Liposomal Curcumin is Better than Curcumin to Alleviate Complications in Experimental Diabetic Mellitus
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Beta Cells and Hepatic Cells Protective Effect of Liposomal Curcumin
3.2. Effects of Liposomal Curcumin on Oxidative Stress Parameters
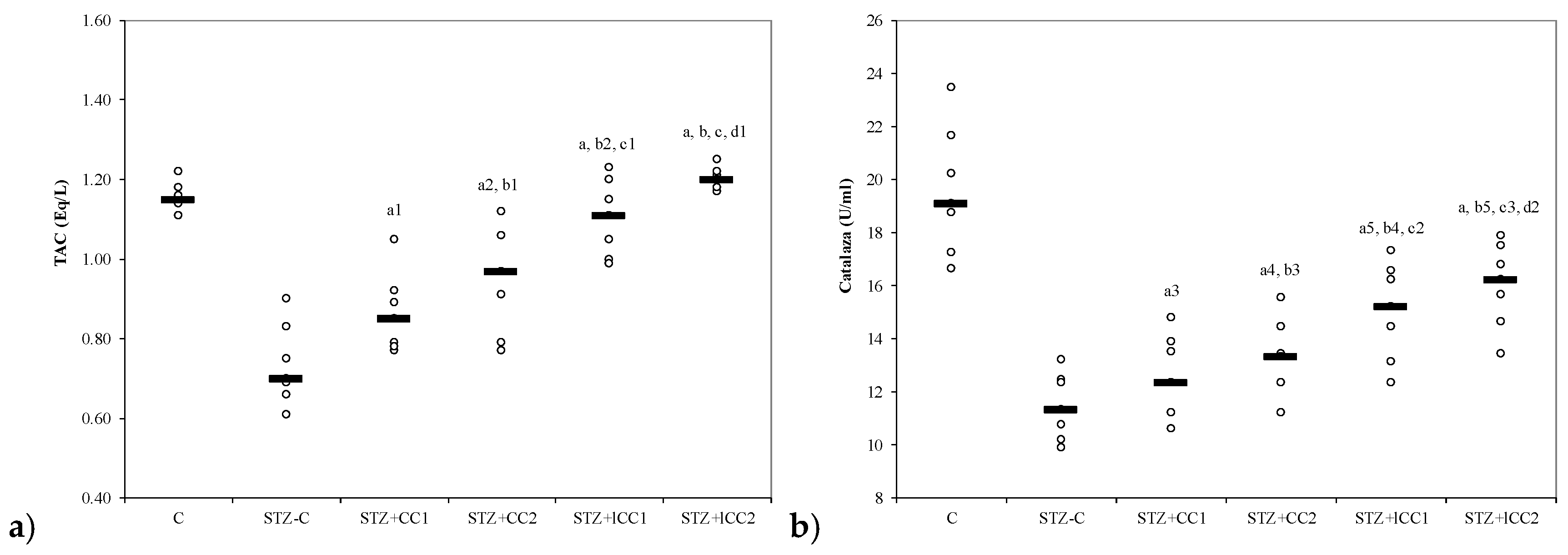
3.3. Effects of Liposomal Curcumin on Antioxidative Status Parameters
3.4. Effect of Liposomal Curcumin on Plasma Matrix Metalloproteinases
3.5. Study Limitations
4. Materials and Methods
4.1. Experimental Design
4.2. Glycemia, Hepatic Enzyme Activities, Oxidative Stress Parameters, and Metalloproteinases Measurements
4.3. Analysis of Data
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American diabetes association diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [CrossRef] [PubMed]
- King, A.J.F. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Barbiers, A.R. Streptozotocin, a new antibiotic. In vitro and in vivo evaluation. Antibiot. Annu. 1959–1960, 7, 247–254. [Google Scholar]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Sandler, S.; Swenne, I. Streptozotocin, but not alloxan, induces DNA repair synthesis in mouse pancreatic islets in vitro. Diabetologia 1983, 25, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Uchigata, Y.; Okamoto, H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature 1981, 294, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Friederich, M.; Hansell, P.; Palm, F. Diabetes, oxidative stress, nitric oxide and mitochondria function. Curr. Diabetes Rev. 2009, 5, 120–144. [Google Scholar] [CrossRef] [PubMed]
- Lukic, M.L.; Stosic-Grujicic, S.; Shahin, A. Effector mechanisms in low-dose streptozotocin-induced diabetes. Dev. Immunol. 1998, 6, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Wu, D.; Elliott, R.B. Low dose streptozotocin causes diabetes in severe combined immunodeficient (SCID) mice without immune cell infiltration of the pancreatic islets. Autoimmunity 1995, 20, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Pickering, R.J.; Rosado, C.J.; Sharma, A.; Buksh, S.; Tate, M.; de Haan, J.B. Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin. Transl. Immunol. 2018, 7, e1016. [Google Scholar] [CrossRef]
- Peeters, S.A.; Engelen, L.; Buijs, J.; Chaturvedi, N.; Fuller, J.H.; Schalkwijk, C.G.; Stehouwer, C.D.; EURODIAB Prospective Complications Study Group. Plasma levels of matrix metalloproteinase-2, -3, -10, and tissue inhibitor of metalloproteinase-1 are associated with vascular complications in patients with type 1 diabetes: The EURODIAB prospective complications study. Cardiovasc. Diabetol. 2015, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000, 14, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, A.D.; Chow, A.K.; Ali, M.A.; Schulz, R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: Beyond the matrix. Cardiovasc. Res. 2010, 85, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sawicki, G.; Schulz, R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase. Cardiovasc. Res. 2002, 53, 165–174. [Google Scholar] [CrossRef]
- Sawicki, G.; Leon, H.; Sawicka, J.; Sariahmetoglu, M.; Schulze, C.J.; Scott, P.G.; Szczesna-Cordary, D.; Schulz, R. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: A new intracellular target for matrix metalloproteinase. Circulation 2005, 112, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.P.; Madjidi, A.; Wilson, M.E.; Nothhelfer, D.A.; Johnson, J.H.; Palma, J.F.; Schweitzer, A.; Burant, C.; Blume, J.E.; Johnson, J.D. Matrix metalloproteinases contribute to insulin insufficiency in Zucker diabetic fatty rats. Diabetes 2005, 54, 2612–2619. [Google Scholar] [CrossRef]
- Liu, C.; Wan, X.; Ye, T.; Fang, F.; Chen, X.; Chen, Y.; Dong, Y. Matrix Metalloproteinase 2 Contributes to Pancreatic Beta Cell Injury Induced by Oxidative Stress. PLoS ONE 2014, 9, e110227. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Odenbach, S. Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes 2004, 53, 3233–3238. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mohammad, G.; Santos, J.M.; Zhong, Q. Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes 2011, 60, 3023–3033. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Madiseh, M.; Malekpour-Tehrani, A.; Bahmani, M.; Rafieian-Kopaei, M. The research and development on the antioxidants in prevention of diabetic complications. Asian Pac. J. Trop. Med. 2016, 9, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, J.O.; Andersson, A.; Eizirik, D.L.; Sandler, S. Interleukin-1 receptor antagonist prevents low dose streptozotocin induced diabetes in mice. Biochem. Biophys. Res. Commun. 1994, 202, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Eren, Z.; Günal, M.Y.; Bakir, E.A.; Coban, J.; Çağlayan, B.; Ekimci, N.; Ethemoglu, S.; Albayrak, O.; Akdeniz, T.; Demirel, G.Y.; et al. Effects of paricalcitol and aliskiren combination therapy on experimental diabetic nephropathy model in rats. Kidney Blood Press Res. 2014, 39, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Flodstrom, M.; Tyrberg, B.; Eizirik, D.L.; Sandler, S. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to multiple low-dose streptozotocin-induced diabetes. Diabetes. 1999, 48, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Bulboacă, A.; Bolboacă, S.D.; Suciu, S. Protective effect of curcumin in fructose-induced metabolic, syndrome and in streptozotocin-induced diabetes in rats. Iran J. Basic Med. Sci. 2016, 19, 585–593. [Google Scholar] [PubMed]
- Miao, M.; Guo, L.; Tian, S.; Wang, T. Effects of curcumin on antioxidation in diabetic rats. Pak. J. Pharm. Sci. 2015, 28, 371–373. [Google Scholar] [PubMed]
- Naijil, G.; Anju, T.R.; Jayanarayanan, S.; Paulose, C.S. Curcumin pretreatment mediates antidiabetogenesis via functional regulation of adrenergic receptor subtypes in the pancreas of multiple low-dose streptozotocin-induced diabetic rats. Nutr. Res. 2015, 35, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Arcaro, C.A.; Gutierres, V.O.; Assis, R.P.; Moreira, T.F.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Piperine, a natural bioenhancer, nullifies the antidiabetic and antioxidant activities of curcumin in streptozotocin-diabetic rats. PLoS ONE 2014, 9, e113993. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Rao, J.; Zou, L.; Zhao, S.; Yi, Z.; Wu, B.; Li, L.; Yuan, H.; Shi, L.; Zhang, C.; et al. Nanoparticle-encapsulated curcumin inhibits diabetic neuropathic pain involving the p2y12 receptor in the dorsal root ganglia. Front. Neurosci. 2018, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Ghayour Mobarhan, M.; Kazemi Oskuee, R. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. Phytomed. 2016, 6, 567–577. [Google Scholar] [PubMed]
- Pan, Y.; Wang, Y.; Cai, L.; Cai, Y.; Hu, J.; Yu, C.; Li, J.; Feng, Z.; Yang, S.; Li, X.; et al. Inhibition of high glucose-induced inflammatory response and macrophage infiltration by a novel curcumin derivative prevents renal injury in diabetic rats. Br. J. Pharmacol. 2012, 166, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Ganugula, R.; Arora, M.; Jaisamut, P.; Wiwattanapatapee, R.; Jørgensen, H.G.; Venkatpurwar, V.P.; Zhou, B.; Rodrigues Hoffmann, A.; Basu, R.; Guo, S.; et al. Nano-curcumin safely prevents streptozotocin-induced inflammation and apoptosis in pancreatic beta cells for effective management of Type 1 diabetes mellitus. Br. J. Pharmacol. 2017, 174, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Morrow, J.D.; Ning, M.; Koroshetz, W.; Lo, E.H.; Terry, E.; Milne, G.L.; Hubbard, J.; Lee, H.; Stevenson, E.; et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: The biomarker evaluation for antioxidant therapies in stroke (BEAT-Stroke) study. Stroke. 2008, 39, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Schulz, R. Activation of MMP-2 as a key event in oxidative stress injury to the heart. Front. Biosci. 2009, 14, 699–716. [Google Scholar]
- Sunil, V.R.; Patel-Vayas, K.; Shen, J.; Laskin, J.D.; Laskin, D.L. Classical and alternative macrophage activation in the lung following ozone-induced oxidative stress. Toxicol. Appl. Pharmacol. 2012, 263, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gallán, P.; Carrascosa, A.; Gussinyé, M.; Domínguez, C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic. Biol. Med. 2003, 34, 1563–1574. [Google Scholar] [CrossRef]
- King, G.L.; Loeken, M.R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell Biol. 2004, 122, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Haluzík, M.; Nedvídková, J. The role of nitric oxide in the development of streptozotocin-induced diabetes mellitus: Experimental and clinical implications. Physiol. Res. 2000, 49, S37–42. [Google Scholar] [PubMed]
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators inspired by nature: A review on curcumin and echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Aktas, C.; Erboga, M. Curcumin attenuates testicular damage, apoptotic germ cell death, and oxidative stress in streptozotocin-induced diabetic rats. Mol. Nutr. Food Res. 2013, 57, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.N.; Barcala Tabarrozzi, A.E.; Winnewisser, J.; Gimeno, M.L.; Antunica Noguerol, M.; Liberman, A.C.; Paz, D.A.; Dewey, R.A.; Perone, M.J. Curcumin ameliorates autoimmune diabetes. Evidence in accelerated murine models of type 1 diabetes. Clin. Exp. Immunol. 2014, 177, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Videla, L.A.; Rodrigo, R.; Orellana, M.; Fernandez, V.; Tapia, G.; Quinones, L.; Varela, N.; Contreras, J.; Lazarte, R.; Csendesm, A. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin. Sci. 2004, 106, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y. Learning toxicology from carbon tetrachloride-induced hepatotoxicity. Yakugaku Zasshi 2006, 126, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zheng, S.; Lin, J.; Ryerse, J.; Chen, A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol. Pharmacol. 2008, 73, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Dogaru, G.; Motricală, M.; Ákos, M.; Rus, V. Effects of mineral water from spring 3 in Băile Tuşnad on experimentally induced alcoholic liver disease. Balneo Res. J. 2017, 8, 125–128. [Google Scholar] [CrossRef]
- Dogaru, G.; Motricală, M.; Ákos, M.; Rus, V. An experimental study regarding the biological effects of mineral water from spring 3 in Băile Tuşnad on some organs after ethyl alcohol administration. Balneo Res. J. 2016, 7, 23–28. [Google Scholar] [CrossRef]
- Schiaffonati, L.; Tiberio, L. Gene expression in liver after toxic injury: Analysis of heat shock response and oxidative stress-inducible genes. Liver 1997, 17, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.S.; Kim, S.P.; Kang, M.Y.; Nam, S.H. Inhibitory effect of curcumin on liver injury in a murine model of endotoxemic shock. Biotechnol. Lett. 2010, 32, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Tell, G.; Vascotto, C.; Tiribelli, C. Alterations in the redox state and liver damage: Hints from the easl basic school of hepatology. J. Hepatol. 2013, 58, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, W.; Yamauchi, S.; Watanabe, K.; Takasaki, W.; Mori, K. Metabolomic analysis of arginine metabolism in acute hepatic injury in rats. J. Toxicol Sci. 2014, 39, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, X.; Tan, Y.; Li, B.; Miao, X.; Jin, L.; Shi, X.; Zhang, X.; Miao, L.; Li, X.; et al. Diabetes-induced hepatic pathogenic damage, inflammation, oxidative stress, and insulin resistance was exacerbated in zinc deficient mouse model. PLoS ONE 2012, 7, e49257. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin–phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007, 330, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Galaly, S.R.; Ahmed, O.M.; Mahmoud, A.M. Thymoquinone and curcumin prevent gentamicin-induced liver injury B,Y attenuating oxidative stress, inflammation and apoptosis. J. Physiol. Pharmacol. 2014, 65, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Muriel, P. NF-κB in liver diseases: A target for drug therapy. J. Appl. Toxicol. 2009, 29, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kanitkar, M.; Gokhale, K.; Galande, S.; Bhonde, R.R. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo. Br. J. Pharmacol. 2008, 155, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Samuhasaneeto, S.; Thong-Ngam, D.; Kulaputana, O.; Suyasunanont, D.; Klaikeaw, N. Curcumin decreased oxidative stress, inhibited NF-kappaB activation, and improved liver pathology in ethanol-induced liver injury in rats. J. Biomed. Biotechnol. 2009, 2009, 981963. [Google Scholar] [CrossRef]
- Soliman, M.M.; Abdo Nassan, M.; Ismail, T.A. Immunohistochemical and molecular study on the protective effect of curcumin against hepatic toxicity induced by paracetamol in Wistar rats. BMC Complement. Altern. Med. 2014, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Karlowicz-Bodalska, K.; Han, S.; Freier, J.; Smolenski, M.; Bodalska, A. Curcuma longa as medicinal herb in the treatment of diabet- ic complications. Acta Pol. Pharm. 2017, 74, 605–610. [Google Scholar] [PubMed]
- Jain, S.K.; Rains, J.; Croad, J.; Larson, B.; Jones, K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid. Redox Signal. 2009, 11, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Satin, K.; Petpiboolthai, H.; Anupunpisit, V. Effect of Curcumin on Characterization and Localization of Interleukin-13 and Tumor Necrosis Factor-alpha in Liver of Diabetic Rats. J. Med. Assoc. Thail. 2016, 99, S187–S195. [Google Scholar]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Kyung, E.J.; Kim, H.B.; Hwang, E.S.; Lee, S.; Choi, B.K.; Kim, J.W.; Kim, H.J.; Lim, S.M.; Kwon, O.I.; Woo, E.J. Evaluation of hepatoprotective effect of curcumin on liver cirrhosis using a combination of biochemical analysis and magnetic resonance-based electrical conductivity imaging. Mediat. Inflamm. 2018, 2018, 5491797. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Lundberg, J.O. Cardiovascular prevention by dietary nitrate and nitrite. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1221–H1223. [Google Scholar] [CrossRef] [PubMed]
- Waltz, P.; Escobar, D.; Botero, A.M.; Zuckerbraun, B.S. Nitrate/nitrite as critical mediators to limit oxidative injury and inflammation. Antioxid. Redox Signal. 2015, 23, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Mohan, I.K.; Das, U.N. Oxidant stress, anti-oxidants and nitric oxide in non-insulin dependent diabetes mellitus. Med. Sci. Res. 1997, 25, 55–57. [Google Scholar] [CrossRef]
- Hung, L.M.; Huang, J.P.; Liao, J.M.; Yang, M.H.; Li, D.E.; Day, Y.J.; Huang, S.S. Insulin renders diabetic rats resistant to acute ischemic stroke by arresting nitric oxide reaction with superoxide to form peroxynitrite. J. Biomed. Sci. 2014, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; Prabu, S.K.; John, A.; Avadhani, N.G. Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2011, 12, 3133–3147. [Google Scholar] [CrossRef] [PubMed]
- Schild, L.; Reinheckel, T.; Reiser, M.; Horn, T.F.; Wolf, G.; Augustin, W. Nitric oxide produced in rat liver mitochondria causes oxidative stress and impairment of respiration after transient hypoxia. FASEB J. 2003, 17, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S.; Oh, J.Y.; Landar, A.L.; Ulasova, E.; Venkatraman, A.; Bailey, S.M.; Darley-Usmar, V.M. Nitroxia: The pathological consequence of dysfunction in the nitric oxide-cytochrome c oxidase signaling pathway. Free Radic. Biol. Med. 2005, 38, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Z.S.; Alkafafy, M.E.; Ahmed, M.M.; Soliman, M.M. Renoprotective effect of curcumin against the combined oxidative stress of diabetes and nicotine in rats. Mol. Med. Rep. 2016, 13, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.J.; Bosch-Morell, F.; Romero, M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Karimi Galougahi, K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, S.; Jasnos, K.; Magierowski, M.; Sliwowski, Z.; Pajdo, R.; Brzozowski, B.; Mach, T.; Wojcik, D.; Brzozowski, T. Lipid peroxidation, reactive oxygen species and antioxidative factors in the pathogenesis of gastric mucosal lesions and mechanism of protection against oxidative stress—Induced gastric injury. J. Physiol. Pharmacol. 2014, 65, 613–622. [Google Scholar] [PubMed]
- Etsuo, N. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Omodanisi, E.I.; Aboua, Y.G.; Chegou, N.N.; Oguntibeju, O.O. Hepatoprotective, antihyperlipidemic, and anti-inflammatory activity of Moringa oleifera in diabetic-induced damage in male wistar rats. Pharmacogn. Res. 2017, 9, 182–187. [Google Scholar] [CrossRef]
- Omodanisi, E.I.; Aboua, Y.G.; Oguntibeju, O.O. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of moringa oleifera in diabetes-induced nephrotoxic male wistar rats. Molecules 2017, 22, 439. [Google Scholar] [CrossRef]
- Patumraj, S.; Wongeakin, N.; Sridulyakul, P.; Jariyapongskul, A.; Futrakul, N.; Bunnag, S. Combined effects of curcumin and vitamin C to protect endothelial dysfunction in the iris tissue of STZ-induced diabetic rats. Clin. Hemorheol. Microcirc. 2006, 35, 481–489. [Google Scholar] [PubMed]
- Abdel Aziz, M.T.; El-Asmar, M.F.; El-Ibrashy, I.N.; Rezq, A.M.; Al-Malki, A.L.; Wassef, M.A.; Fouad, H.H.; Ahmed, H.H.; Taha, F.M.; Hassouna, A.A.; et al. Effect of novel water soluble curcumin derivative on experimental type-1 diabetes mellitus (short term study). Diabetol. Metab. Syndr. 2012, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Joo, S.H. Downregulation of reactive oxygen species in apoptosis. J. Cancer Prev. 2016, 21, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Pigeolet, E.; Corbisier, P.; Houbion, A.; Lambert, D.; Michiels, C.; Raes, M.; Zachary, M.D.; Remacle, J. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech. Ageing Dev. 1990, 51, 283–297. [Google Scholar] [CrossRef]
- Saravanan, G.; Ponmurugan, P. Ameliorative potential of S-allyl cysteine on oxidative stress in STZ induced diabetic rats. Chem. Biol. Interact. 2011, 189, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wu, B.; Shen, G.; Li, X.; Wu, Q. Curcumin alleviates liver oxidative stress in type 1 diabetic rats. Mol. Med. Rep. 2018, 17, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Assis, R.P.; Arcaro, C.A.; Gutierres, V.O.; Oliveira, J.O.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Combined effects of curcumin and lycopene or bixin in yoghurt on inhibition of ldl oxidation and increases in hdl and paraoxonase levels in streptozotocin-diabetic rats. Int. J. Mol. Sci. 2017, 18, 332. [Google Scholar] [CrossRef]
- Kaur, G.; Invally, M.; Chintamaneni, M. Influence of piperine and quercetin on antidiabetic potential of curcumin. J. Complement. Integr. Med. 2016, 13, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Srivastava, S.K.; Chaudhuri, T.K.; Upadhyay, G. Multifaceted role of matrix metalloproteinases (MMPs). Front. Mol. Biosci. 2015, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jörns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54, S97–S107. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Daskalopoulou, S.S.; Perrea, D.; Liapis, C.D. Matrix metalloproteinases and diabetic vascular complications. Angiology 2005, 56, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Mandrup-Poulsen, T. A choice of death-the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 2001, 44, 2115–2133. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, S.M.; Henschel, A.M.; Hessner, M.J. Innate inflammation in type 1 diabetes. Transl. Res. 2016, 167, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Schönbein, G.W. The damaging potential of leukocyte activation in the microcirculation. Angiology 1993, 44, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gupta, S.; Pandey, R.M.; Sahni, P.; Chauhan, S.S.; Saraya, A. Prognostic significance of plasma matrix metalloprotease-2 in pancreatic cancer patients. Indian J. Med. Res. 2017, 146, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’angelo, A.; Tinelli, C.; Devangelio, E.; Consoli, A.; Miccoli, R.; Cicero, A.F.G. Evaluation of metalloproteinase 2 and 9 levels and their inhibitors in diabetic and healthy subjects. Diabetes Metab. 2007, 33, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, I.; Nakamura, T.; Shimada, N.; Koide, H. Increased plasma metalloproteinase-9 concentrations precede development of microalbuminuria in non-insulin-dependent diabetes mellitus. Am. J. Kidney Dis. 1998, 32, 544–550. [Google Scholar] [CrossRef]
- Kłysik, A.B.; Naduk-Kik, J.; Hrabec, Z.; Goś, R.; Hrabec, E. Intraocular matrix metalloproteinase 2 and 9 in patients with diabetes mellitus with and without diabetic retinopathy. Arch. Med. Sci. 2010, 6, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, B.; Zhou, S.; Zhang, B.; Xu, Y. The protective effect of fasudil on the structure and function of cardiac mitochondria from rats with type 2 diabetes induced by streptozotocin with a high-fat diet is mediated by the attenuation of oxidative stress. Biomed. Res. Int. 2013, 2013, 430791. [Google Scholar] [CrossRef] [PubMed]
- Porfire, A.S.; Parvu, A.E.; Daicoviciu, D.; Leucuta, S.E. Evaluation of antiinflamatory activity of liposome encapsulated superoxide dismutase in rats peritonitis. Farmacia 2009, 57, 412–423. [Google Scholar]
- Tefas, L.R.; Sylvester, B.; Tomuta, I.; Sesarman, A.; Licarete, E.; Banciu, M.; Porfire, A. Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. Drug Des. Dev. Ther. 2017, 11, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Bulboacă, A.E.; Bolboacă, S.D.; Stănescu, I.C.; Sfrângeu, C.A.; Porfire, A.; Tefas, L.; Bulboacă, A.C. The effect of intravenous administration of liposomal curcumin in addition to sumatriptan treatment in an experimental migraine model in rats. Int. J. Nanomed. 2018, 13, 3093–3103. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; Ali, N.; Reza, B.; Ali, K. Effect of ascorbic acid supplementation on nitric oxide metabolites and systolic blood pressure in rats exposed to lead. Indian J. Pharmacol. 2010, 42, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Parvu, A.E.; Parvu, M.; Vlase, L.; Miclea, P.; Mot, A.C.; Silaghi-Dumitrescu, R. Anti-inflammatory effects of Allium schoenoprasum L. leaves. J. Physiol. Pharmacol. 2014, 65, 309–315. [Google Scholar] [PubMed]
- Yagi, K. Assay for blood plasma and serum peroxides. Methods Enzymol. 1984, 105, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.L.; Milic, N.M.; Winham, S.J.; Garovic, V.D. Beyond bar and line graphs: Time for a new data presentation paradigm. PLoS Biol. 2015, 13, e1002128. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The datasets generated during the current study are available from the corresponding authors on request. |



| Group Abbreviation (7 Rats per Group) | ||||||
|---|---|---|---|---|---|---|
| C | STZ-C | STZ + CC1 | STZ + CC2 | STZ + lCC1 | STZ + lCC2 | |
| Oxidative stress | ||||||
| MDA (nmol/mL) | 2.08 (0.31) | 20.74 (1.23) | 13.50 (1.47) | 11.00 (1.23) | 6.92 (0.27) | 4.52 (0.31) |
| NO (μmol/L) | 26.29 (3.15) | 61.29 (2.29) | 44.00 (2.94) | 42.14 (2.54) | 33.71 (2.93) | 28.86 (2.61) |
| TOS (μmol/L) | 17.43 (1.72) | 72.14 (4.02) | 48.86 (1.57) | 49.29 (3.04) | 42.00 (3.27) | 32.29 (2.29) |
| Antioxidants | ||||||
| TAC (mEq/L) | 1.16 (0.03) | 0.73 (0.10) | 0.86 (0.10) | 0.94 (0.13) | 1.10 (0.09) | 1.20 (0.03) |
| Catalase (U/mL) | 19.59 (2.40) | 11.46 (1.26) | 12.51 (1.58) | 13.24 (1.45) | 15.04 (1.84) | 16.03 (1.58) |
| Marker of pancreatic damage | ||||||
| Glycemia (mmol/L) | 3.42 (0.24) | 23.99 (0.47) | 22.21 (0.43) | 21.49 (0.32) | 20.36 (0.37) | 18.95 (0.74) |
| Markers of hepatic damage—hepatic enzymes | ||||||
| AST (U/L) | 33.14 (5.37) | 167.71 (8.36) | 129.43 (5.26) | 116.57 (4.04) | 83.14 (3.34) | 70.71 (2.63) |
| ALT (U/L) | 34.57 (3.36) | 216.43 (5.86) | 108.00 (7.14) | 95.29 (4.11) | 70.86 (4.78) | 54.57 (2.51) |
| Matrix metalloproteinases | ||||||
| MMP-2 (ng/mL) | 86.00 (8.47) | 215.71 (10.70) | 187.86 (7.76) | 174.86 (8.36) | 127.29 (5.09) | 105.29 (9.05) |
| MMP-9 (ng/mL) | 20.57 (1.62) | 34.86 (2.12) | 32.00 (1.41) | 30.43 (2.37) | 27.29 (2.43) | 25.00 (1.83) |
| Group (Abbreviation) | Administration Route, Dose [ref] |
|---|---|
| Control (C) | 1 mL i.p. saline solution, 0.9% |
| Streptozotocin control (STZ-C) | 1 mL i.p. STZ * [2,24,100] |
| STZ and curcumin (1 mg/100 g bw) solution pre-treatment (STZ + CC1) | 1 mL i.p. STZ * 1 mL i.p. Curcumin solution, 1 mg/100 g bw |
| STZ and curcumin (2 mg/100 g bw) solution pre-treatment (STZ + CC2) | 1 mL i.p. STZ * 1 mL i.p. Curcumin solution, 2 mg/100 g bw |
| STZ and liposomal-curcumin (1 mg/100 g bw) solution pre-treatment (STZ + lCC1) | 1 mL i.p. STZ * 1 mL i.p. Liposomal-curcumin, 1 mg/100 g bw |
| STZ and liposomal-curcumin (2 mg/100 g bw) solution pre-treatment (STZ + lCC2) | 1 mL i.p. STZ * 1 mL i.p. Liposomal-curcumin, 2 mg/100 g bw |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulboacă, A.E.; Porfire, A.S.; Tefas, L.R.; Boarescu, P.M.; Bolboacă, S.D.; Stănescu, I.C.; Bulboacă, A.C.; Dogaru, G. Liposomal Curcumin is Better than Curcumin to Alleviate Complications in Experimental Diabetic Mellitus. Molecules 2019, 24, 846. https://doi.org/10.3390/molecules24050846
Bulboacă AE, Porfire AS, Tefas LR, Boarescu PM, Bolboacă SD, Stănescu IC, Bulboacă AC, Dogaru G. Liposomal Curcumin is Better than Curcumin to Alleviate Complications in Experimental Diabetic Mellitus. Molecules. 2019; 24(5):846. https://doi.org/10.3390/molecules24050846
Chicago/Turabian StyleBulboacă, Adriana Elena, Alina S. Porfire, Lucia R. Tefas, Paul Mihai Boarescu, Sorana D. Bolboacă, Ioana C. Stănescu, Angelo Corneliu Bulboacă, and Gabriela Dogaru. 2019. "Liposomal Curcumin is Better than Curcumin to Alleviate Complications in Experimental Diabetic Mellitus" Molecules 24, no. 5: 846. https://doi.org/10.3390/molecules24050846
APA StyleBulboacă, A. E., Porfire, A. S., Tefas, L. R., Boarescu, P. M., Bolboacă, S. D., Stănescu, I. C., Bulboacă, A. C., & Dogaru, G. (2019). Liposomal Curcumin is Better than Curcumin to Alleviate Complications in Experimental Diabetic Mellitus. Molecules, 24(5), 846. https://doi.org/10.3390/molecules24050846









