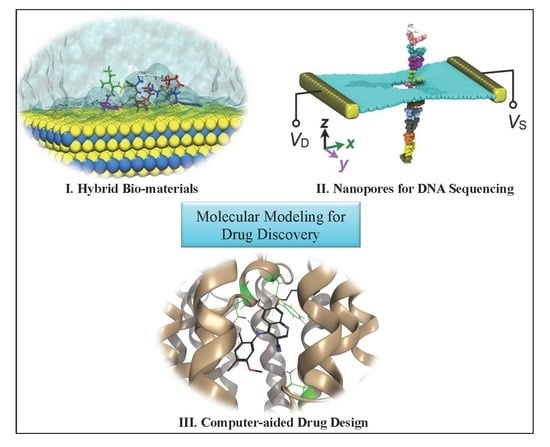
An Overview of Molecular Modeling for Drug Discovery with Specific Illustrative Examples of Applications
Abstract
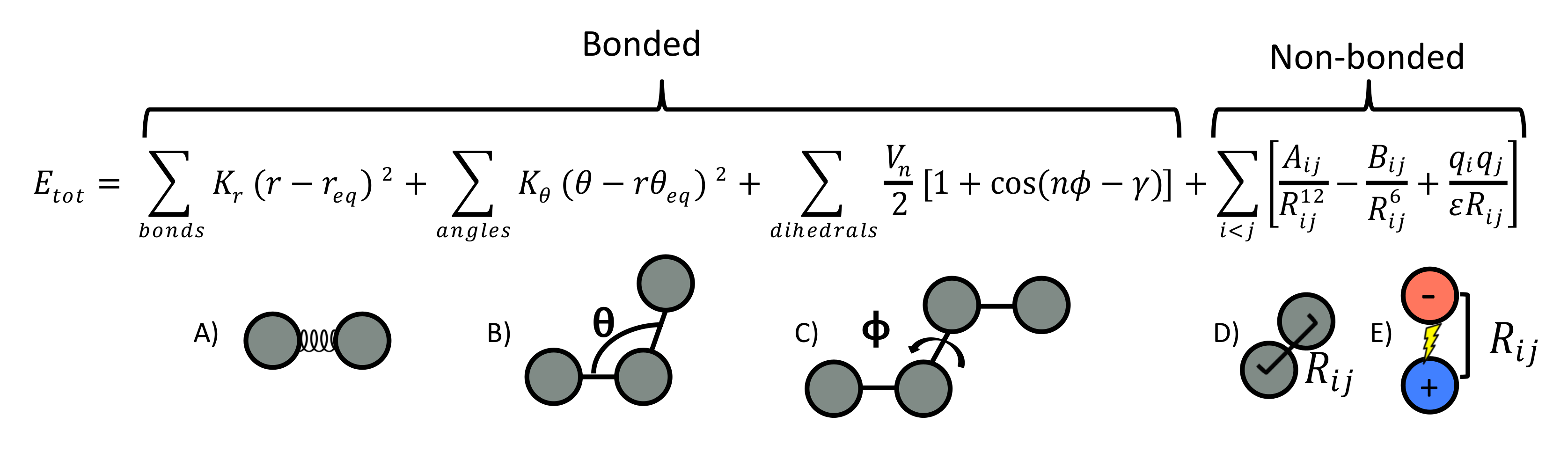
:1. Introduction to Molecular Modeling Methods
Length and Time Scale Limitations in Molecular Dynamics Simulations
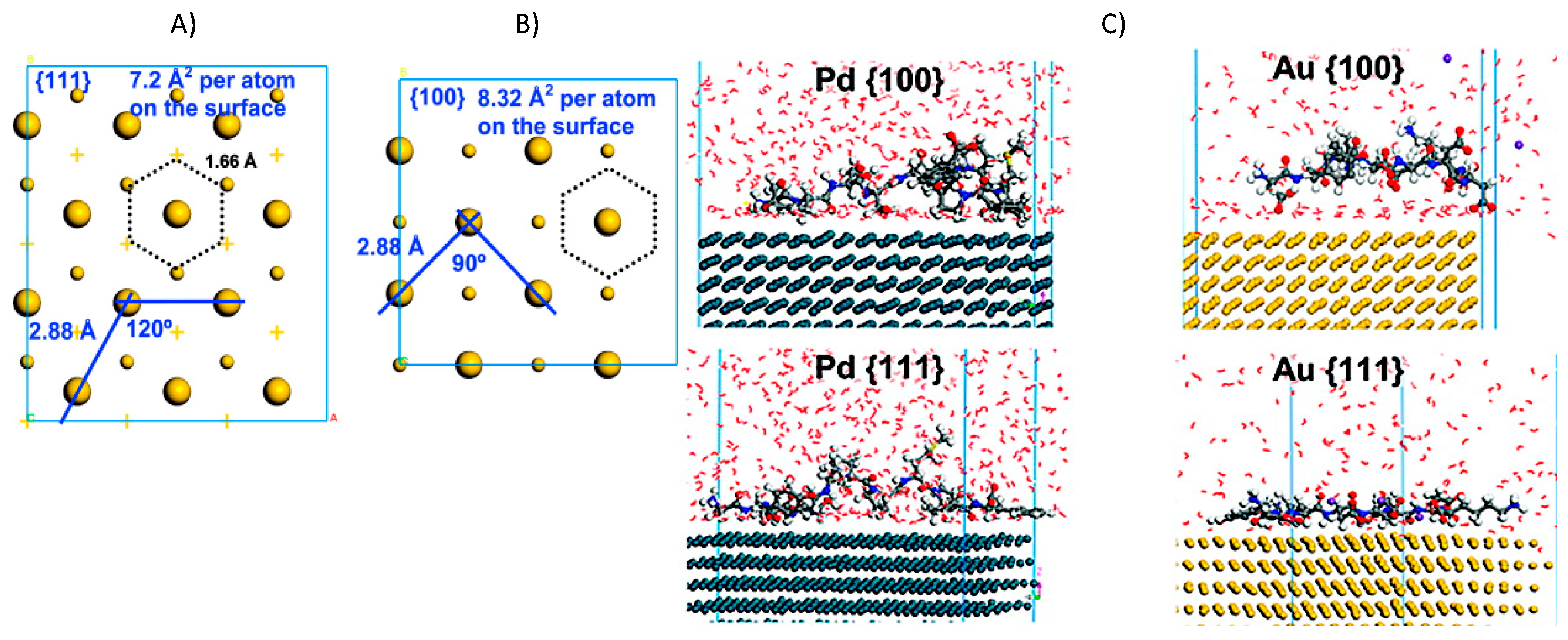
2. Organic–Inorganic Interface Simulations for Smart Novel Material Discoveries
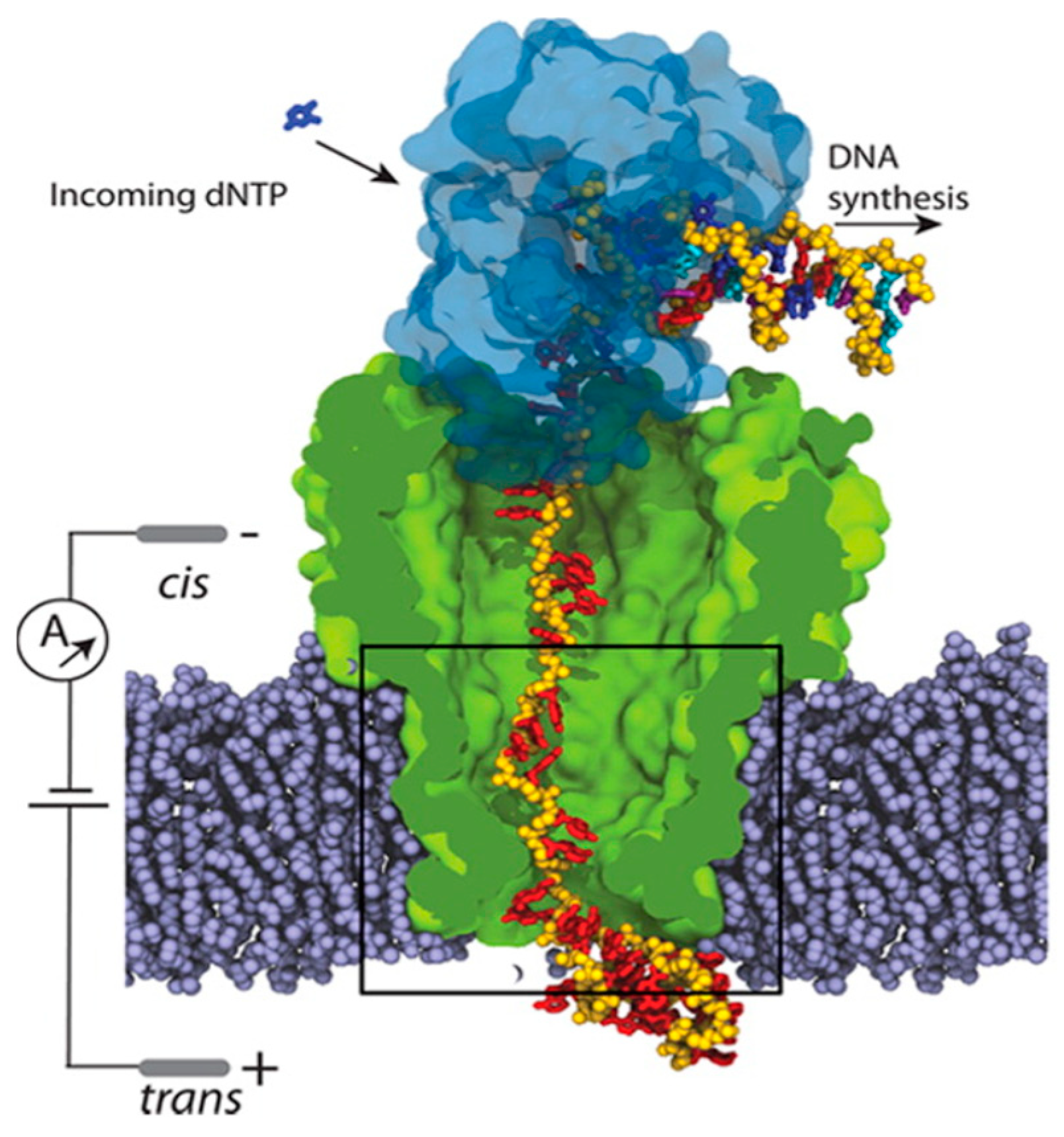
3. Modeling of Nanopores for DNA Sequencing Applications
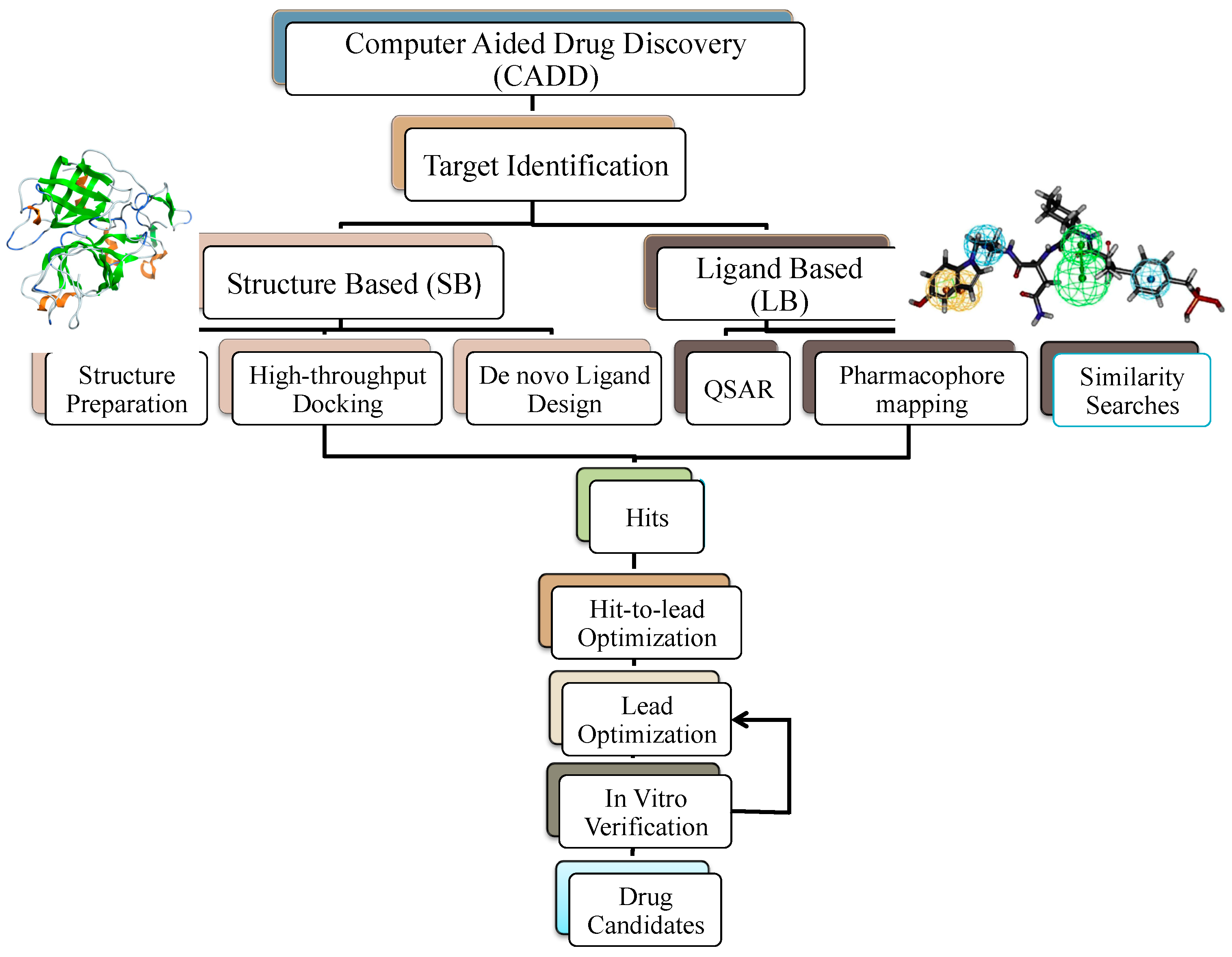
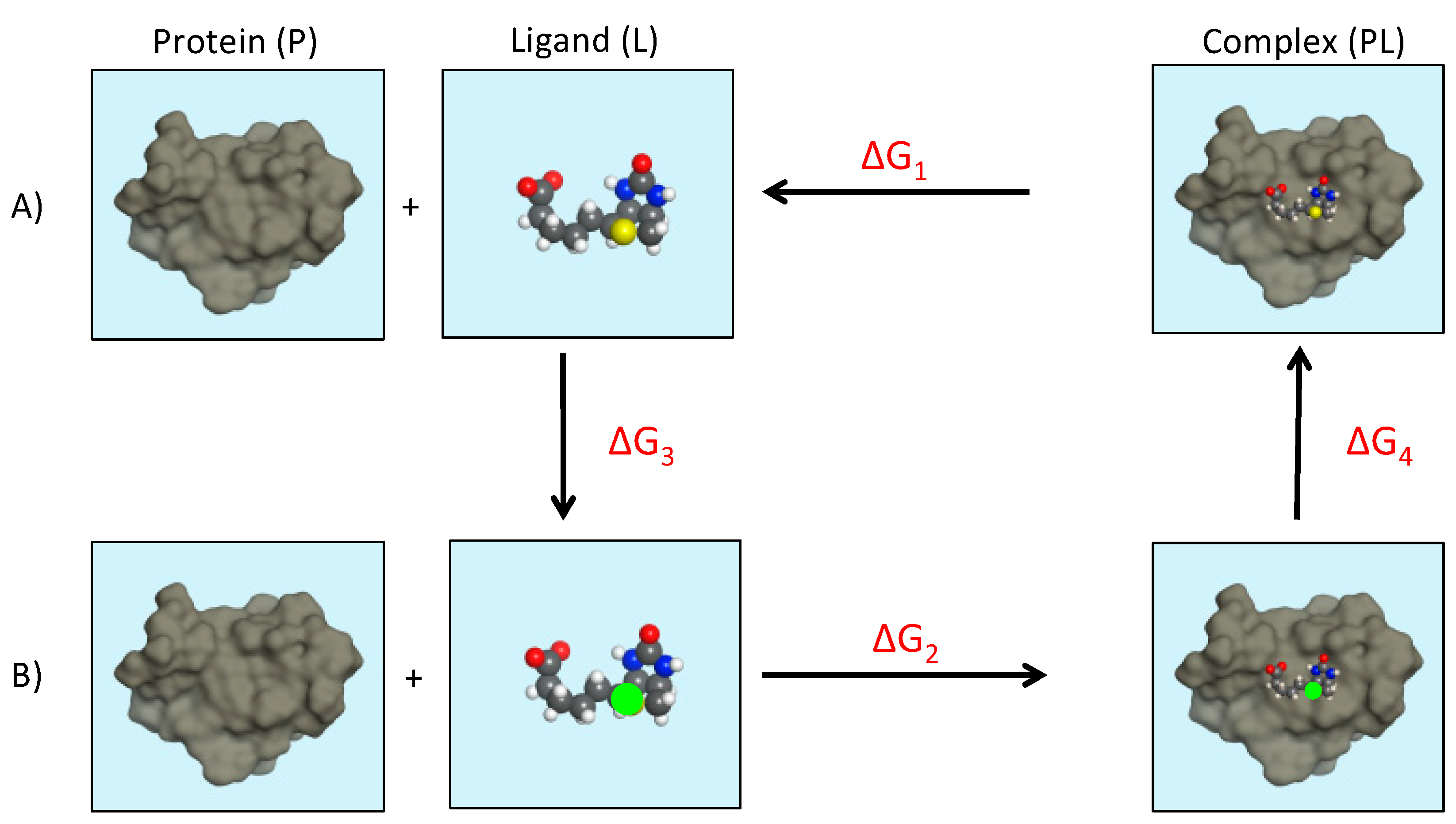
4. Computer-Aided Drug Design
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pollack, L. Klaus Schulten Reflects on his 2015 National Lecture. Available online: http://www.ks.uiuc.edu/events/NationalLecture2015/reflection/ (accessed on 21 March 2019).
- Feynman, R.P.; Leighton, R.B.; Sands, M. The Feynman Lectures in Physics; Addison-Wesley: Reading, MA, USA, 1963; Volume I. [Google Scholar]
- McCammon, J.A.; Gelin, B.R.; Karplus, M. Perspective on “Dynamics of folded proteins”. Nature 1977, 267, 585–590. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Dreele, R.B.; Stephens, P.W.; Smith, G.D.; Blessing, R.H. The first protein crystal structure determined from high-resolution X-ray powder diffraction data: A variant of T3R3 human insulin-zinc complex produced by grinding. Acta Cryst. D 2000, 56, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, U.F.; Guidoni, L.; Laio, A.; Frank, I.; Rothlisberger, U. A Molecular Spring for Vision. J. Am. Chem. Soc. 2004, 126, 15328–15329. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, R.; Kleinschmidt, V.; Schmitt, U.W.; Marx, D. Modeling protonated water networks in bacteriorhodopsin. Phys. Chem. Chem. Phys. 2004, 6, 1848–1859. [Google Scholar] [CrossRef]
- Molteni, C.; Frank, I.; Parrinello, M. An Excited State Density Functional Theory Study of the Rhodopsin Chromophore. J. Am. Chem. Soc. 1999, 121, 12177–12183. [Google Scholar] [CrossRef]
- Guidoni, L.; Carloni, P. Potassium permeation through the KcsA channel: A density functional study. Biochim. Biophys. Acta 2002, 1563, 1–6. [Google Scholar] [CrossRef]
- Silvestrelli, P.L.; Bernasconi, M.; Parrinello, M. Ab initio infrared spectrum of liquid water. Chem. Phys. Lett. 1997, 277, 478–482. [Google Scholar] [CrossRef]
- Zhu, Z.; Tuckerman, M.E. Ab Initio Molecular Dynamics Investigation of the Concentration Dependence of Charged Defect Transport in Basic Solutions via Calculation of the Infrared Spectrum. J. Phys. Chem. B 2002, 106, 8009–8018. [Google Scholar] [CrossRef]
- Putrino, A.; Sebastiani, D.; Parrinello, M. Generalized variational density functional perturbation theory. J. Chem. Phys. 2000, 113, 7102–7109. [Google Scholar] [CrossRef]
- Putrino, A.; Parrinello, M. Anharmonic Raman Spectra in High-Pressure Ice from Ab Initio Simulations. Phys. Rev. Lett. 2002, 88, 176401. [Google Scholar] [CrossRef]
- Piana, S.; Sebastiani, D.; Carloni, P.; Parrinello, M. Ab Initio Molecular Dynamics-Based Assignment of the Protonation State of Pepstatin A/HIV-1 Protease Cleavage Site. J. Am. Chem. Soc. 2001, 123, 8730–8737. [Google Scholar] [CrossRef]
- Raugei, S.a.C. Scientific Highlight of the Month: “ab Initio Modeling of Biological Systems” Ab Initio Modeling of Biological Systems. p. 10. Available online: https://psi-k.net/download/highlights/Highlight_71.pdf (accessed on 24 April 2019).
- Car, R.; Parrinello, M. Unified Approach for Molecular Dynamics and Density-Functional Theory. Phys. Rev. Lett. 1985, 55, 2471–2474. [Google Scholar] [CrossRef]
- Warshel, A. Energetics of enzyme catalysis. Proc. Natl. Acad. Sci. USA 1978, 75, 5250–5254. [Google Scholar] [CrossRef]
- Sousa, S.F.; Fernandes, P.A.; Ramos, M.J. Computational enzymatic catalysis clarifying enzymatic mechanisms with the help of computers. Phys. Chem. Chem. Phys. 2012, 14, 12431–12441. [Google Scholar] [CrossRef]
- Verlet, L. Computer Experiments on Classical Fluids.I. Thermodynamical Properties of Lennard-Jones Molecules. Phys. Rev. 1967, 159, 98. [Google Scholar] [CrossRef]
- Huang, J.; Lopes, P.E.M.; Roux, B.; MacKerell, A.D. Recent Advances in Polarizable Force Fields for Macromolecules: Microsecond Simulations of Proteins Using the Classical Drude Oscillator Model. J. Phys. Chem. Lett. 2014, 5, 3144–3150. [Google Scholar] [CrossRef]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M.; et al. The ReaxFF reactive force-field: Development, applications and future directions. Npj Comput. Mat. 2016, 2, 15011. [Google Scholar] [CrossRef]
- Leach, A.R. Molecular Modeling: Principles and Applications; Addison Wesley Longman: Essex, UK, 1996. [Google Scholar]
- Bronowska, A.K. Thermodynamics of Ligand-Protein Interactions: Implications for Molecular Design, Thermodynamics—Interaction Studies—Solids, Liquids and Gases; Moreno-Pirajan, J.C., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Villa, E.; Sengupta, J.; Trabuco, L.G.; LeBarron, J.; Baxter, W.T.; Shaikh, T.R.; Grassucci, R.A.; Nissen, P.; Ehrenberg, M.; Schulten, K.; et al. Ribosome-induced changes in elongation factor Tu conformation control GTP hydrolysis. Proc. Natl. Acad. Sci. USA 2009, 106, 1063–1068. [Google Scholar] [CrossRef]
- Sanbonmatsu, K.Y.; Tung, C.S. High performance computing in biology: Multimillion atom simulations of nanoscale systems. J. Struct. Biol. 2007, 157, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Freddolino, P.L.; Arkhipov, A.S.; Larson, S.B.; McPherson, A.; Schulten, K. Molecular dynamics simulations of the complete satellite tobacco mosaic virus. Structure 2006, 14, 437–449. [Google Scholar] [CrossRef]
- Zink, M.; Grubmuller, H. Mechanical Properties of the Icosahedral Shell of Southern Bean Mosaic Virus: A Molecular Dynamics Study. Biophys. J. 2009, 96, 1350–1363. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.P.; Perilla, J.R.; Yufenyuy, E.L.; Meng, X.; Chen, B.; Ning, J.Y.; Ahn, J.; Gronenborn, A.M.; Schulten, K.; Aiken, C.; et al. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature 2013, 497, 643–646. [Google Scholar] [CrossRef] [Green Version]
- Freddolino, P.L.; Liu, F.; Gruebele, M.; Schulten, K. Ten-Microsecond Molecular Dynamics Simulation of a Fast-Folding WW Domain. Biophys. J. 2008, 94, L75–L77. [Google Scholar] [CrossRef] [Green Version]
- Freddolino, P.L.; Schulten, K. Common Structural Transitions in Explicit-Solvent Simulations of Villin Headpiece Folding. Biophys. J. 2009, 97, 2338–2347. [Google Scholar] [CrossRef] [Green Version]
- Klepeis, J.L.; Lindorff-Larsen, K.; Dror, R.O.; Shaw, D.E. Long-timescale molecular dynamics simulations of protein structure and function. Curr. Opin. Struc. Biol. 2009, 19, 120–127. [Google Scholar] [CrossRef]
- Cartron, M.L.; Olsen, J.D.; Sener, M.; Jackson, P.J.; Brindley, A.A.; Qian, P.; Dickman, M.J.; Leggett, G.J.; Schulten, K.; Neil Hunter, C. Integration of energy and electron transfer processes in the photosynthetic membrane of Rhodobacter sphaeroides. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1837, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Shih, A.Y.; Arkhipov, A.; Freddolino, P.L.; Schulten, K. Coarse Grained Protein−Lipid Model with Application to Lipoprotein Particles. J. Phys. Chem. B 2006, 110, 3674–3684. [Google Scholar] [CrossRef]
- Arkhipov, A.; Freddolino, P.L.; Schulten, K. Stability and Dynamics of Virus Capsids Described by Coarse-Grained Modeling. Structure 2006, 14, 1767–1777. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.L.; Shinoda, W. Large-Scale Molecular Dynamics Simulations of Self-Assembling Systems. Science 2008, 321, 798. [Google Scholar] [CrossRef]
- Yin, Y.; Arkhipov, A.; Schulten, K. Simulations of Membrane Tubulation by Lattices of Amphiphysin N-BAR Domains. Structure 2009, 17, 882–892. [Google Scholar] [CrossRef]
- Levitt, M.; Warshel, A. Computer simulation of protein folding. Nature 1975, 253, 694–698. [Google Scholar] [CrossRef]
- Levitt, M. A simplified representation of protein conformations for rapid simulation of protein folding. J. Mol. Biol. 1976, 104, 59–107. [Google Scholar] [CrossRef] [Green Version]
- Torrie, G.M.; Valleau, J.P. Nonphysical sampling distributions in Monte Carlo free-energy estimation: Umbrella sampling. J. Comput. Phys. 1977, 23, 187–199. [Google Scholar] [CrossRef]
- Laio, A.; Parrinello, M. Escaping free-energy minima. Proc. Natl. Acad. Sci. USA 2002, 99, 12562–12566. [Google Scholar] [CrossRef] [Green Version]
- Isralewitz, B.; Gao, M.; Schulten, K. Steered molecular dynamics and mechanical functions of proteins. Curr. Opin. Struc. Biol. 2001, 11, 224–230. [Google Scholar] [CrossRef]
- Grubmüller, H.; Heymann, B.; Tavan, P. Ligand Binding: Molecular Mechanics Calculation of the Streptavidin-Biotin Rupture Force. Science 1996, 271, 997. [Google Scholar] [CrossRef]
- Isralewitz, B.; Baudry, J.; Gullingsrud, J.; Kosztin, D.; Schulten, K. Steered molecular dynamics investigations of protein function. J. Mol. Graph. Mod. 2001, 19, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Florin, E.L.; Moy, V.T.; Gaub, H.E. Adhesion forces between individual ligand-receptor pairs. Science 1994, 264, 415. [Google Scholar] [CrossRef]
- Svoboda, K.; Schmidt, C.F.; Schnapp, B.J.; Block, S.M. Direct observation of kinesin stepping by optical trapping interferometry. Nature 1993, 365, 721–727. [Google Scholar] [CrossRef]
- Smith, S.B.; Cui, Y.; Bustamante, C. Overstretching B-DNA: The Elastic Response of Individual Double-Stranded and Single-Stranded DNA Molecules. Science 1996, 271, 795. [Google Scholar] [CrossRef] [PubMed]
- Cluzel, P.; Lebrun, A.; Heller, C.; Lavery, R.; Viovy, J.-L.; Chatenay, D.; Caron, F. DNA: An Extensible Molecule. Science 1996, 271, 792. [Google Scholar] [CrossRef]
- Rief, M.; Gautel, M.; Oesterhelt, F.; Fernandez, J.M.; Gaub, H.E. Reversible Unfolding of Individual Titin Immunoglobulin Domains by AFM. Science 1997, 276, 1109. [Google Scholar] [CrossRef] [PubMed]
- Kellermayer, M.S.Z.; Smith, S.B.; Granzier, H.L.; Bustamante, C. Folding-Unfolding Transitions in Single Titin Molecules Characterized with Laser Tweezers. Science 1997, 276, 1112. [Google Scholar] [CrossRef]
- Kumar, S.; Rosenberg, J.M.; Bouzida, D.; Swendsen, R.H.; Kollman, P.A. THE weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J. Comput. Chem. 1992, 13, 1011–1021. [Google Scholar] [CrossRef]
- Ge, X.; Roux, B. Absolute Binding Free Energy Calculations of Sparsomycin Analogs to the Bacterial Ribosome. J. Phys. Chem. B 2010, 114, 9525–9539. [Google Scholar] [CrossRef] [PubMed]
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W. Computational Methods in Drug Discovery. Pharmacol. Rev. 2013, 66, 334. [Google Scholar] [CrossRef] [PubMed]
- Perilla, J.R.; Goh, B.C.; Cassidy, C.K.; Liu, B.; Bernardi, R.C.; Rudack, T.; Yu, H.; Wu, Z.; Schulten, K. Molecular dynamics simulations of large macromolecular complexes. Curr. Opin. Struc. Biol. 2015, 31, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Yu, I.; Mori, T.; Ando, T.; Harada, R.; Jung, J.; Sugita, Y.; Feig, M. Biomolecular interactions modulate macromolecular structure and dynamics in atomistic model of a bacterial cytoplasm. eLife 2016, 5, e19274. [Google Scholar] [CrossRef]
- Shaw, D.E.; Maragakis, P.; Lindorff-Larsen, K.; Piana, S.; Dror, R.O.; Eastwood, M.P.; Bank, J.A.; Jumper, J.M.; Salmon, J.K.; Shan, Y.; et al. Atomic-Level Characterization of the Structural Dynamics of Proteins. Science 2010, 330, 341–346. [Google Scholar] [CrossRef]
- Feig, M.; Nawrocki, G.; Yu, I.; Wang, P.-H.; Sugita, Y. Challenges and opportunities in connecting simulations with experiments via molecular dynamics of cellular environments. J. Phys. Conf. Series 2018, 1036, 012010. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Asahi, R. Molecular dynamics simulations with machine learning potential for Nb-doped lithium garnet-type oxide Li 7− x La 3 (Zr 2− x Nb x) O 12. Phy. Rev. Mat. 2018, 2, 105404. [Google Scholar]
- Hollingsworth, S.A.; Dror, R.O. Molecular Dynamics Simulation for All. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Caflisch, A. Molecular dynamics in drug design. Eur. J. Med. Chem. 2015, 91, 4–14. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, M.; Masetti, M.; Bottegoni, G.; Cavalli, A. Role of Molecular Dynamics and Related Methods in Drug Discovery. J. Med. Chem. 2016, 59, 4035–4061. [Google Scholar] [CrossRef] [PubMed]
- Heinz, H.; Ramezani-Dakhel, H. Simulations of inorganic–bioorganic interfaces to discover new materials: insights, comparisons to experiment, challenges, and opportunities. Chem. Soc. Rev. 2016, 45, 412–448. [Google Scholar] [CrossRef] [PubMed]
- Maffeo, C.; Yoo, J.; Comer, J.; Wells, D.B.; Luan, B.; Aksimentiev, A. Close encounters with DNA. J. Phys. 2014, 26, 413101. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Sharma, B.K.; Hwang, H.S.; Erhan, S.Z.; Perez, J.M. Development of Biobased Synthetic Fluids: Application of Molecular Modeling to Structure−Physical Property Relationship. Ind. Eng. Chem. Res. 2006, 45, 928–933. [Google Scholar] [CrossRef]
- Thompson, D.W. On Growth and Form; Cambridge University Press: Cambridge, UK, 1942. [Google Scholar]
- Maier, I.; Morgan, M.R.A.; Lindner, W.; Pittner, F. Optical Resonance-Enhanced Absorption-Based Near-Field Immunochip Biosensor for Allergen Detection. Anal. Chem. 2008, 80, 2694–2703. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cao, Q.; Svec, F.; Fréchet, J.M.J. Porous Polymer Monolithic Column with Surface-Bound Gold Nanoparticles for the Capture and Separation of Cysteine-Containing Peptides. Anal. Chem. 2010, 82, 3352–3358. [Google Scholar] [CrossRef]
- Hartmann, M. Ordered Mesoporous Materials for Bioadsorption and Biocatalysis. Chem. Mat. 2005, 17, 4577–4593. [Google Scholar] [CrossRef]
- Yguerabide, J.; Yguerabide, E.E. Resonance light scattering particles as ultrasensitive labels for detection of analytes in a wide range of applications. J. Cellular Biochem. 2001, 84, 71–81. [Google Scholar] [CrossRef]
- Wright, M.; Uddin, A. Organic—inorganic hybrid solar cells: A comparative review. Solar Energy Mat. Solar Cells 2012, 107, 87–111. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Kinnear, C.; Moore, T.L.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Petri-Fink, A. Form Follows Function: Nanoparticle Shape and Its Implications for Nanomedicine. Chem. Rev. 2017, 117, 11476–11521. [Google Scholar] [CrossRef]
- Penna, M.J.a.B.; Mark, J. The Binding Mechanism of an Experimentally Identified Platinum-binding Peptide by Molecular Dynamics Simulation. In Proceedings of the Chemeca 2010: Engineering at the Edge, Hilton Adelaide, Australia, 26–29 September 2010; Engineers Australia: Barton, Australia; pp. 289–298. [Google Scholar]
- Penna, M.J.; Mijajlovic, M.; Biggs, M.J. Molecular-Level Understanding of Protein Adsorption at the Interface between Water and a Strongly Interacting Uncharged Solid Surface. J. Am. Chem. Soc. 2014, 136, 5323–5331. [Google Scholar] [CrossRef]
- Brancolini, G.; Kokh, D.B.; Calzolai, L.; Wade, R.C.; Corni, S. Docking of Ubiquitin to Gold Nanoparticles. ACS Nano 2012, 6, 9863–9878. [Google Scholar] [CrossRef]
- Hildebrand, N.; Köppen, S.; Derr, L.; Li, K.; Koleini, M.; Rezwan, K.; Colombi Ciacchi, L. Adsorption Orientation and Binding Motifs of Lysozyme and Chymotrypsin on Amorphous Silica. J. Phys. Chem. C 2015, 119, 7295–7307. [Google Scholar] [CrossRef]
- Heinz, H.; Lin, T.-J.; Kishore Mishra, R.; Emami, F.S. Thermodynamically Consistent Force Fields for the Assembly of Inorganic, Organic, and Biological Nanostructures: The INTERFACE Force Field. Langmuir 2013, 29, 1754–1765. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Iori, F.; Corni, S. Including image charge effects in the molecular dynamics simulations of molecules on metal surfaces. J. Comput. Chem. 2008, 29, 1656–1666. [Google Scholar] [CrossRef]
- Wright, L.B.; Rodger, P.M.; Corni, S.; Walsh, T.R. GolP-CHARMM: First-Principles Based Force Fields for the Interaction of Proteins with Au(111) and Au(100). J. Chem. Theory Comp. 2013, 9, 1616–1630. [Google Scholar] [CrossRef]
- Monti, S.; Barcaro, G.; Sementa, L.; Carravetta, V.; Ågren, H. Dynamics and self-assembly of bio-functionalized gold nanoparticles in solution: Reactive molecular dynamics simulations. Nano Res. 2018, 11, 1757–1767. [Google Scholar] [CrossRef]
- Watabe, M.; Yamada, H.; Miyakawa, T.; Morikawa, R.; Takasu, M.; Uchida, T.; Yamagishi, A. Structural Analysis of Metal-Binding Peptides Using Molecular Dynamics. Conf. Proc. ICBBB 2018. [Google Scholar]
- Ma, W.; Saccardo, A.; Roccatano, D.; Aboagye-Mensah, D.; Alkaseem, M.; Jewkes, M.; Di Nezza, F.; Baron, M.; Soloviev, M.; Ferrari, E. Modular assembly of proteins on nanoparticles. Nature Commu. 2018, 9, 1489. [Google Scholar] [CrossRef]
- Heinz, H.; Farmer, B.L.; Pandey, R.B.; Slocik, J.M.; Patnaik, S.S.; Pachter, R.; Naik, R.R. Nature of Molecular Interactions of Peptides with Gold, Palladium, and Pd−Au Bimetal Surfaces in Aqueous Solution. J. Am. Chem. Soc. 2009, 131, 9704–9714. [Google Scholar] [CrossRef]
- Ruan, L.; Ramezani-Dakhel, H.; Chiu, C.-Y.; Zhu, E.; Li, Y.; Heinz, H.; Huang, Y. Tailoring Molecular Specificity Toward a Crystal Facet: a Lesson From Biorecognition Toward Pt{111}. Nano Lett. 2013, 13, 840–846. [Google Scholar] [CrossRef]
- Cetinel, S.; Shen, W.-Z.; Aminpour, M.; Bhomkar, P.; Wang, F.; Borujeny, E.R.; Sharma, K.; Nayebi, N.; Montemagno, C. Biomining of MoS(2) with Peptide-based Smart Biomaterials. Sci. Rep. 2018, 8, 3374. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Accounts Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Salzmann, C.G.; Ward, M.A.H.; Jacobs, R.M.J.; Tobias, G.; Green, M.L.H. Interaction of Tyrosine-, Tryptophan-, and Lysine-Containing Polypeptides with Single-Wall Carbon Nanotubes and Its Relevance for the Rational Design of Dispersing Agents. J. Phys. Chem. C 2007, 111, 18520–18524. [Google Scholar] [CrossRef]
- Kim, S.N.; Kuang, Z.; Slocik, J.M.; Jones, S.E.; Cui, Y.; Farmer, B.L.; McAlpine, M.C.; Naik, R.R. Preferential Binding of Peptides to Graphene Edges and Planes. J. Am. Chem. Soc. 2011, 133, 14480–14483. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Z.; Tian, X.; Xiu, P.; Zhou, R. Amino acid analogues bind to carbon nanotube via π-π interactions: Comparison of molecular mechanical and quantum mechanical calculations. J. Chem. Phys. 2012, 136, 025103. [Google Scholar] [CrossRef]
- Ramakrishnan, S.K.; Martin, M.; Cloitre, T.; Firlej, L.; Gergely, C. Molecular Mechanism of Selective Binding of Peptides to Silicon Surface. J. Chem. Informat. Modeling 2014, 54, 2117–2126. [Google Scholar] [CrossRef]
- Welch, C.M.; Camden, A.N.; Barr, S.A.; Leuty, G.M.; Kedziora, G.S.; Berry, R.J. Computation of the binding free energy of peptides to graphene in explicit water. J. Chem. Phys. 2015, 143, 045104. [Google Scholar] [CrossRef]
- Kim, S.S.; Kuang, Z.; Ngo, Y.H.; Farmer, B.L.; Naik, R.R. Biotic–Abiotic Interactions: Factors that Influence Peptide–Graphene Interactions. ACS Appl. Mat. Interfaces 2015, 7, 20447–20453. [Google Scholar] [CrossRef]
- Patwardhan, S.V.; Emami, F.S.; Berry, R.J.; Jones, S.E.; Naik, R.R.; Deschaume, O.; Heinz, H.; Perry, C.C. Chemistry of Aqueous Silica Nanoparticle Surfaces and the Mechanism of Selective Peptide Adsorption. J. Am. Chem. Soc. 2012, 134, 6244–6256. [Google Scholar] [CrossRef]
- Tosaka, R.; Yamamoto, H.; Ohdomari, I.; Watanabe, T. Adsorption Mechanism of Ribosomal Protein L2 onto a Silica Surface: A Molecular Dynamics Simulation Study. Langmuir 2010, 26, 9950–9955. [Google Scholar] [CrossRef]
- Rimola, A.; Sodupe, M.; Ugliengo, P. Affinity Scale for the Interaction of Amino Acids with Silica Surfaces. J. Phy. Chem. C 2009, 113, 5741–5750. [Google Scholar] [CrossRef]
- Nonella, M.; Seeger, S. Investigating Alanine–Silica Interaction by Means of First-Principles Molecular-Dynamics Simulations. ChemPhysChem 2008, 9, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Han, G.; Lindgren, M.; Shen, Z.; Laaksonen, A. Adhesion of lactoferrin and bone morphogenetic protein-2 to a rutile surface: dependence on the surface hydrophobicity. Biomat. Sci. 2014, 2, 1090–1099. [Google Scholar] [CrossRef]
- Monti, S.; Carravetta, V.; Battocchio, C.; Iucci, G.; Polzonetti, G. Peptide/TiO2 Surface Interaction: A Theoretical and Experimental Study on the Structure of Adsorbed ALA-GLU and ALA-LYS. Langmuir 2008, 24, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Carravetta, V.; Monti, S.; Zhang, W. Interaction of biomolecular systems with titanium-based materials: Computational investigations. Theor. Chem. Acc. 2009, 123, 299–309. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mat. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Ambrogio, M.W.; Thomas, C.R.; Zhao, Y.-L.; Zink, J.I.; Stoddart, J.F. Mechanized Silica Nanoparticles: A New Frontier in Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 903–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Cai, Y.; Sun, Z.; Liu, J.; Liu, C.; Wei, J.; Li, W.; Liu, C.; Wang, Y.; Zhao, D. Multifunctional Mesoporous Composite Microspheres with Well-Designed Nanostructure: A Highly Integrated Catalyst System. J. Am. Chem. Soc. 2010, 132, 8466–8473. [Google Scholar] [CrossRef] [PubMed]
- Jouault, N.; Vallat, P.; Dalmas, F.; Said, S.; Jestin, J.; Boué, F. Well-Dispersed Fractal Aggregates as Filler in Polymer−Silica Nanocomposites: Long-Range Effects in Rheology. Macromolecules 2009, 42, 2031–2040. [Google Scholar] [CrossRef] [Green Version]
- Tangney, P.; Scandolo, S. An ab initio parametrized interatomic force field for silica. J. Chem. Phys. 2002, 117, 8898–8904. [Google Scholar] [CrossRef]
- Kermode, J.R.; Cereda, S.; Tangney, P.; De Vita, A. A first principles based polarizable O(N) interatomic force field for bulk silica. J. Chem. Phys. 2010, 133, 094102. [Google Scholar] [CrossRef] [Green Version]
- Butenuth, A.; Moras, G.; Schneider, J.; Koleini, M.; Köppen, S.; Meißner, R.; Wright, L.B.; Walsh, T.R.; Ciacchi, L.C. Ab initio derived force-field parameters for molecular dynamics simulations of deprotonated amorphous-SiO2/water interfaces. Phys. Status Solidi B 2012, 249, 292–305. [Google Scholar] [CrossRef]
- Lopes, P.E.M.; Murashov, V.; Tazi, M.; Demchuk, E.; MacKerell, A.D. Development of an Empirical Force Field for Silica. Application to the Quartz−Water Interface. J. Phys. Chem. B 2006, 110, 2782–2792. [Google Scholar] [CrossRef]
- Pedone, A.; Malavasi, G.; Menziani, M.C.; Segre, U.; Musso, F.; Corno, M.; Civalleri, B.; Ugliengo, P. FFSiOH: a New Force Field for Silica Polymorphs and Their Hydroxylated Surfaces Based on Periodic B3LYP Calculations. Chem. Mat. 2008, 20, 2522–2531. [Google Scholar] [CrossRef]
- Ramakrishnan, S.K.; Estephan, E.; Martin, M.; Cloitre, T.; Gergely, C. Probing the mechanism of material specific peptides for optical biosensors. Int. Soc. Opt. Photonics 2013. [Google Scholar] [CrossRef]
- Heinz, H. Adsorption of biomolecules and polymers on silicates, glasses, and oxides: mechanisms, predictions, and opportunities by molecular simulation. Curr. Opin. Chem. Eng. 2016, 11, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Emami, F.S.; Puddu, V.; Berry, R.J.; Varshney, V.; Patwardhan, S.V.; Perry, C.C.; Heinz, H. Prediction of Specific Biomolecule Adsorption on Silica Surfaces as a Function of pH and Particle Size. Chem. Mat. 2014, 26, 5725–5734. [Google Scholar] [CrossRef] [Green Version]
- Carravetta, V.; Monti, S. Peptide−TiO2 Surface Interaction in Solution by Ab Initio and Molecular Dynamics Simulations. J. Phys. Chem. B 2006, 110, 6160–6169. [Google Scholar] [CrossRef]
- Chenoweth, K.; van Duin, A.C.T.; Goddard, W.A. ReaxFF Reactive Force Field for Molecular Dynamics Simulations of Hydrocarbon Oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef] [Green Version]
- van der Kamp, M.W.; Mulholland, A.J. Combined Quantum Mechanics/Molecular Mechanics (QM/MM) Methods in Computational Enzymology. Biochemistry 2013, 52, 2708–2728. [Google Scholar] [CrossRef]
- Ghadari, R. A study on the interactions of amino acids with nitrogen doped graphene; docking, MD simulation, and QM/MM studies. Phys. Chem. Chem. Phys. 2016, 18, 4352–4361. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akeson, M.; Branton, D.; Church, G.; Deamer, D.W. Characterization of individual polymer molecules based on monomer-interface interactions. U.S. Patent US20120160687A1, 28 June 2018. [Google Scholar]
- Bond, P.J.; Guy, A.T.; Heron, A.J.; Bayley, H.; Khalid, S. Molecular Dynamics Simulations of DNA within a Nanopore: Arginine−Phosphate Tethering and a Binding/Sliding Mechanism for Translocation. Biochemistry 2011, 50, 3777–3783. [Google Scholar] [CrossRef] [PubMed]
- Guy, A.T.; Piggot, T.J.; Khalid, S. Single-Stranded DNA within Nanopores: Conformational Dynamics and Implications for Sequencing; a Molecular Dynamics Simulation Study. Biophys. J. 2012, 103, 1028–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkin, J.; Khalid, S. Atomistic Molecular-Dynamics Simulations Enable Prediction of the Arginine Permeation Pathway through OccD1/OprD from Pseudomonas aeruginosa. Biophys. J. 2014, 107, 1853–1861. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Derrington, I.M.; Pavlenok, M.; Niederweis, M.; Gundlach, J.H.; Aksimentiev, A. Molecular Dynamics Study of MspA Arginine Mutants Predicts Slow DNA Translocations and Ion Current Blockades Indicative of DNA Sequence. ACS Nano 2012, 6, 6960–6968. [Google Scholar] [CrossRef]
- Haider, S.; Neidle, S. Molecular Modeling and Simulation of G-Quadruplexes and Quadruplex-Ligand Complexes. In G-Quadruplex DNA: Methods and Protocols; Baumann, P., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 17–37. [Google Scholar] [CrossRef]
- Kang, X.-F.; Gu, L.-Q.; Cheley, S.; Bayley, H. Single Protein Pores Containing Molecular Adapters at High Temperatures. Angew. Chem. Int. Ed. 2005, 44, 1495–1499. [Google Scholar] [CrossRef]
- Cherf, G.M.; Lieberman, K.R.; Rashid, H.; Lam, C.E.; Karplus, K.; Akeson, M. Automated forward and reverse ratcheting of DNA in a nanopore at 5-A precision. Nat. Biotech. 2012, 30, 344–348. [Google Scholar] [CrossRef]
- Menestrina, G. Ionic channels formed byStaphylococcus aureus alpha-toxin: Voltage-dependent inhibition by divalent and trivalent cations. J. Mem. Biol. 1986, 90, 177–190. [Google Scholar] [CrossRef]
- Korchev, Y.E.; Alder, G.M.; Bakhramov, A.; Bashford, C.L.; Joomun, B.S.; Sviderskaya, E.V.; Usherwood, P.N.R.; Pasternak, C.A. Staphylococcus aureus alpha-toxin-induced pores: Channel-like behavior in lipid bilayers and patch clamped cells. J. Membrane Biol. 1995, 143, 143–151. [Google Scholar] [CrossRef]
- Mathé, J.; Aksimentiev, A.; Nelson, D.R.; Schulten, K.; Meller, A. Orientation discrimination of single-stranded DNA inside the α-hemolysin membrane channel. Proc. Natl. Acad. Sci. USA USA 2005, 102, 12377–12382. [Google Scholar] [CrossRef] [Green Version]
- Derrington, I.M.; Butler, T.Z.; Collins, M.D.; Manrao, E.; Pavlenok, M.; Niederweis, M.; Gundlach, J.H. Nanopore DNA sequencing with MspA. Proc. Natl. Acad. Sci. USA 2010, 107, 16060. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Yoo, J.; Aksimentiev, A. Water Mediates Recognition of DNA Sequence via Ionic Current Blockade in a Biological Nanopore. ACS Nano 2016, 10, 4644–4651. [Google Scholar] [CrossRef] [Green Version]
- Merchant, C.A.; Healy, K.; Wanunu, M.; Ray, V.; Peterman, N.; Bartel, J.; Fischbein, M.D.; Venta, K.; Luo, Z.; Johnson, A.T.C.; et al. DNA Translocation through Graphene Nanopores. Nano Lett. 2010, 10, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.F.; Kowalczyk, S.W.; Calado, V.E.; Pandraud, G.; Zandbergen, H.W.; Vandersypen, L.M.K.; Dekker, C. DNA Translocation through Graphene Nanopores. Nano Lett. 2010, 10, 3163–3167. [Google Scholar] [CrossRef] [Green Version]
- Garaj, S.; Hubbard, W.; Reina, A.; Kong, J.; Branton, D.; Golovchenko, J.A. Graphene as a subnanometre trans-electrode membrane. Nature 2010, 467, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Stein, D.; McMullan, C.; Branton, D.; Aziz, M.J.; Golovchenko, J.A. Ion-beam sculpting at nanometre length scales. Nature 2001, 412, 166–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storm, A.J.; Chen, J.H.; Zandbergen, H.W.; Dekker, C. Translocation of double-strand DNA through a silicon oxide nanopore. Phy. Rev. E 2005, 71, 051903. [Google Scholar] [CrossRef] [Green Version]
- Heng, J.B.; Ho, C.; Kim, T.; Timp, R.; Aksimentiev, A.; Grinkova, Y.V.; Sligar, S.; Schulten, K.; Timp, G. Sizing DNA Using a Nanometer-Diameter Pore. Biophys. J. 2004, 87, 2905–2911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heng, J.B.; Aksimentiev, A.; Ho, C.; Marks, P.; Grinkova, Y.V.; Sligar, S.; Schulten, K.; Timp, G. Stretching DNA Using the Electric Field in a Synthetic Nanopore. Nano Lett. 2005, 5, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Heng, J.B.; Aksimentiev, A.; Ho, C.; Marks, P.; Grinkova, Y.V.; Sligar, S.; Schulten, K.; Timp, G. The Electromechanics of DNA in a Synthetic Nanopore. Biophys. J. 2006, 90, 1098–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Comer, J.; Dimitrov, V.; Yemenicioglu, S.; Aksimentiev, A.; Timp, G. Stretching and unzipping nucleic acid hairpins using a synthetic nanopore. Nucleic Acids Res. 2008, 36, 1532–1541. [Google Scholar] [CrossRef] [Green Version]
- Fologea, D.; Uplinger, J.; Thomas, B.; McNabb, D.S.; Li, J. Slowing DNA Translocation in a Solid-State Nanopore. Nano Lett. 2005, 5, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Gu, J.; Brandin, E.; Kim, Y.-R.; Wang, Q.; Branton, D. Probing Single DNA Molecule Transport Using Fabricated Nanopores. Nano Lett. 2004, 4, 2293–2298. [Google Scholar] [CrossRef]
- Gershow, M.; Golovchenko, J.A. Recapturing and trapping single molecules with a solid-state nanopore. Nat. Nano 2007, 2, 775–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanunu, M.; Sutin, J.; McNally, B.; Chow, A.; Meller, A. DNA Translocation Governed by Interactions with Solid-State Nanopores. Biophys. J. 2008, 95, 4716–4725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.; Kosari, F.; Andreadakis, G.; Alam, M.A.; Vasmatzis, G.; Bashir, R. DNA-Mediated Fluctuations in Ionic Current through Silicon Oxide Nanopore Channels. Nano Lett. 2004, 4, 1551–1556. [Google Scholar] [CrossRef] [Green Version]
- Smeets, R.M.M.; Keyser, U.F.; Krapf, D.; Wu, M.-Y.; Dekker, N.H.; Dekker, C. Salt Dependence of Ion Transport and DNA Translocation through Solid-State Nanopores. Nano Lett. 2006, 6, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Trepagnier, E.H.; Radenovic, A.; Sivak, D.; Geissler, P.; Liphardt, J. Controlling DNA Capture and Propagation through Artificial Nanopores. Nano Lett. 2007, 7, 2824–2830. [Google Scholar] [CrossRef] [Green Version]
- Fologea, D.; Gershow, M.; Ledden, B.; McNabb, D.S.; Golovchenko, J.A.; Li, J. Detecting Single Stranded DNA with a Solid State Nanopore. Nano Lett. 2005, 5, 1905–1909. [Google Scholar] [CrossRef] [Green Version]
- Han, A.; Schürmann, G.; Mondin, G.; Bitterli, R.A.; Hegelbach, N.G.; de Rooij, N.F.; Staufer, U. Sensing protein molecules using nanofabricated pores. Appl. Phys. Lett. 2006, 88, 093901. [Google Scholar] [CrossRef]
- Fologea, D.; Ledden, B.; McNabb, D.S.; Li, J. Electrical characterization of protein molecules by a solid-state nanopore. Appl. Phys. Lett. 2007, 91, 053901. [Google Scholar] [CrossRef]
- Fan, R.; Karnik, R.; Yue, M.; Li, D.; Majumdar, A.; Yang, P. DNA Translocation in Inorganic Nanotubes. Nano Lett. 2005, 5, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- McNally, B.; Wanunu, M.; Meller, A. Electromechanical Unzipping of Individual DNA Molecules Using Synthetic Sub-2 nm Pores. Nano Lett. 2008, 8, 3418–3422. [Google Scholar] [CrossRef]
- Harrell, C.C.; Choi, Y.; Horne, L.P.; Baker, L.A.; Siwy, Z.S.; Martin, C.R. Resistive-Pulse DNA Detection with a Conical Nanopore Sensor. Langmuir 2006, 22, 10837–10843. [Google Scholar] [CrossRef]
- Bayley, H. Nanotechnology: Holes with an edge. Nature 2010, 467, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-s.; Lu, X.; Ding, H.-m.; Ma, Y.-q. Computational investigation on DNA sequencing using functionalized graphene nanopores. Phy. Chem. Chem. Phy. 2018, 20, 9063–9069. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Liu, K.; Sarathy, A.; Leburton, J.-P.; Radenovic, A. Transverse Detection of DNA in a MoS2 Nanopore. Biophys. J. 2018, 114, 180a. [Google Scholar] [CrossRef]
- Li, H.; Carrion-Vazquez, M.; Oberhauser, A.F.; Marszalek, P.E.; Fernandez, J.M. Point mutations alter the mechanical stability of immunoglobulin modules. Nat. Struct. Mol. Biol 2000, 7, 1117–1120. [Google Scholar]
- Storm, A.J.; Chen, J.H.; Ling, X.S.; Zandbergen, H.W.; Dekker, C. Fabrication of solid-state nanopores with single-nanometre precision. Nat. Mater. 2003, 2, 537–540. [Google Scholar] [CrossRef]
- Sathe, C.; Zou, X.; Leburton, J.-P.; Schulten, K. Computational Investigation of DNA Detection Using Graphene Nanopores. ACS Nano 2011, 5, 8842–8851. [Google Scholar] [CrossRef]
- Wells, D.B.; Belkin, M.; Comer, J.; Aksimentiev, A. Assessing Graphene Nanopores for Sequencing DNA. Nano Lett. 2012, 12, 4117–4123. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Cui, P.; Wang, Q.; Wu, T.; Agren, H.; Tu, Y. Theoretical study on key factors in DNA sequencing with graphene nanopores. RSC Adv. 2013, 3, 2445–2453. [Google Scholar] [CrossRef]
- Qiu, H.; Sarathy, A.; Leburton, J.-P.; Schulten, K. Intrinsic Stepwise Translocation of Stretched ssDNA in Graphene Nanopores. Nano Lett. 2015, 15, 8322–8330. [Google Scholar] [CrossRef]
- Wanunu, M.; Meller, A. Chemically Modified Solid-State Nanopores. Nano Lett. 2007, 7, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.M.; Akin, D.; Bashir, R. Solid-state nanopore channels with DNA selectivity. Nat. Nano 2007, 2, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Keyser, U.F. Controlling molecular transport through nanopores. J. Royal Soc. Interface 2011, 8, 1369. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.R.; Scott, A.; Rotem, D.; Mehta, K.K.; Bayley, H.; Dekker, C. Hybrid pore formation by directed insertion of [alpha]-haemolysin into solid-state nanopores. Nat. Nano 2010, 5, 874–877. [Google Scholar] [CrossRef]
- Van Drie, J.H. Computer-aided drug design: the next 20 years. J. Comput.-Aided Mol. Des. 2007, 21, 591–601. [Google Scholar] [CrossRef]
- Yang, S.-Y. Pharmacophore modeling and applications in drug discovery: challenges and recent advances. Drug Dis. Today 2010, 15, 444–450. [Google Scholar] [CrossRef]
- Bajorath, J. (Ed.) Chemoinformatics and Computational Chemical Biology; Humana Press: Totowa, NJ, USA, 2011. [Google Scholar]
- Jitender, V.; Vijay, M.K.; Evans, C.C. 3D-QSAR in Drug Design—A Review. Curr. Topics Med. Chem. 2010, 10, 95–115. [Google Scholar]
- Lin, S.-K. Pharmacophore Perception, Development and Use in Drug Design. Edited by Osman F. Güner. Molecules 2000, 5, 987. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.J.; Sterling, T.; Mysinger, M.M.; Bolstad, E.S.; Coleman, R.G. ZINC: A Free Tool to Discover Chemistry for Biology. J. Chem. Inform. Modeling 2012, 52, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.J.; Shoichet, B.K. ZINC–A Free Database of Commercially Available Compounds for Virtual Screening. J. Chem. Inform. Modeling 2005, 45, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Li, Q.; Zhou, Z.; Wang, Y.; Bryant, S.H. Structure-Based Virtual Screening for Drug Discovery: A Problem-Centric Review. AAPS J. 2012, 14, 133–141. [Google Scholar] [CrossRef]
- Lengauer, T.; Rarey, M. Computational methods for biomolecular docking. Curr. Opin. Struc. Biol. 1996, 6, 402–406. [Google Scholar] [CrossRef]
- Barreiro, G.; Kim, J.T.; Guimarães, C.R.W.; Bailey, C.M.; Domaoal, R.A.; Wang, L.; Anderson, K.S.; Jorgensen, W.L. From Docking False-Positive to Active Anti-HIV Agent. J. Med. Chem. 2007, 50, 5324–5329. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.K.; Johnson, A.P.; Fishwick, C.W.G. Synthesis of de novo designed small-molecule inhibitors of bacterial RNA polymerase. Tetrahedron 2008, 64, 10049–10054. [Google Scholar] [CrossRef]
- Stephanie, B.A.d.B.; Nico, P.E.V.; Chris, O. The Role of Water Molecules in Computational Drug Design. Curr. Topics Med. Chem. 2010, 10, 55–66. [Google Scholar]
- Pan, A.C.; Borhani, D.W.; Dror, R.O.; Shaw, D.E. Molecular determinants of drug–receptor binding kinetics. Drug Dis. Today 2013, 18, 667–673. [Google Scholar] [CrossRef]
- Dror, R.O.; Pan, A.C.; Arlow, D.H.; Borhani, D.W.; Maragakis, P.; Shan, Y.; Xu, H.; Shaw, D.E. Pathway and mechanism of drug binding to G-protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 13118–13123. [Google Scholar] [CrossRef]
- Zwanzig, R.W. High-Temperature Equation of State by a Perturbation Method. I. Nonpolar Gases. Chem. Phys. 1954, 22, 1420–1426. [Google Scholar] [CrossRef]
- Straatsma, T.P.; Berendsen, H.J.C.; Postma, J.P.M. Free energy of hydrophobic hydration: A molecular dynamics study of noble gases in water. Chem. Phys. 1986, 85, 6720–6727. [Google Scholar] [CrossRef]
- Straatsma, T.P.; Berendsen, H.J.C. Free energy of ionic hydration: Analysis of a thermodynamic integration technique to evaluate free energy differences by molecular dynamics simulations. Chem. Phys. 1988, 89, 5876–5886. [Google Scholar] [CrossRef]
- Durrant, J.D.; McCammon, J.A. Molecular dynamics simulations and drug discovery. BMC Biol. 2011, 9, 71. [Google Scholar] [CrossRef]
- Ferguson, D.M.; Radmer, R.J.; Kollman, P.A. Determination of the relative binding free energies of peptide inhibitors to the HIV-1 protease. J. Med. Chem. 1991, 34, 2654–2659. [Google Scholar] [CrossRef]
- Merz, K.M.R.D.; Reynolds, C.H. Drug Design: Structure-and Ligand-Based Approaches; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Boresch, S.; Tettinger, F.; Leitgeb, M.; Karplus, M. Absolute Binding Free Energies: A Quantitative Approach for Their Calculation. J. Phy. Chem. B 2003, 107, 9535–9551. [Google Scholar] [CrossRef]
- Rombouts, F.J.R.; Tresadern, G.; Buijnsters, P.; Langlois, X.; Tovar, F.; Steinbrecher, T.B.; Vanhoof, G.; Somers, M.; Andrés, J.-I.; Trabanco, A.A. Pyrido[4,3-e][1,2,4]triazolo[4,3-a]pyrazines as Selective, Brain Penetrant Phosphodiesterase 2 (PDE2) Inhibitors. ACS Med. Chem. Lett. 2015, 6, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Ciordia, M.; Pérez-Benito, L.; Delgado, F.; Trabanco, A.A.; Tresadern, G. Application of Free Energy Perturbation for the Design of BACE1 Inhibitors. J. Chem. Inform. Modeling 2016, 56, 1856–1871. [Google Scholar] [CrossRef] [PubMed]
- Keränen, H.; Pérez-Benito, L.; Ciordia, M.; Delgado, F.; Steinbrecher, T.B.; Oehlrich, D.; van Vlijmen, H.W.T.; Trabanco, A.A.; Tresadern, G. Acylguanidine Beta Secretase 1 Inhibitors: A Combined Experimental and Free Energy Perturbation Study. J. Chem. Theory Comput. 2017, 13, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L. Computer-aided discovery of anti-HIV agents. Bioorg. Med. Chem. 2016, 24, 4768–4778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, W.L.; Ruiz-Caro, J.; Tirado-Rives, J.; Basavapathruni, A.; Anderson, K.S.; Hamilton, A.D. Computer-aided design of non-nucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg. Med. Chem. Lett. 2006, 16, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Caro, J.; Basavapathruni, A.; Kim, J.T.; Bailey, C.M.; Wang, L.; Anderson, K.S.; Hamilton, A.D.; Jorgensen, W.L. Optimization of diarylamines as non-nucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg. Med. Chem. Lett. 2006, 16, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Hamilton, A.D.; Bailey, C.M.; Domoal, R.A.; Wang, L.; Anderson, K.S.; Jorgensen, W.L. FEP-Guided Selection of Bicyclic Heterocycles in Lead Optimization for Non-Nucleoside Inhibitors of HIV-1 Reverse Transcriptase. J. Am. Chem. Soc. 2006, 128, 15372–15373. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Bollini, M.; Thakur, V.V.; Domaoal, R.A.; Spasov, K.A.; Anderson, K.S. Efficient Discovery of Potent Anti-HIV Agents Targeting the Tyr181Cys Variant of HIV Reverse Transcriptase. J. Am. Chem. Soc. 2011, 133, 15686–15696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollini, M.; Domaoal, R.A.; Thakur, V.V.; Gallardo-Macias, R.; Spasov, K.A.; Anderson, K.S.; Jorgensen, W.L. Computationally-Guided Optimization of a Docking Hit to Yield Catechol Diethers as Potent Anti-HIV Agents. J. Med. Chem. 2011, 54, 8582–8591. [Google Scholar] [CrossRef] [Green Version]
- Lovering, F.; Aevazelis, C.; Chang, J.; Dehnhardt, C.; Fitz, L.; Han, S.; Janz, K.; Lee, J.; Kaila, N.; McDonald, J.; et al. Imidazotriazines: Spleen Tyrosine Kinase (Syk) Inhibitors Identified by Free-Energy Perturbation (FEP). ChemMedChem 2016, 11, 217–233. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Deng, Y.; Kim, B.; Pierce, L.; Krilov, G.; Lupyan, D.; Robinson, S.; Dahlgren, M.K.; Greenwood, J.; et al. Accurate and Reliable Prediction of Relative Ligand Binding Potency in Prospective Drug Discovery by Way of a Modern Free-Energy Calculation Protocol and Force Field. J. Am. Chem. Soc. 2015, 137, 2695–2703. [Google Scholar] [CrossRef] [Green Version]
- Abel, R.; Mondal, S.; Masse, C.; Greenwood, J.; Harriman, G.; Ashwell, M.A.; Bhat, S.; Wester, R.; Frye, L.; Kapeller, R.; et al. Accelerating drug discovery through tight integration of expert molecular design and predictive scoring. Curr. Opin. Struc. Biol. 2017, 43, 38–44. [Google Scholar] [CrossRef]
- Williams-Noonan, B.J.; Yuriev, E.; Chalmers, D.K. Free Energy Methods in Drug Design: Prospects of “Alchemical Perturbation” in Medicinal Chemistry. J. Med. Chem. 2018, 61, 638–649. [Google Scholar] [CrossRef]
- Cournia, Z.; Allen, B.; Sherman, W. Relative Binding Free Energy Calculations in Drug Discovery: Recent Advances and Practical Considerations. J. Chem. Inform. Modeling 2017, 57, 2911–2937. [Google Scholar] [CrossRef]
- Gupta, S.; Jhawat, V. Quality by design (QbD) approach of pharmacogenomics in drug designing and formulation development for optimization of drug delivery systems. J. Control. Release 2017, 245, 15–26. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, J.; Han, D.; Zhu, H. From machine learning to deep learning: progress in machine intelligence for rational drug discovery. Drug Disco. Today 2017, 22, 1680–1685. [Google Scholar] [CrossRef]
- Lavecchia, A.; Cerchia, C. In silico methods to address polypharmacology: current status, applications and future perspectives. Drug Disco. Today 2016, 21, 288–298. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminpour, M.; Montemagno, C.; Tuszynski, J.A. An Overview of Molecular Modeling for Drug Discovery with Specific Illustrative Examples of Applications. Molecules 2019, 24, 1693. https://doi.org/10.3390/molecules24091693
Aminpour M, Montemagno C, Tuszynski JA. An Overview of Molecular Modeling for Drug Discovery with Specific Illustrative Examples of Applications. Molecules. 2019; 24(9):1693. https://doi.org/10.3390/molecules24091693
Chicago/Turabian StyleAminpour, Maral, Carlo Montemagno, and Jack A. Tuszynski. 2019. "An Overview of Molecular Modeling for Drug Discovery with Specific Illustrative Examples of Applications" Molecules 24, no. 9: 1693. https://doi.org/10.3390/molecules24091693
APA StyleAminpour, M., Montemagno, C., & Tuszynski, J. A. (2019). An Overview of Molecular Modeling for Drug Discovery with Specific Illustrative Examples of Applications. Molecules, 24(9), 1693. https://doi.org/10.3390/molecules24091693