Abstract
The heat capacities on two minerals of hungchaoite (MgB4O7·9H2O, Hu) and mcallisterite (MgB6O10·7.5H2O, Mc) have been measured with a precision calorimeter at temperatures ranging from 306.15 to 355.15 K, experimentally. It was found that there are no phase transition and thermal anomalies, and the molar heat capacities against temperature for the minerals of hungchaoite and mcallisterite were fitted as and , respectively. The molar heat capacities and thermodynamic functions of (HT-H298.15), (ST-S298.15), and (GT-G298.15) at intervals of 1 K for the two minerals were obtained for the first time. These results are significant in order to understand the thermodynamic properties of those minerals existing in nature salt lakes, as well as applying them to the chemical engineering process design.
1. Introduction
Magnesium borates and their superior performances are widely used in chemical industries, alkali-free glass fiber, ceramic, and agriculture [1,2]. China is rich in boron resources with solid boron mineral in the east and liquid boron mineral in the brine of salt lakes in the west. Especially, the Qinghai-Tibet Plateau Salt Lake is famous for abundant boron-magnesium resources [3,4], and it has been found to have 14 kinds of natural borate minerals [5,6]. Among them, several kinds of the most common magnesium borate hydrates, which are pinnoite (MgB2O4·3H2O), mcallisterite (MgB6O10·7.5H2O), hungchaoite (MgB4O7·9H2O), inderite (Mg2B6O11·15H2O), and kurnakovite (Mg2B6O11·15H2O) [7,8,9,10,11,12]. However, the thermodynamic properties on these normal magnesium borates are scarce. Therefore, studies on the thermodynamic properties of different forms of magnesium borates are essential to provide important basic thermodynamic data for the development and utilization of the salt lake brine resources in the western region of China.
Heat capacity means the basic thermodynamic property, which can reflect the interaction between solute and solvent in the solution system [13,14]. The apparent molar enthalpy at different concentrations was calculated by the heat capacity at different temperatures. In addition, it is also one of the essential basic data of chemical production [15,16]. According to the heat capacity data, accurate information about the materialized treatment and reaction device design can be obtained [17,18]. At the same time, basic properties such as enthalpy, entropy, and Gibbs free energy can be calculated by heat capacity, and the structure and phase transition can also be analyzed by it.
The heat capacity and thermodynamic properties of magnesium borates are basic thermodynamic parameters, which provide basic data for the comprehensive utilization of magnesium and boron resources. According to the Tarasov theory of the heat capacity of solids [13], which is a particular case of the fractal heat capacity theory, the dependence of T is proportional to T for solids with chain structures, T2 for layer solids, and T3 for framework structures.
In this research, the heat capacities and thermodynamic properties on two minerals of hungchaoite (MgB4O7·9H2O) and mcallisterite (MgB6O10·7.5H2O) in the temperature range of 306.15~365.15 K were measured by a precision calorimeter for the first time.
2. Experimental
2.1. Chemicals
(MgCO3)4·Mg(OH)2·5H2O (A.R., Macklin Chemical Reagent Co., Ltd., Shanghai, China); H3BO3 (A.R., Sinopharm Chemical Reagent Co., Ltd., Shanghai, China); double deionized water (DDW) with its conductivity less than 0.055 μS·m−1 was produced by the deionized water machine (ULUP-II-10T, Chongqing Jiuyang Co. Lt., Chongqing, China).
2.2. Synthesis
Hungchaoite (MgB4O7·9H2O) and mcallisterite (MgB6O10·7.5H2O) were synthesized according to the reference [19]. Briefly, hungchaoite and mcallisterite were both synthesized with basic magnesium carbonate, magnesium oxide, and boric acid. Initially, active magnesium oxide was made from basic magnesium carbonate in a Muffle Furnace (SX2-5-12, Tianjing Zhonghuan Co., Tianjin, China) at 873.15 K for 6 h. Then, magnesium oxide, basic magnesium carbonate, and boric acid were dissolved at 298.15 K and stirred for 3.5 h to filter the liquid. The magnetic stirring was paused for 4 h. Finally, the solid phase was separated by washing three times with absolute ethyl alcohol and DDW separately. All the samples were dried in a desiccator before using.
2.3. Identification and Analytical Methods
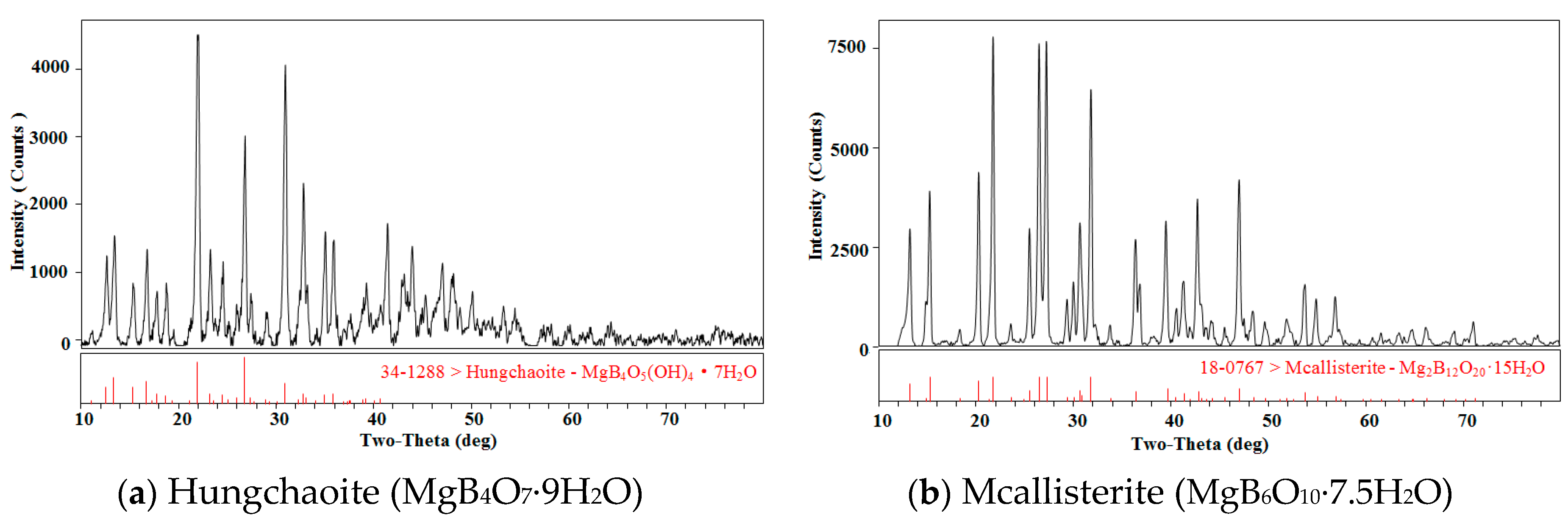
MgB4O7·9H2O and MgB6O10·7.5H2O were identified by X-ray diffractometer (MSAL XD-3, Beijing Purkinje General Instrument Co., Ltd., Beijing, China) with Cu-Kα radiation at 4°·min−1, and the results are shown in Figure 1.
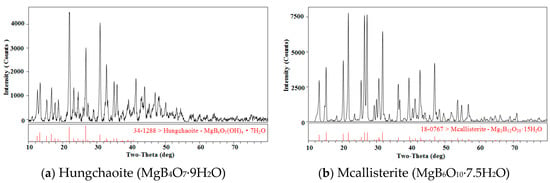
Figure 1.
The X-ray diffraction patterns for minerals hungchaoite and mcallisterite. The codes “34-1288” and “18-0767” denote standard codes of X-ray of hungchaoite and mcallisterite.
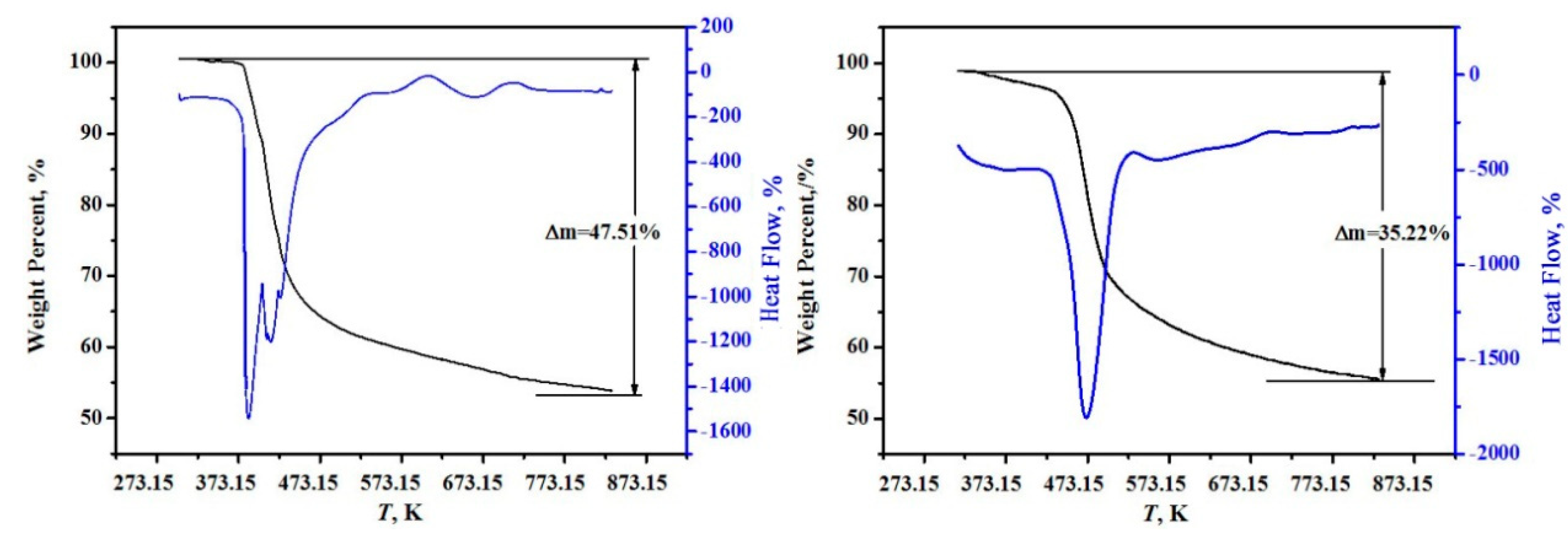
The high-precision thermal analysis (TG-DSC, Labsys, Setaram, France) under argon atmosphere with a heating rate of 10 K/min was used, and the TG-DSC curves for the two minerals are shown in Figure 2. It can be found from Figure 2 that the samples were continuously weightless at 368.15~748.15 K, the total weightlessness rates were 47.51% and 35.22% for MgB4O7·9H2O and MgB6O10·7.5H2O, respectively. A comparison of dehydrated crystal waters between the theoretical values and the experimental values agree well and the deviations are within 0.17% in Table 1.
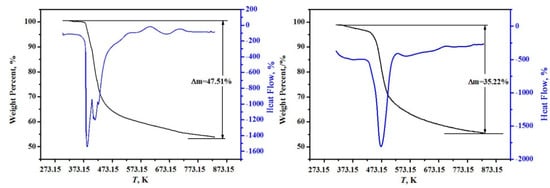
Figure 2.
The high-precision thermal analysis (TG-DSC) curves of hungchaoite (on the left) and mcallisterite (on the right).

Table 1.
Analytical results of hungchaoite and mcallisterite.
The content of magnesium was determined by EDTA titration with an uncertainty of 0.003 [20], and the content of B2O3 was analyzed by the method of mannitol weight titration with an uncertainty of 0.0005 [21]. The chemical analysis results for MgB4O7·9H2O and MgB6O10·7.5H2O are presented in Table 1.
2.4. Calorimetry and Experiment Method
The high-precision calorimeter (TG-DSC Labsys, Setaram, France) was used for a heat capacity experiment, which requires three groups of experiments, namely, blank experiment, reference experiment, and sample experiment, and Alumina as reference experiment. To verify the performance, the heat capacity of KCl was measured, and the average experimental value for five times of 0.6860 J·g−1·K−1 is in accordance with 0.6879 J·g−1·K−1 reported in the literature [22], whose deviation is 0.28%. The heat capacities were carried out from 303 K to 355 K with a heating rate of 5 K/min and a flow rate of N2 is 20 mL/min, and weighting 16.02 ± 0.01 and 16.65 ± 0.01 mg samples of hungchaoite and mcallisterite were used, respectively.
3. Results and Discussion
3.1. Heat Capacities
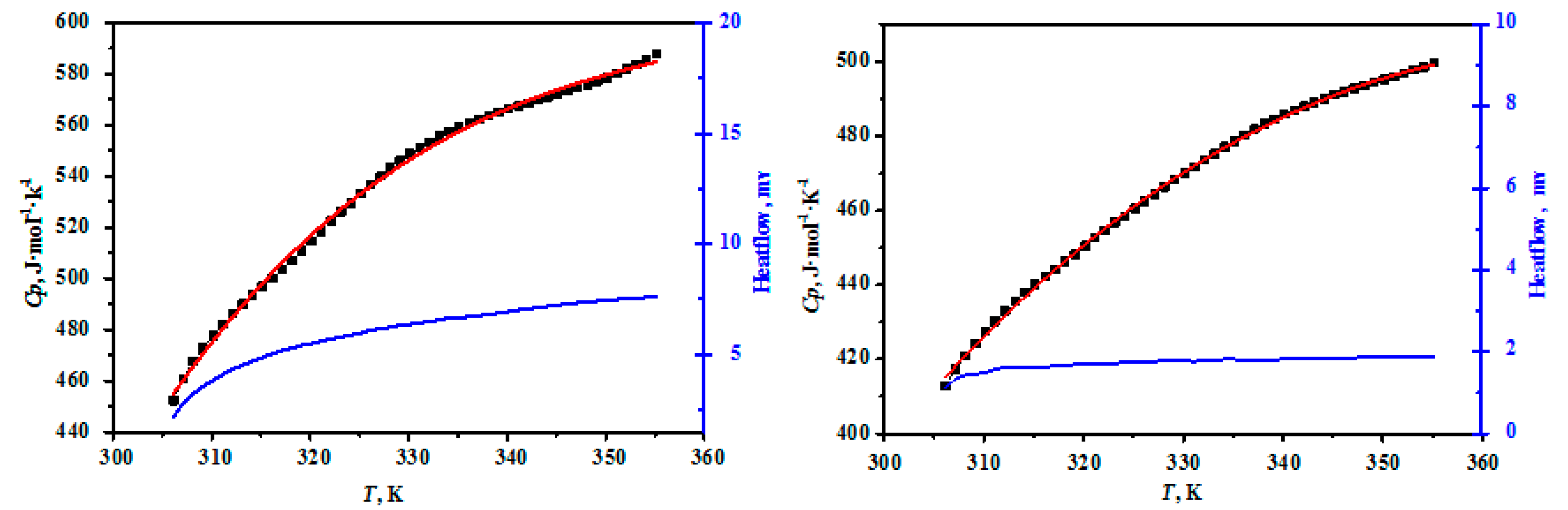
The heat capacities of MgB4O7·9H2O and MgB6O10·7.5H2O are presented in Table 2 and the comparisons between the experimental and fitting results for the compounds were plotted in Figure 3. As can be seen in Figure 3, the heat capacity curves of the hungchaoite and mcallisterite increase slowly with the increasing temperature, which showed that the structure of the hungchaoite and mcallisterite were smooth within the temperature range. There is no phase change, association, and thermal decomposition occurring from 306.15 K to 355.15 K for hungchaoite and mcallisterite. Molar heat capacities polynomial equations of hungchaoite and mcallisterite are expressed as Equations (1) and (2), respectively.

Table 2.
Experimental values of molar heat capacities of hungchaoite (MgB4O7·9H2O) and mcallisterite (MgB6O10·7.5H2O) at 306.15~355.15 K and 101.325 kPa a.
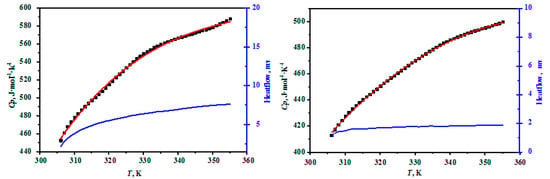
Figure 3.
Comparisons on the experimental and fitting results for the compounds of hungchaoite (on the left) and mcallisterite (on the right) at temperatures ranging from 306.15 to 355.15 K.
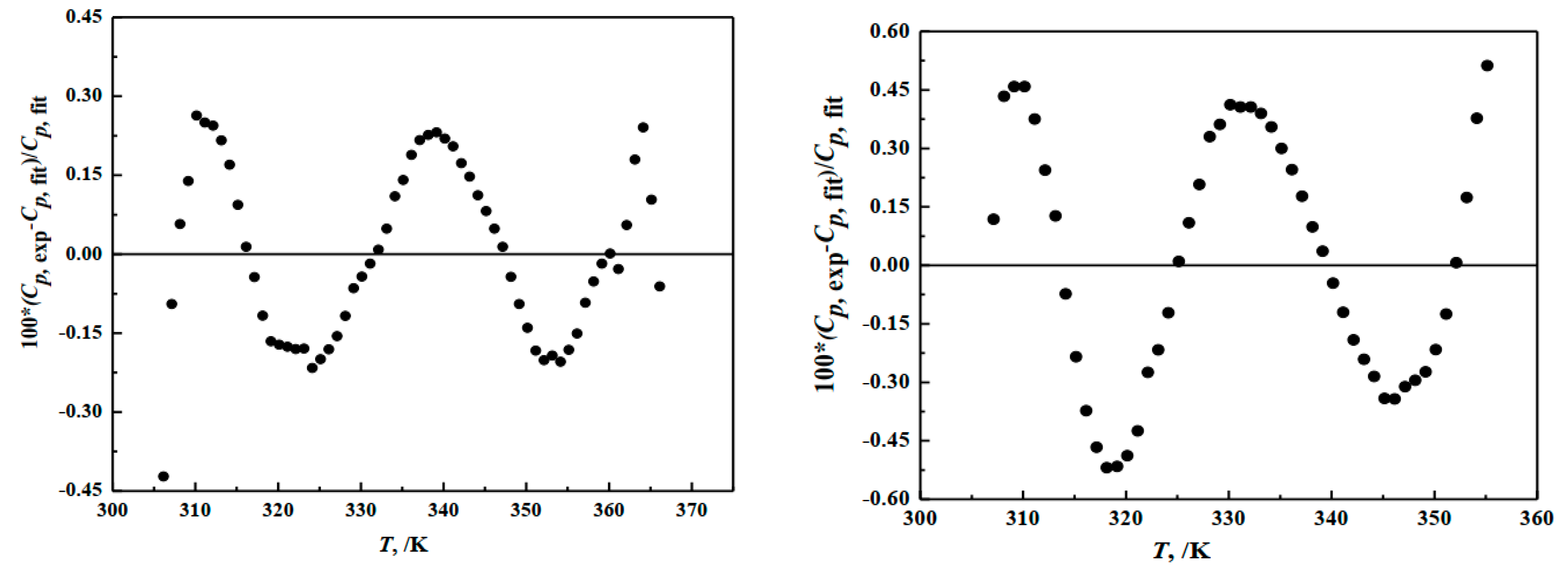
The regression analysis results are shown in Table 2 and Figure 4 and the determination coefficient of the fitting curves are = 0.9975 and = 0.9992. The deviation between the fitting values and the experimental values of hungchaoite and mcallisterite are 0.32% and 1.8%, separately. The relative deviation between the fitting value and the experimental value of two compounds are both less than 0.47%.
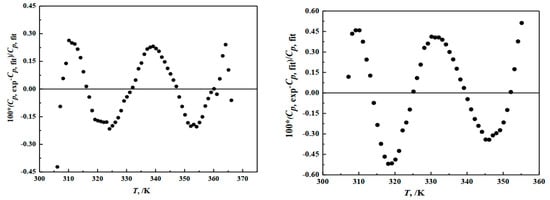
Figure 4.
Deviation of heat capacities of the compounds of hungchaoite (on the left) and mcallisterite (on the right) between experimental Cp, exp and calculated Cp, fit.
3.2. Enthalpy, Entropy, and Gibbs Free Energy
The molar heat capacity is calculated according to the fitting heat capacity curve. The standard reference temperature of the thermodynamic function is 298.15 K, (HT-H298.15), (ST-S298.15), and (GT-G298.15) calculated according to the following thermodynamic functions. The values of (HT-H298.15), (ST-S298.15), and (GT-G298.15) for the hungchaoite and mcallisterite are listed in Table 3.

Table 3.
Molar heat capacities obtained on the fitted Equations (1) and (2), as well as the thermodynamic functions (HT-H298.15), (ST-S298.15), and (GT-G298.15) for MgB4O7·9H2O and MgB6O10·7.5H2O from 306.15 to 355.15 K.
4. Conclusions
The heat capacities of hungchaoite (MgB4O7·9H2O) and mcallisterite (MgB6O10·7.5H2O) were measured using the precision calorimetry, and the molar heat capacities of hungchaoite and mcallisterite were derived experimentally. The experimental results showed that the molar heat capacities of the two minerals are increased with the increasing of temperature, and no phase change, association, and thermal decomposition were found at temperatures ranging from 306.15 to 355.15 K. At the same time, the polynomial equations of the molar heat capacities against the temperature for the two minerals were fitted by the least square method. The thermodynamic functions of (HT-H298.15), (ST-S298.15), and (GT-G298.15) at intervals of 1 K for the two minerals were obtained for the first time.
Author Contributions
Investigation and writing—original draft preparation, J.S.; resources and writing—review and editing, F.Y.; data curation, L.L.; visualization, L.L.; supervision, Y.G.; project administration and funding acquisition, T.D.
Funding
This research was funded by the National Natural Science Foundation of China (U1607123 and 21773170), the Key Projects of Natural Science Foundation of Tianjin (18JCZDJC10040), the Yangtze Scholars and Innovative Research Team in Chinese University (IRT_17R81).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, J.G.; Wang, F.F.; Zhang, Y.J. Microstructure and mechanical properties of magnesium matrix composite reinforced with magnesium borate whisker. J. Compos. Mater. 2012, 24, 3011–3016. [Google Scholar] [CrossRef]
- Shao, S.N.; Xiong, X.X. Discussion on main Ore collection area of boron ore in China and its resource potential. Chem. Miner. Geol. 2010, 32, 65–74. [Google Scholar]
- Han, J.W.; Li, F.Q.; Wang, X.W. Advances in the extraction of boron from liquid mines. Salt Lake Res. 2007, 15, 57–61. [Google Scholar]
- Zhao, W.; Guo, Y.F.; Gao, J. The general situation of boron resources and the research progress of boron extraction in China. World Sci. Technol. Res. Dev. 2011, 33, 29–32. [Google Scholar]
- Han, X.N.; Zhao, M.L.; Wang, S.Q. Phase Equilibria of the Aqueous Systems Containing Magnesium and Borate Ions. Trans. Tech. 2014, 1015, 409–412. [Google Scholar] [CrossRef]
- Yuan, G.H. Structural Characteristics and Classification of Boric Acid Salt; Dalian University of Technology: Liaoning, China, 2007. [Google Scholar]
- Lin, F.; Dong, Y.P.; Peng, J.Y. Synthesis and formation mechanism of pinnoite by the phase transition process. Phase Transit. 2015, 89, 558–567. [Google Scholar] [CrossRef]
- Xu, G.M. Shortening the reaction time is the only way to produce the high yield and low consumption of borax by carbon-alkali method. Dandong Chem. 1995, 12, 1–3. [Google Scholar]
- Meng, L.Z.; Deng, T.L.; Duan, C.W. Study on synthesis method of three-way boron magnesium stone. World Sci. Technol. Res. Dev. 2010, 32, 825–826. [Google Scholar]
- Derun, E.M.; Senberber, F.T. Characterization and thermal dehydration kinetics of highly crystalline mcallisterite, synthesized at low temperatures. Sci. World J. 2014, 2014, 985185. [Google Scholar]
- Frost, R.L.; López, A.; Xi, Y.F. The molecular structure of the borate mineral inderite Mg(H4B3O7)(OH)5H2O—A vibrational spectroscopic study. Mol. Biomol. Spectrosc. 2013, 116, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.H.; Han, W.T.; Qian, Z.Q. Three ways boron magnesium stone a new borate mineral. Geology 1965, 45, 298–305. [Google Scholar]
- Marochkina, M.N.; Smirnova, N.N.; Knyazev, A.V. The low-temperature heat capacity of alkali and alkaline-earth metal uranoborates. Russ. J. Phys. Chem. 2008, 82, 1516–1520. [Google Scholar] [CrossRef]
- Zhang, S.S. Thermochemical Properties of Aqueous System Containing Alkalis Borates; Tianjin University of Science and Technology: Tianjin, China, 2017. [Google Scholar]
- Bespyatov, M.A.; Chernyaikin, I.S.; Naumov, V.N. Low-temperature heat capacity of Al(C11H19O2)3. Thermochim. Acta 2014, 596, 40–41. [Google Scholar] [CrossRef]
- Gao, S.Y.; Xu, K.F.; Li, G. The behavior of boric acid in the chemical v-of boron-containing concentrated halogen dilution of halogen borate. J. Chem. 1986, 44, 1229–1233. [Google Scholar]
- Calvar, N.; Gómez, E.; Macedo, E.A. Thermal analysis and heat capacities of pyridinium and imidazolium ionic liquids. Thermochim. Acta 2013, 565, 178–182. [Google Scholar] [CrossRef]
- Hu, R.Z.; Zhao, F.Q.; Gao, H.X. Fundamentals and Applications of Quantitative Thermodynamics; Science Press: Beijing, China, 2011. [Google Scholar]
- Ge, H.W. Study on Multi-Warm Mechanical Properties of MgB4O7-H2O System; University of Technology: Chengdu, China, 2011. [Google Scholar]
- Qinghai Institute of Salt Lakes, Chinese Academy of Sciences. Analysis Methods for Brines and Salts, 2nd ed.; Science Press: Beijing, China, 1998.
- Li, H.X.; Dong, O.Y.; Yao, Y. The mass titration analytical method and its application. J. Salt Lake Res. 2011, 19, 31–36. [Google Scholar]
- Speight, J.G. Lange’s Handbook of Chemistry, 16th ed.; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).