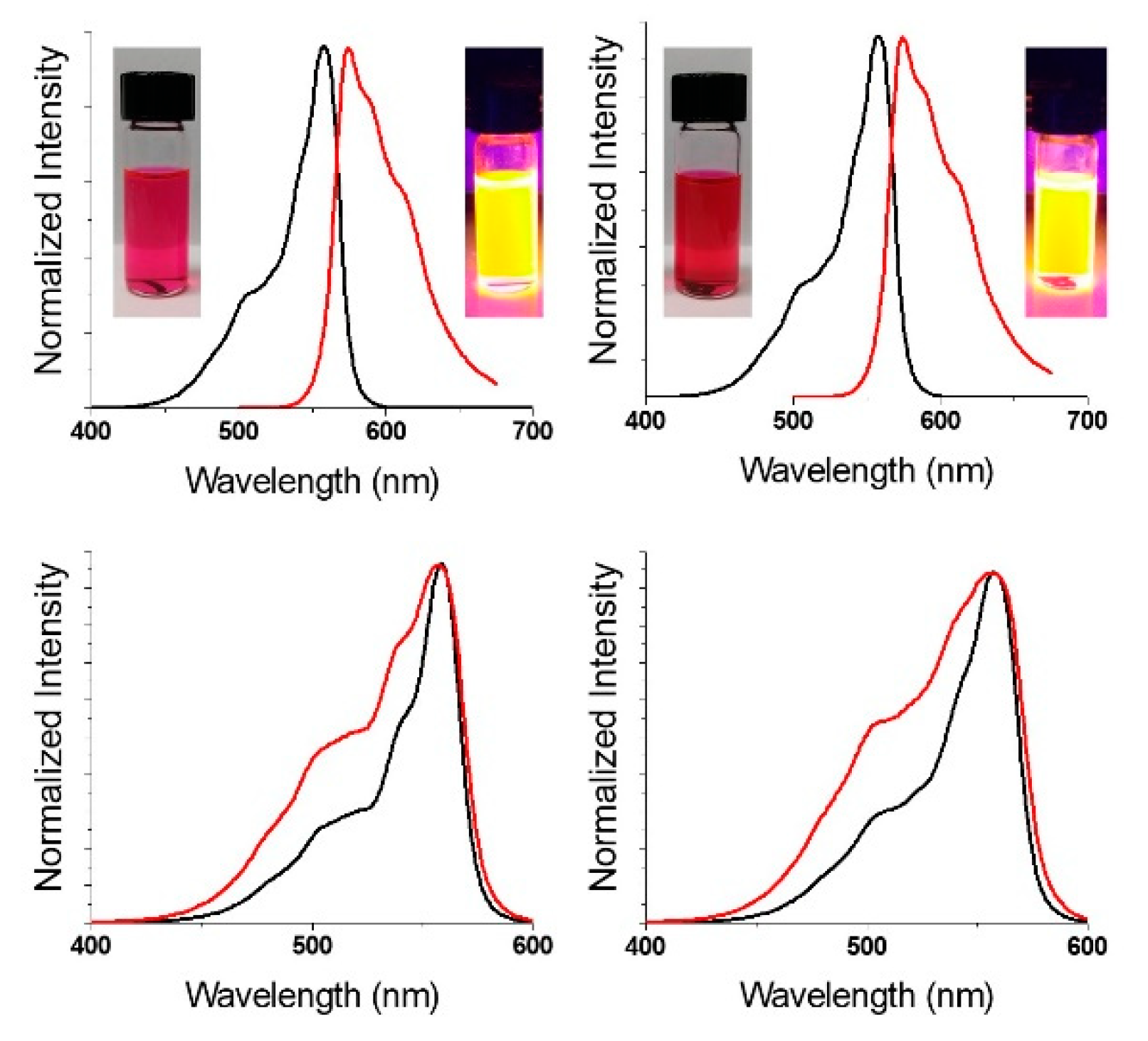
Analogous to other pyrrolic macrocycles [28], SPcs have two major absorption features, the Soret band (2nd HOMO → LUMO; λmax < 350 nm) and the Q-band (HOMO → LUMO; λmax > 500 nm). The F3SPc(C3) and F3SPc(C1) exhibit identical Q-band absorptions at 557 nm, representing a ~15 nm hypsochromic shift from previously reported perfluorinated SPcs (Figure 3, black) [20]. Upon excitation into the Soret band (λex = 325 nm), intense orange fluorescence is observed (λem = 575 nm) (Figure 3, upper, red). Excitation spectra (λem = 625 nm) match absorption spectra, as expected (Figure 3, lower). Quantum yields of fluorescence (Φf) were determined by the relative method, using boron subphthalocyanine chloride as a standard (Φf = 0.29). Quantum yields were found to be identical for the constitutional isomers (Φf = 0.27–0.28) within the error of the measurement. Furthermore, these values are comparable to other fluorinated SPcs, providing sufficient brightness for fluorescence imaging studies, discussed below.
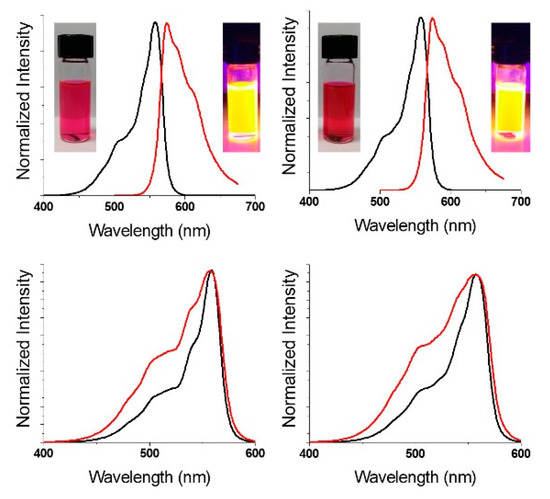
Figure 3.
Normalized absorbance (black), emission (λex = 325 nm (red; upper)) and excitation (λem = 625 nm (red; lower)) spectra of C3 (left) and C1 (right) F3-SPcs. Samples in THF solution.
2.3. Epifluorescence Microscopy of Subphthalocyanines in MDA-MB-231 Breast Tumor Cells
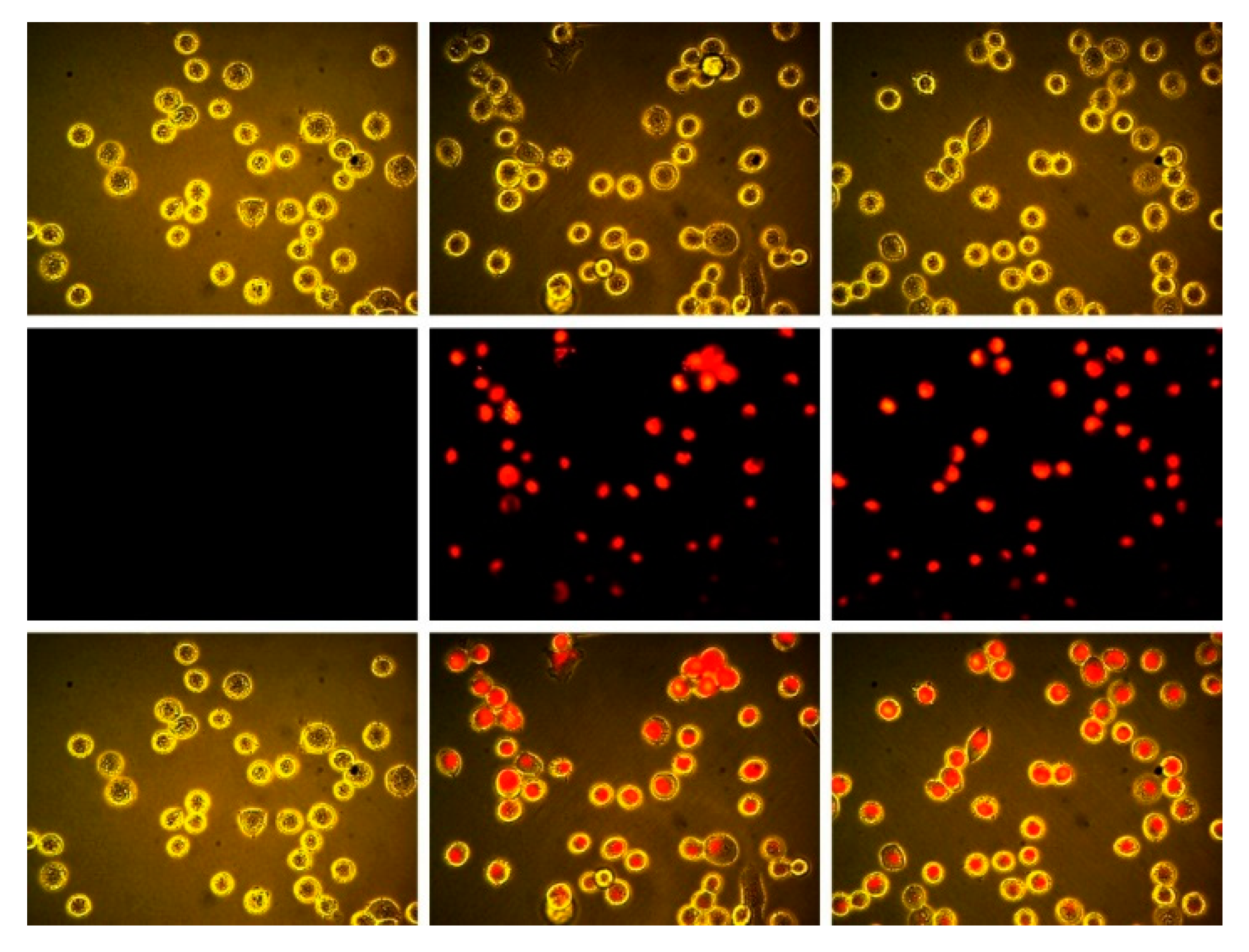
As discussed above, we had previously reported that the inclusion of F-atoms at the periphery of boron subpthalocyanine chloride macrocycles led to enchanced intracellular fluorescence from the SPcs in MDA-MB-231 cells. In these examples, each isoindoline sub-unit was perfluorinated, yielding SPcs with either 4, 8, or 12 F-atoms at the periphery. To further test this platform, trifluorosubphtlaocyanines reported here were tested in MDA-MB-231 cells. The difference reported herein is that SPcs have fewer F-atoms overall (3 vs. 4–12) and are situated only in the β positions, as opposed to both α and β positions. Cells were plated and allowed to grow to 50% confluency before being treated with 50 μM F3SPc(C3) (Figure 4, center) and 50 μM F3SPc(C1) (Figure 4, right), using 1% DMSO as a solubilizing vehicle. Control cells were treated with the 1% DMSO vehicle alone (Figure 4, left).
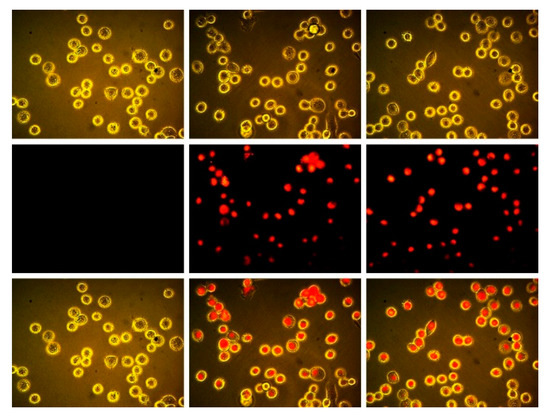
Figure 4.
Brightfield (upper), epifluorescence ((middle) λex = BP 528–553 nm, λem = BP 578–633 nm), and (lower) overlay images of MDA-MB 231 breast tumor cells. DMSO control (left), treated with 50 μM F3SPc(C3) (center), and 50 μM F3SPc(C1) (right) for 15 min (400×).
Treated cells showed marked intracellular fluorescence compared to the vehicle control. Therefore, even as few as three F-atoms at the SPc periphery can lead to enhanced cellular uptake, and possession of F-atoms in the α position on the isoindoline is not a necessary requirement.
3. Materials and Methods
3.1. General Considerations
Reagents and chemicals, including silica gel (60 Å, 230–400 mesh), were purchased from VWR (Radnor, PA, USA) and used without further purification unless otherwise noted. Nuclear magnetic resonance (NMR) spectra were recorded on an Avance III (400 MHz, Bruker, Billerica, MA, USA) spectrophotometer. 19F NMR spectra were recorded using trifluoroacetic acid as a standard (δ = −76.55 ppm). Mass spectrometry was performed on an LC/MS 6545 Q-TOF (Agilent, Santa Clara, CA, USA) in APCI mode. Purity analysis by HPLC was performed on an Agilent 1100 system with a diode array detector and C8 ZORBAX Eclipse Plus column (Agilent). Absorption data was collected on a Cary-100 UV-vis spectrophotometer (Agilent) in double-beam mode using 1-cm path quartz cuvettes. Corrected fluorescence spectra were collected on a Fluorolog 3 fluorometer (Horiba Jobin-Yvon, Kyoto, Japan) equipped with an R928 PMT (Hamamatsu, Bridgewater Township, NJ, USA). Solutions were prepared such that absorption remained below 0.1 AU to prevent reabsorption and self-quenching.
3.2. Synthesis
Boron trifluorosubphthalocyanine chloride. To a 25 mL round-bottomed flask was added 4-fluorophthalonitrile (0.134 g, 0.92 mmol). The flask was purged with N2, p-xylene (4 mL) was added and the flask was heated to 140 °C. A solution of BCl3 (1 M in p-xylene, 0.75 mL, 0.75 mmol) was added, resulting in a yellow solution. After approximately five minutes, the solution changed color to dark purple. The reaction was stirred for 40 min, after which the solvent was reduced in vacuo. The resulting dark purple solid was dissolved in dichloromethane and filtered through a plug of silica gel. The resulting sample was purified by flash chromatography on silica gel (0–5% ethyl acetate gradient in hexane).
F3SPc(C3)—Boron 2,9,16-trifluorosubphthalocyanine chloride. First elution, purple solid (0.007 g, 0.015 mmol, 4.7% yield). 1H NMR (400 MHz, CDCl3) δ (ppm): 8.87 (dd, 3H, JH–H(ortho) = 8.7 Hz, JH–F(meta) = 4.7 Hz), 8.53 (dd, 3H, JH–F(ortho) = 8.2 Hz, JH–H(meta) = 2.2 Hz), 7.69 (ddd, 3H, JH–H(ortho) = JH–F(ortho) = 8.7 Hz, JH–H(meta) = 2.3 Hz). 19F NMR (376 MHz, CDCl3) δ (ppm): −106.74 (m). HR-LCMS APCI: calcd. for (C24H10BClF3N6) [M + H]+, 485.0701; found, 485.0702.
F3SPc(C1)—Boron 2,9,17-trifluorosubphthalocyanine chloride. Second elution, purple solid (0.011 g, 0.025 mmol, 7.4% yield). 1H NMR (400 MHz, CDCl3) δ (ppm): 8.87 (m, 3H), 8.52 (m, 3H), 7.68 (m, 3H). 19F NMR (376 MHz, CDCl3) δ (ppm): −106.60 (m), −106.66 (m), −106.86 (m). HR-LCMS APCI: calcd. for (C24H10BClF3N6) [M + H]+, 485.0701; found, 485.0704.
3.3. Fluorescence Quantum Yield Determination
Fluorescence quantum yields were determined by the relative method [29], and analysis with Equation (1):
where r and x denote the standard and unknown, respectively, A is the absorption intensity at the excitation wavelength, F is the integrated fluorescence intensity and n is the refractive index of the solvent. Cross-calibration determined less than 10% error for this method and instrumentation.
3.4. Cell Culture and Fluorescence Imaging
Cell culture reagents were obtained from Hyclone (GE, Boston, MA, USA) unless otherwise noted. MDA-MB-231 breast tumor cells were obtained from the American Type Culture Collection and maintained in Dulbecco′s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum. For imaging assays, cells were plated in 12-well plates (Corning, Corning, NY, USA) and allowed to grow to ~50% confluency before being treated with solutions of SPcs in media (1% dimethylsulfoxide). Images were collected with a VWR Inverted Fluorescence Microscope, Moticam 5.0 (Motic, Richmond, BC, Canada), and TRITC filter set (λex = 540 nm ± 12 nm, λem = 605 nm ± 27 nm).
Supplementary Materials
The following are available online. HR APCI-MS, 1H NMR, 19F NMR, and HPLC chromatograms with DAD purity reports.
Author Contributions
Conceptualization, K.J.M. and E.R.T.; methodology, E.R.T.; investigation, R.L.C., K.J.M. and L.E.D.; writing—original draft preparation, E.R.T.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meller, A.; Ossko, A. Phthalocyaninartige Bor-Komplexe. Mon. Für Chem. Chem. Mon. 1972, 103, 150–155. [Google Scholar] [CrossRef]
- Torres, T. From subphthalocyanines to subporphyrins. Angew. Chem. Int. Ed. 2006, 45, 2834–2837. [Google Scholar] [CrossRef] [PubMed]
- Claessens, C.G.; Gonzalez-Rodriguez, D.; Rodriguez-Morgade, M.S.; Medina, A.; Torres, T. Subphthalocyanines, subporphyrazines, and subporphyrins: Singular nonplanar aromatic systems. Chem. Rev. 2014, 114, 2192–2277. [Google Scholar] [CrossRef] [PubMed]
- Montero-Campillo, M.M.; Lamsabhi, A.M.; Mó, O.; Yáñez, M. Photochemical behavior of beryllium complexes with subporphyrazines and subphthalocyanines. J. Phys. Chem. A 2016, 120, 4845–4852. [Google Scholar] [CrossRef]
- Morse, G.E.; Bender, T.P. Boron subphthalocyanines as organic electronic materials. Acs Appl. Mater. Interfaces 2012, 4, 5055–5068. [Google Scholar] [CrossRef]
- Plint, T.G.; Lessard, B.H.; Bender, T.P. Doping chloro boron subnaphthalocyanines and chloro boron subphthalocyanine in simple OLED architectures yields warm white incandescent-like emissions. Opt. Mater. 2018, 75, 710–718. [Google Scholar] [CrossRef]
- Morse, G.E.; Helander, M.G.; Maka, J.F.; Lu, Z.-H.; Bender, T.P. Fluorinated phenoxy boron subphthalocyanines in organic light-emitting diodes. Acs Appl. Mater. Interfaces 2010, 2, 1934–1944. [Google Scholar] [CrossRef]
- Sullivan, P.; Duraud, A.; Hancox, l.; Beaumont, N.; Mirri, G.; Tucker, J.H.R.; Hatton, R.A.; Shipman, M.; Jones, T.S. Halogenated boron subphthalocyanines as light harvesting electron acceptors in organic photovoltaics. Adv. Energy Mater. 2011, 1, 352–355. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, X.J.; Chan, E.Y.M.; Fong, W.P.; Ng, D.K.P. Synthesis, photophysical properties and in vitro photodynamic activity of axially substituted subphthalocyanines. Org. Biomol. Chem. 2007, 5, 3987–3992. [Google Scholar] [CrossRef]
- Winckel, E.; Mascaraque, M.; Zamarrón, A.; Juarranz de la Fuente, Á.; Torres, T.; Escosura, A. Dual role of subphthalocyanine dyes for optical imaging and therapy of cancer. Adv. Funct. Mater. 2018, 28, 1705938. [Google Scholar] [CrossRef]
- Spesia, M.B.; Durantini, E.N. Synthesis and antibacterial photosensitizing properties of a novel tricationic subphthalocyanine derivative. Dyes Pigment. 2008, 77, 229–237. [Google Scholar] [CrossRef]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Simultaneous photodiagnosis and photodynamic treatment of metastatic melanoma. Molecules 2019, 24, 3153. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.C.H.; Lo, P.-C.; Ng, D.K.P. Stimuli responsive phthalocyanine-based fluorescent probes and photosensitizers. Coord. Chem. Rev. 2019, 379, 30–46. [Google Scholar] [CrossRef]
- Awaji, A.I.; Köksoy, B.; Durmuş, M.; Aljuhani, A.; Alraqa, S.Y. Novel hexadeca-substituted metal free and zinc(II) phthalocyanines; design, synthesis and photophysicochemical properties. Molecules 2018, 24, 77. [Google Scholar] [CrossRef]
- Bonnett, R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar] [CrossRef]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: New structural trends and therapeutic areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in pharmaceutical industry: Fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef]
- Mei, H.; Han, J.; Fustero, S.; Medio-Simon, M.; Sedgwick, D.M.; Santi, C.; Ruzziconi, R.; Soloshonok, V.A. Fluorine-containing drugs approved by the FDA in 2018. Chem. A Eur. J. 2019, 25, 11797–11819. [Google Scholar] [CrossRef]
- McAuliffe, K.J.; Kaster, M.A.; Szlag, R.G.; Trivedi, E.R. Low-symmetry mixed fluorinated subphthalocyanines as fluorescence imaging probes in MDA-MB-231 breast tumor cells. Int. J. Mol. Sci. 2017, 18, 1177. [Google Scholar] [CrossRef]
- Sejdarasi, L.; McAuliffe, K.J.; Corbin, B.A.; Trivedi, E.R. Synthesis and characterization of mixed fluorinated phenylthio- subphthalocyanines. Chem. Sel. 2017, 2, 7417–7420. [Google Scholar] [CrossRef]
- Hamdoush, M.; Skvortsov, I.A.; Mikhailov, M.S.; Pakhomov, G.; Stuzhin, P.A. Perfluorinated subphthalocynine analogues containing fused 1,2,5-thiadiazole fragments. J. Fluor. Chem. 2017, 204, 31–36. [Google Scholar] [CrossRef]
- Farley, C.; Bhupathiraju, N.V.S.D.K.; John, B.K.; Drain, C.M. Tuning the structure and photophysics of a fluorous phthalocyanine platform. J. Phys. Chem. A 2016, 120, 7451–7464. [Google Scholar] [CrossRef] [PubMed]
- Guilleme, J.; González-Rodríguez, D.; Torres, T. Triflate-subphthalocyanines: Versatile, reactive intermediates for axial functionalization at the boron atom. Angew. Chem. Int. Ed. 2011, 50, 3506–3509. [Google Scholar] [CrossRef] [PubMed]
- Hanack, M.; Geyer, M. Synthesis and separation of structural isomers of tri-tert-butylsubphthalocyaninatophenylboron(III). J. Chem. Soc. Chem. Commun. 1994, 2253–2254. [Google Scholar] [CrossRef]
- Claessens, C.G.; Torres, T. Subphthalocyanine enantiomers: First resolution of a C-3 aromatic compound by HPLC. Tetrahedron Lett. 2000, 41, 6361–6365. [Google Scholar] [CrossRef]
- Morse, G.E.; Helander, M.G.; Stanwick, J.; Sauks, J.M.; Paton, A.S.; Lu, Z.-H.; Bender, T.P. Experimentally validated model for the prediction of the HOMO and LUMO energy levels of boronsubphthalocyanines. J. Phys. Chem. C 2011, 115, 11709–11718. [Google Scholar] [CrossRef]
- Gouterman, M.; Snyder, L.C.; Wagniere, G.H. Spectra of porphyrins: Part II. Four orbital model. J. Mol. Spectrosc. 1963, 11, 108–127. [Google Scholar] [CrossRef]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds may be available from the authors upon request. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).