Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments
Abstract
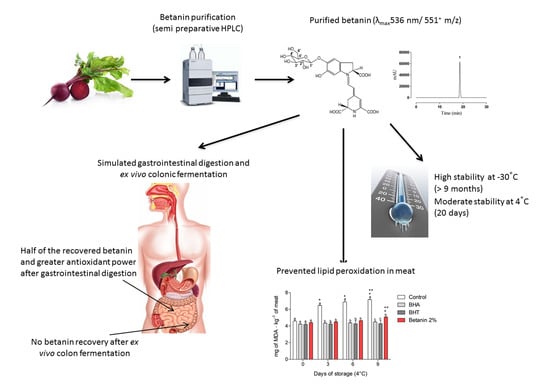
:1. Introduction
2. Results and Discussion
2.1. Betanin Purification
2.2. HPLC-ESI(+)-MS/MS Analysis
2.3. Storage Stability
2.4. Lipid Peroxidation Inhibition in Meat Matrices
2.5. Betanin Chemical Stability during In Vitro Simulated Gastrointestinal Digestion
2.6. Betanin Antioxidant Activity throughout Simulated Human Gastrointestinal Digestion
3. Material and Methods
3.1. Standards and Reagents
3.2. Betanin Purification
3.2.1. Sample Preparation
3.2.2. HPLC Betanin Purification
3.3. Betanin Identification by Liquid Chromatography Positive Ion Electrospray Ionization Tandem Mass Spectrometry (LC-ESI(+)-MS/MS)
3.4. Storage Stability
3.5. Betanin Ability to Inhibit Lipid Peroxidation in Meat
3.6. TAP Determination
3.7. Antioxidant Activity Determination by Different Assays
3.7.1. FRAP Determination
3.7.2. TEAC Determination
3.7.3. ORAC Determination
3.8. Simulated Betanin In Vitro Human Gastrointestinal Digestion and Ex Vivo Colon Fermentation (Supplementary File—Figure S5)
Ex vivo Colon Fermentation
3.9. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baião, D.S.; da Silva, D.V.; Del Aguila, E.M.; Paschoalin, V.M.F. Nutritional, Bioactive and Physicochemical Characteristics of Different Beetroot Formulations, Food Additives. 6 September 2017. Available online: https://www.intechopen.com/books/food-additives/nutritional-bioactive-and-physicochemical-characteristics-of-different-beetroot-formulations (accessed on 3 December 2018).
- Azeredo, M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Saponjac, V.T.; Canadanovic-Brunet, J.; Cetkovic, G.; Jakisic, M.; Djilas, S.; Vulic, J.; Stajcic, S. Encapisulation of beetroot pomace extract: Rsm optimization, storage and gastrointestinal stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on the re-evaluation of beetroot red (E 162) as a food additive. EFSA J. 2015, 13, 4318. [Google Scholar] [CrossRef]
- US Food and Drug Administration (FDA). Code of Federal Regulations. 2009. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=73.260 (accessed on 28 January 2019).
- Nemzer, B.; Pietrzkowski, Z.; Sporna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Gómez, M.; Lorenzo, J.M. Effect of packaging conditions on shelf-life of foal fresh meat. Meat Sci. 2012, 91, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Ito, N.; Fukushima, S.; Tsuda, H.; Shirai, T.; Tatematsu, M.; Imaida, K. Modification of chemical carcinogenesis by antioxidants. Princess Takamatsu Symp. 1983, 14, 381–389. [Google Scholar] [CrossRef]
- Williams, G.M.; Wang, C.X.; Iatropoulos, M.J. Toxicity studies of butylated hydroxanisole and butylated hydroxytoluene. II. Chronic feeding studies. Food Chem. Toxicol. 1990, 28, 799–806. [Google Scholar] [CrossRef]
- Cunha, L.C.M.; Monteiro, M.L.G.; Costa-Lima, B.R.C.; Guedes-Oliveira, J.M.; Alves, V.H.M.; Almeida, A.L.; Tonon, R.V.; Rosenthal, A.; Conte-Junior, C.A. Effect of microencapsulated extract of pitaya (Hylocereuscostaricensis) peel on color, texture and oxidative stability of refrigerated ground pork patties submitted to high pressure processing. Innov. Food Sci. Emerg. Technol. 2018, 49, 136–145. [Google Scholar] [CrossRef]
- Muíño, I.; Fuente, J.; Pérez, C.; Apeleo, E.; Pérez-Santaescolástica, C.; Cañeque, V.; Lauzurica, S.; Bermejo-Poza, R.; Díaz, A.M.T. Use of red wine polyphenols as a natural preservative in health-promoting omega-3 fatty acids-enriched lamb patties. Molecules 2018, 23, 3080. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I. Plant betalain: Safety, antioxidant activity, clinical efficacy, and bioavailability. Compr. Rev. Food Sci. Food Saf. 2015, 15, 316–330. [Google Scholar] [CrossRef]
- Gentile, C.; Tesoriere, L.; Allegra, M.; Livrea, M.A.; D’Alessio, P. Antioxidant betalains from cactus pear (Opuntiaficus-indica) inhibit endothelial ICAM-1 expression. Ann. N. Y. Acad. Sci. 2004, 1028, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, G.J.; Azuine, M.A.; Rao, G.S.; Arai, T.; Lida, A.; Tokuda, H. Cytotoxic effect of the red beetroot (Beta vulgaris L.) extract compared to doxorubicin (Adriamycin) in the human prostate (PC-3) and breast (MCF-7) cancer cell lines. Anticancer Agents Med. Chem. 2011, 11, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Paluszczak, J.; Szaefer, H.; Baer-Dubowska, W. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br. J. Nutr. 2013, 110, 2138–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, L.C.P.; Trassi, M.A.S.; Lopes, N.B.; Dör, F.A.; dos Santos, M.T.; Baader, J.W.; Oliveira Jr, V.X.; Bastos, E.L. A comparative study of the purification of betanin. Food Chem. 2012, 131, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation—Structural and chromatic aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef]
- Netzel, M.; Stintzing, F.C.; Quaas, D.; Stra, B.G.; Carle, R.; Bitsch, R.; Bitsch, I.; Frank, T. Renal excretion of antioxidative constituents from red beet in humans. Food Res. Int. 2005, 38, 1051–1058. [Google Scholar] [CrossRef]
- Belhadj, S.I.; Najar, T.; Abderrabba, M. Chemical and antioxidant properties of betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef]
- Kaimainen, M.; Laaksonen, O.; Järvenpää, E.; Sandell, M.; Huopalahti, R. Consumer acceptance and stability of spray dried betanin in model juices. Food Chem. 2015, 187, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Kim, S.J.; Kim, S.H.; Kim, D.M. Repeated regeneration of degraded red beet juice pigments in the presence of antioxidants. J. Food Sci. 1998, 63, 69–72. [Google Scholar] [CrossRef]
- Kujala, T.S.; Loponen, J.M.; Klika, K.D.; Pihlaja, K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef] [PubMed]
- FDA (US Food and Drug Administration)—Refrigerator & Freezer Storage Chart. Available online: Chttps://www.fda.gov/downloads/food/resourcesforyou/healtheducators/ucm109315.pdf. (accessed on 2 December 2018).
- Raikos, V.; McDonagh, A.; Ranawana, V.; Duthie, G. Processed beetroot (Beta vulgaris L.) as a natural antioxidant in mayonnaise: Effects on physical stability, texture and sensory attributes. Food Sci. Hum. Wellness 2016, 5, 191–198. [Google Scholar] [CrossRef]
- Sucu, C.; Yildiz, G.T. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Sci. 2018, 140, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Krantz, C.; Monier, M.; Wahlström, B. Absorption, excretion, metabolism and cardiovascular effects of beetroot extract in the rat. Food Cosmet. Toxicol. 1980, 18, 363–366. [Google Scholar] [CrossRef]
- Watts, A.R.; Lennard, M.S.; Mason, S.L.; Tucker, G.T.; Woods, H.F. Beeturia and the biological fate of beetroot pigments. Pharmacogenetics 1993, 3, 302–311. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, R.C. Impact of thermal treatment on color and pigment pattern of Red beet (Beta vulgaris L.) preparations. J. Food Sci. 2004, 69, 491–498. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Angileri, F.; Gentile, C.; Livrea, M.A. In vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J. Agric. Food Chem. 2008, 56, 10487–11092. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin—A food colorant with biological activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Nagler, R.M.; Klein, I.; Zarzhevsky, N.; Drigues, N.; Reznick, A.Z. Characterization of the differentiated antioxidant profile of human saliva. Free Rad. Biol. Med. 2002, 32, 268–277. [Google Scholar] [CrossRef]
- Frank, T.; Stintzing, F.C.; Carle, R.; Bitsch, I.; Quaas, D.; Strass, G.; Bitsch, R.; Netzel, M. Urinary pharmacokinetics of betalains following consumption of red beet juice in healthy humans. Pharmacol. Res. 2005, 52, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska-Swigło, A.; Szymusiak, H.; Malinowska, P. Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical-scavenging activity. Food Addit. Contam. 2006, 23, 1079–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanner, J.; Harel, S.; Granit, R. Betalains–A new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, H.; Corke, H. HPLC Characterization of betalains from plants in the Amaranthaceae. J. Chromatogr. Sci. 2005, 43, 454–460. [Google Scholar] [CrossRef]
- Jung, S.; Nam, K.C.; Jo, C. Detection of malondialdehyde in processed meat products without interference from the ingredients. Food Chem. 2016, 209, 90–94. [Google Scholar] [CrossRef]
- Silva, D.V.; Silva, F.O.; Perrone, D.; Pierucci, A.P.T.R.; Conte-Junior, C.A.; Alvares, T.S.; Del Aguila, E.M.; Paschoalin, V.M.F. Physicochemical, nutritional, and sensory analyses of a nitrate-enriched beetroot gel and its effects on plasmatic nitric oxide and blood pressure. Food Nut. Res. 2016, 60, 29909. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Ana. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zuleta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Oomen, A.G.; Rompelberg, C.J.; Bruil, M.A.; Dobbe, C.J.; Pereboom, D.P.; Sips, A.J. Development of an in vitro digestion model for estimating the bioaccessibility of soil contaminants. Arch. Environ. Contam. Toxicol. 2003, 44, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Sagratini, G.; Caprioli, G.; Maggi, F.; Font, G.; Giardinà, D.; Mañes, J.; Meca, G.; Ricciutelli, M.; Sirocchi, V.; Torregiani, E.; Vittori, S. Determination of soya saponins I and βg in raw and cooked legumes by solid phase extraction (SPE) coupled to liquid chromatography (LC)-mass spectrometry (MS) and assessment of their bioaccessibility by an in vitro digestion model. J. Agric. Food Chem. 2013, 61, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zheng, Y.L.; Hyde, W.; Hendrich, S.; Murphy, P.A. Human fecal metabolism of soya saponin I. J. Agric. Food Chem. 2004, 52, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.A.; Schroeter, K.; Fuentes, S.; Heikamp-Dejong, I.; Khursigara, C.M.; de Vos, W.M.; Allen-Vercoe, E. Evaluation of microbial community reproducibility, stability and composition in a human distal gut chemostat model. J. Microbiol. Methods 2013, 95, 167–174. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Pre-Digestion | Oral Fluid | Gastric Fluid | Small Intestine Fluid | Colon Fermentation Fluid | |
|---|---|---|---|---|---|
| Betanin content (mg∙mL−1) | 23.05 ± 0.61 a | 21.44 ± 2.03 a | 14.84 ± 0.11 b | 12.42 ± 0.01 c | 0.0 |
| Loss (mg∙mL−1) and loss percentage after pre-digestion | - | 1.6 (≈7%) | 8.2 (≈35%) | 10.6 (≈46%) | - |
| TAP (%) | FRAP µmoL (Fe2+∙L−1) | TEAC µmoL (Trolox∙L−1) | ORAC µmoL (Trolox∙L−1) | ||
|---|---|---|---|---|---|
| Pre-digestion | Betanin | 75.42 ± 5.91 b | 518.31 ± 3.31 c | 3932.02 ± 94.42 a | 1992.44 ± 214.31 ab |
| Post-digestion | Oral fluid | 80.71 ± 0.92 b | 585.82 ± 13.23 b | 4964.03 ± 5.31 a | 2217.53 ± 10.31 a |
| Gastric fluid | 55.11 ± 9.23 c | 400.02 ± 12.43 d | 1382.94 ± 4.91 b | 1475.41 ± 18.73 c | |
| Small intestine fluid | 96.63 ± 0.61 a | 1053.81 ± 164.64 a | 4312.71 ± 651.81 a | 2199.71 ± 19.75 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira Teixeira da Silva, D.; dos Santos Baião, D.; de Oliveira Silva, F.; Alves, G.; Perrone, D.; Mere Del Aguila, E.; M. Flosi Paschoalin, V. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. https://doi.org/10.3390/molecules24030458
Vieira Teixeira da Silva D, dos Santos Baião D, de Oliveira Silva F, Alves G, Perrone D, Mere Del Aguila E, M. Flosi Paschoalin V. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules. 2019; 24(3):458. https://doi.org/10.3390/molecules24030458
Chicago/Turabian StyleVieira Teixeira da Silva, Davi, Diego dos Santos Baião, Fabrício de Oliveira Silva, Genilton Alves, Daniel Perrone, Eduardo Mere Del Aguila, and Vania M. Flosi Paschoalin. 2019. "Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments" Molecules 24, no. 3: 458. https://doi.org/10.3390/molecules24030458
APA StyleVieira Teixeira da Silva, D., dos Santos Baião, D., de Oliveira Silva, F., Alves, G., Perrone, D., Mere Del Aguila, E., & M. Flosi Paschoalin, V. (2019). Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules, 24(3), 458. https://doi.org/10.3390/molecules24030458