Terpenoids in the Essential Oil and Concentrated Aromatic Products Obtained from Nicotiana glutinosa L. Leaves
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Obtaining and Analysis of Aromatic Products
4.3. Olfactory Evaluation of the EO and Extracts
4.4. Chemical Composition of the EO and Extracts
4.4.1. GC-MS Analysis of the Extracts (Concrete and Resinoid)
4.4.2. GC-MS Analysis of the EO
4.5. Antimicrobial Activity of Sclareol
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goodspeed, T. On the evaluation of the genus Nicotiana. Proc. Natl. Acad. Sci. USA 1947, 33, 158–171. [Google Scholar]
- Chase, M.; Knapp, S.; Cox, A.; Clarkson, J.; Butsko, Y.; Joseph, J.; Savolainen, V.; Parokonny, A. Molecular systematic, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae). Ann. Bot. 2003, 92, 107–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapp, S.; Chase, M.; Clarkson, J. Nomenclatural changes and a new sectional classification in Nicotiana (Solanaceae). Taxon 2004, 53, 73–82. [Google Scholar] [CrossRef]
- Lewis, R.; Nicholson, J. Aspects of the evolution of Nicotiana tabacum L. and the status of the United States Nicotiana Germplasm Collection. Genet. Resour. Crop. Evol. 2007, 54, 727–740. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zare, S.; Asadollahi, M.; Schuman, M. Ecological roles and biological activities of specialized metabolites from the genus Nicotiana. Chem. Rev. 2017, 117, 12227–12280. [Google Scholar] [CrossRef]
- Raghavan, T.; Srinivasan, A. Cytogenetical studies in Nicotiana. Part II. Morphological features of Nicotiana glutinosa and the hybrid between Nicotiana glutinosa and N. tabacum. Proc. Indian Acad. Sci. B. 1941, 14, 35–46. [Google Scholar]
- Glater, R.; Solberg, R.; Scott, F. A developmental study of the leaves of Nicotiana glutinosa as related to their smog-sensitivity. Am. J. Bot. 1962, 49, 954–970. [Google Scholar] [CrossRef]
- Jackson, M.; Severson, R.; Sisson, V.; Stephenson, M. Ovipositional response of tobacco budworm moths (Lepidoptera: Noctuidae) to cuticular labdanes and sucrose esters from the green leaves of Nicotiana glutinosa L. (Solanaceae). J. Chem. Ecol. 1991, 17, 2489–2506. [Google Scholar] [CrossRef]
- Kennedy, B.; Nielsen, M.; Severson, R.; Sisson, V.; Stephenson, M.; Jackson, D. Leaf surface chemicals from Nicotiana affecting germination of Peronospora Tabacina. J. Chem. Ecol. 1992, 18, 1467–1479. [Google Scholar] [CrossRef]
- Bailey, J.; Carter, G.; Burden, R.; Wain, R. Control of rust diseases by diterpenes from Nicotiana glutinosa. Nature 1975, 255, 328–329. [Google Scholar] [CrossRef]
- Bailey, J.; Vincent, G.; Burden, R. Diterpenes from Nicotiana glutinosa and their effect on fungal growth. J. Gen. Microbiol. 1974, 85, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Sisson, V.; Severson, R. Alkaloid composition of the Nicotiana species. Beitr. Tabakforsch. Int. 1990, 14, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Arrendale, R.; Severson, R.; Sisson, V.; Costello, C.; Leary, J.; Himmelsbach, D.; van Halbeek, H. Characterization of the sucrose ester fraction from Nicotiana glutinosa. J. Agric. Food Chem. 1990, 38, 75–85. [Google Scholar] [CrossRef]
- Guo, Z.; Wagner, G. Biosynthesis of labdenediol and sclareol in cell-free extracts from trichomes of Nicotiana glutinosa. Planta 1995, 197, 627–632. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Koeshi, K.; Koiwai, A. Germination and growth inhibition of surface lipids from Nicotiana species and identification of sucrose esters. Agric. Biol. Chem. 1988, 52, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- Dawson, R. An experimental analysis of alkaloid production in Nicotiana: The origin of nornicotine. Am. J. Bot. 1945, 32, 416–423. [Google Scholar] [CrossRef]
- Nottingham, S.; Chortyk, O.; Stephenson, M. Sugar esters from Nicotiana species as potential insecticides against the sweetpotato whitefly (Homoptera: Aleyrodidae). J. Entomol. Sci. 1996, 31, 331–339. [Google Scholar] [CrossRef]
- Jackson, D.; Chortyk, O.; Stephenson, M.; Johnson, A.; Harlow, C.; Simmons, A.; Sisson, V. Potential of Nicotiana species for production of sugar esters. Tob. Sci. 1998, 42, 1–9. [Google Scholar]
- Jackson, D.M.; Danehower, D.A. Integrated case study: Nicotiana leaf surface components and their effects on insect pests and disease. In Plant Cuticles: An Integrated Functional Approach; Kerstiens, G., Ed.; BIOS Scientific Publishers, Ltd.: Oxford, UK, 1996; pp. 231–254. [Google Scholar]
- Reid, W. The diterpenes of Nicotiana species and N. tabacum cultivars. In The Biology and Taxonomy of the Solanaceae. Linnean Society Symposium Series; Hawkes, J.G., Lester, R.N., Skelding, A.D., Eds.; Academic Press: New York, NY, USA, 1979; Number 7; pp. 273–278. [Google Scholar]
- Lawrence, B. Progress in essential oils. Perfum. Flavor. 1986, 21, 57–68. [Google Scholar]
- Demetzos, C.; Stahl, B.; Anastassaki, T.; Gazouli, M.; Tzouvelekis, L.; Rallis, M. Chemical analysis and antimicrobial activity of the resin Ladano, of its essential oil and of the isolated compounds. Planta Med. 1999, 65, 76–78. [Google Scholar] [CrossRef]
- McNeil, M.; Porter, R.; Williams, L.; Rainford, L. Chemical composition and antimicrobial activity of the essential oils from Cleome spinose. Nat. Prod. Commun. 2010, 5, 1301–1306. [Google Scholar] [PubMed] [Green Version]
- Severson, R.; Jackson, D.; Johnson, A.; Sisson, V.; Stephenson, M. Ovipositional behavior of tobacco budworm and tobacco hornworm. Effects of cuticular components from Nicotiana species. In Naturally Occuring Pest Bioregulators. ACS Symposium Series; Hedin, P.A., Ed.; American Chemistry Society: Washington, DC, USA, 1991; Volume 449, pp. 264–277. [Google Scholar]
- Hayashi, T.; Kobayashi, D.; Kariu, T.; Tahara, M.; Hada, K.; Kouzuma, Y.; Kimura, M. Genomic cloning of ribonucleases in Nicotiana glutinosa leaves, as induced in response to wounding or to TMV-infection, and characterization of their promoters. Biosci. Biotechnol. Biochem. 2003, 67, 2574–2583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.-X.; Stansly, P.A.; Chortyk, O.T. Insecticidal activity of natural and synthetic sugar esters against Bemisia argentifolii (Homoptera: Aleyrodidae). J. Econ. Entomol. 1996, 89, 1233–1239. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, B.; Nielsen, M.; Severson, R. Biorationals from Nicotiana protect cucumbers against Colletotrichum lagenarium (pass.) Ell. & Halst disease development. J. Chem. Ecol. 1995, 21, 221–231. [Google Scholar]
- Cohen, Y.; Eyal, H.; Goldschmid, Z.; Sklarz, B. A preformed chemical inhibitor of tobacco powdery mildew on leaves of Nicotiana glutinosa. Physiol. Plant. Pathol. 1983, 22, 143–150. [Google Scholar] [CrossRef]
- Kroumova, A.; Artiouchine, I.; Wagner, G. Use of several natural products from selected Nicotiana species to prevent black shank disease in tobacco. Beitr. Tabakforsch. Int. 2016, 27, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.; Gomi, K.; Kaku, H.; Abe, H.; Seto, H.; Nakatsu, S.; Neya, M.; Kobayashi, M.; Nakaho, K.; Ichinose, Y.; et al. Identification of natural diterpenes that inhibit bacterial wilt disease in tobacco, tomato and Arabidopsis. Plant. Cell Physiol. 2012, 53, 1432–1444. [Google Scholar] [CrossRef] [Green Version]
- Georgiev, E.; Stoyanova, A. A Guide for the Specialist in Aromatic Industry, 1st ed.; UFT Acad. Publ. House: Plovdiv, Bulgaria, 2006. [Google Scholar]
- Popova, V.; Gochev, V.; Girova, T.; Iliev, I.; Ivanova, T.; Stoyanova, A. Extraction products from tobacco –aroma and bioactive compounds and activities. Curr. Bioact. Compd. 2015, 11, 31–37. [Google Scholar] [CrossRef]
- Bauer, K.; Garbe, D.; Surburg, H. Common Fragrance and Flavor Materials. Preparation, Properties and Uses, 4th ed.; Wiley-VCH: Weinheim, NY, USA, 2001. [Google Scholar]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics—a critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization Home Page. Available online: https://www.iso.org/standard/51017.html (accessed on 19 December 2019).
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Popova, V.; Ivanova, T.; Stoyanova, A.; Georgiev, V.; Hristeva, T.; Nikolova, V.; Docheva, M.; Nikolov, N.; Damyanova, S. Phytochemicals in leaves and extracts of the variety “Plovdiv 7” of Bulgarian oriental tobacco (Nicotiana tabacum L.). Trends Phytochem. Res. 2018, 2, 27–36. [Google Scholar]
- Popova, V.; Ivanova, T.; Prokopov, T.; Nikolova, M.; Stoyanova, A.; Zheljazkov, V.D. Carotenoid-related volatile compounds of tobacco (Nicotiana tabacum L.) essential oils. Molecules 2019, 24, 3446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popova, V.; Ivanova, T.; Nikolova, V.; Stoyanova, A.; Docheva, M.; Hristeva, T.; Damyanova, S.; Nikolov, N. Biologically active and volatile compounds in leaves and extracts of Nicotiana alata Link & Otto from Bulgaria. J. Pharm. Sci. Res. 2017, 9, 2045–2051. [Google Scholar]
- Di Bella, G.; Saitta, M.; La Pera, L.; Alfa, M.; Dugo, G. Pesticide and plasticizer residues in bergamot essential oils from Calabria (Italy). Chemosphere 2004, 56, 777–782. [Google Scholar] [CrossRef]
- Saeidnia, S.; Abdollahi, M. Are medicinal plants polluted with phthalates? DARU J. Pharm. Sci. 2013, 21, 43. [Google Scholar] [CrossRef] [Green Version]
- Manayi, A.; Kurepaz-mahmoodabadi, M.; Gohari, A.R.; Ajani, Y.; Saeidnia, S. Presence of phthalate derivatives in the essential oils of a medicinal plant Achillea tenuifolia. DARU J. Pharm. Sci. 2014, 22, 78. [Google Scholar] [CrossRef] [Green Version]
- Leffingwell, J.C.; Alford, E.D. Volatile constituents of Perique tobacco. Electron. J. Environ. Agric. Food Chem. 2005, 4, 899–915. [Google Scholar]
- Jia, Z.H.; Yi, J.H.; Su, Y.R.; Shen, H. Autotoxic substances in the root exudates from continuous tobacco cropping. Allelopath. J. 2011, 27, 87–96. [Google Scholar]
- Liu, L.; Huang, Y.; Wang, J.; Tang, Z.; Lu, L.; Wu, R.; Lei, Q. Study on discriminating flue-cured tobacco by volatile compounds related to geographical origin and cultivar. Asian J. Chem. 2013, 25, 7587–7592. [Google Scholar] [CrossRef]
- Rodgman, A.; Perfetti, T. (Eds.) The Chemical Components of Tobacco and Tobacco Smoke, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant sources, extraction methods, and uses of squalene. Int. J. Agron. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Murkute, A.; Sahu, M.; Mali, P.; Rangari, V. Development and evaluation of formulations of microbial biotransformed extract of tobacco leaves for hair growth potential. Pharmacogn. Res. 2010, 2, 300–303. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Skalicka-Wo’zniak, K.; Georgiev, M.I. Pharmacognosy: Fundamentals, Applications and Strategies; Badal, S., Delgoda, R., Eds.; Elsevier Inc.: Amsterdam, Netherlands, 2017; pp. 233–266. [Google Scholar]
- Sandjo, L.P.; Kuete, V. Diterpenoids from the medicinal plants of Africa. In Medicinal Plant Research in Africa: Pharmacology and Chemistry; Kuete, V., Ed.; Elsevier: London, UK, 2013; pp. 105–133. [Google Scholar]
- Lawrence, B. Production of clary sage oil and sclareol in North America. In Proceedings of the Remes Recontres Internationales Nyons, Nyons, France, 5–7 December 1994; pp. 41–50. [Google Scholar]
- Caniard, A.; Zerbe, P.; Legrand, S.; Cohade, A.; Valot, N.; Magnard, J.-L.; Bohlmann, J.; Legendre, L. Discovery and functional characterization of two diterpene synthases for sclareol biosynthesis in Salvia sclarea (L.) and their relevance for perfume manufacture. Bmc Plant. Biol. 2012, 12, 119. [Google Scholar] [CrossRef] [Green Version]
- Mookherijee, B.; Wilson, R. Tobacco constituents—Their importance in flavor and fragrance chemistry. Perfum. Flavor. 1990, 15, 27–49. [Google Scholar]
- Leffingwell, J.C.; Leffingwell, D. Chemical and sensory aspects of tobacco flavor—An overview. Rec. Adv. Tob. Sci. 1988, 14, 169–218. [Google Scholar]
- Ohloff, G. The importance of minor components in flavors and fragrances. In Proceedings of the 7th International Congress of Essential Oils, Kyoto, Japan, 7–11 October 1977; pp. 69–74. [Google Scholar]
- Tsai, S.-W.; Hsieh, M.-C.; Li, S.; Lin, S.-C.; Wang, S.-P.; Lehman, C.W.; Lien, C.Z.; Lin, C.-C. Therapeutic potential of sclareol in experimental models of rheumatoid arthritis. Int. J. Mol. Sci. 2018, 19, 1351. [Google Scholar] [CrossRef] [Green Version]
- Goossens, A. Cosmetic contact allergens. Cosmetics 2016, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, 342, 59. [Google Scholar]
- Baser, K.H.C. Production of salvia oil in Mediterranean countries. In Sage: The Genus Salvia; Kintzios, S.E., Ed.; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; pp. 252–257. [Google Scholar]
- Stoyanova, A.; Georgiev, E.; Atanasova, T. A Handbook for Laboratory Practice in Essential Oils; UFT Acad. Publ. House: Plovdiv, Bulgaria, 2007. [Google Scholar]
- Balinova-Tzvetkova, A.; Diakov, G. On improved apparatus for microdistillation of rose flowers. Plant. Sci. 1974, 11, 79–85. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas. In Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Clinical Laboratory Standard Institute. Reference Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts. In Approved Guideline M44-A2, 2nd ed.; CLSI: Wayne, PA, USA, 2009. [Google Scholar]
- Clinical Laboratory Standard Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. In Approved Standard M27-A3, 3rd ed.; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical Laboratory Standard Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests. In Approved Standard M2-A9, 9th ed.; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- Clinical Laboratory Standard Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. In Approved Standard M7-A7, 7th ed.; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
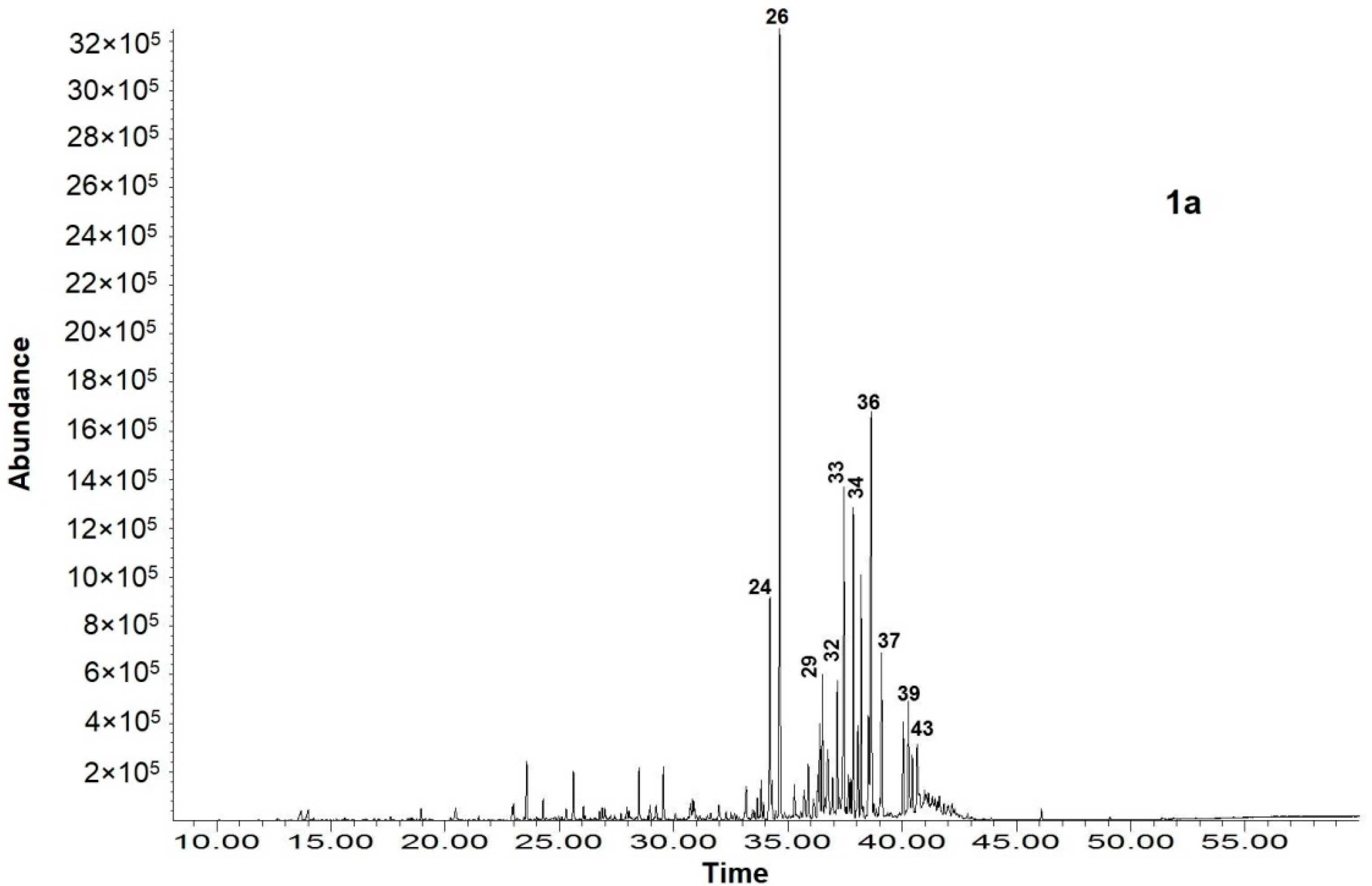
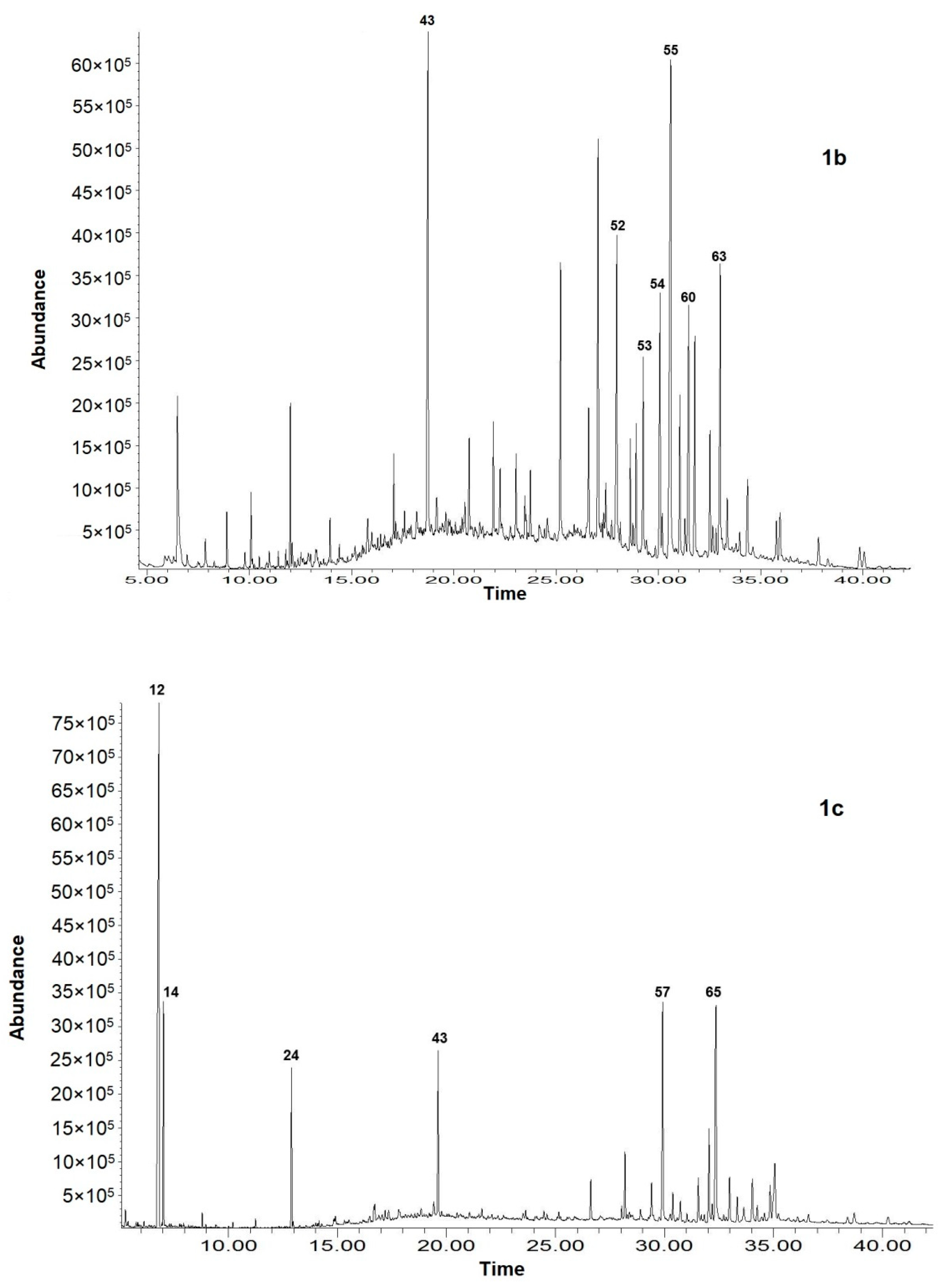

| No | Compounds | RI 1 | Content, % of TIC 2 | ||
|---|---|---|---|---|---|
| EO 3 | Concrete | Resinoid | |||
| 1. | n-Hexanoic acid | 975 | 0.13 ± 0.00 4 | nd 5 | nd |
| 2. | 4-Methylhexanoic acid | 1009 | 0.12 ± 0.00 | nd | nd |
| 3. | Isophorone | 1118 | 0.17 ± 0.00 | nd | nd |
| 4. | Neral ((Z)-3,7-dimethyl-2,6-Octadienal) | 1238 | nd | nd | 0.53 ± 0.01 |
| 5. | Octanoic acid tms 6 | 1258 | nd | 3.88 ± 0.02 | nd |
| 6. | Geranial ((E)-3,7-dimethyl-2,6-Octadienal) | 1267 | nd | nd | 0.10 ± 0.00 |
| 7. | n-Nonanoic acid | 1268 | 0.15 ± 0.00 | nd | nd |
| 8. | 4-[(2E)-2-Butenyl]-1,2-dimethylbenzene | 1312 | 0.43 ± 0.01 | nd | nd |
| 9. | Dehydro-ar-ionene (Naphthalene, 1,2-dihydro-1,1,6-trimethyl-) | 1333 | 0.55 ± 0.01 | nd | nd |
| 10. | α-Ionone | 1340 | 1.62 ± 0.01 | nd | nd- |
| 11. | Nonanoic acid tms | 1355 | nd | 0.20 ± 0.01 | nd |
| 12. | Nicotine | 1366 | 0.18 ± 0.00 | 0.46 ± 0.01 | 32.92 ± 0.16 |
| 13. | trans-β-Damascenone | 1369 | 1.60 ± 0.02 | nd | nd |
| 14. | Solanone | 1374 | 1.46 ± 0.02 | nd | 6.85 ± 0.03 |
| 15. | Naphthalene, 1,2-dihydro-1,5,8-trimethyl- | 1377 | 0.62 ± 0.00 | nd | nd |
| 16. | 3,4-Dehydro-β-ionone ((3E)-4-(2,6,6-Trimethyl-1,3-cyclohexadien-1-yl)-3-buten-2-one) | 1457 | 2.11 ± 0.01 | nd | nd |
| 17. | Malic acid tms | 1501 | nd | 0.22 ± 0.01 | nd |
| 18. | Lilial (3-(4-(tert-Butyl)phenyl)-2-methylpropanal) | 1528 | nd | nd | 0.31 ± 0.01 |
| 19. | n-Hexyl benzoate | 1545 | 1.21 ± 0.01 | nd | nd |
| 20. | Megastigmtrienone 1 | 1559 | nd | 0.84 ± 0.02 | nd |
| 21. | Megastigmtrienone 2 | 1582 | nd | 0.30 ± 0.02 | nd |
| 22. | Megastigmtrienone 3 | 1629 | nd | 1.12 ± 0.07 | nd |
| 23. | Megastigmtrienone 4 | 1656 | nd | 0.26 ± 0.01 | nd |
| 24. | Hexahydrofarnesyl acetone | 1831 | 6.01 ± 0.03 | 0.30 ± 0.01 | 6.51 ± 0.03 |
| 25. | Tetradecanoic acid tms | 1841 | nd | 0.41 ± 0.02 | nd |
| 26. | Diisobutyl phthalate | 1856 | 17.61 ± 0.09 | nd | nd |
| 27. | Dihydroxyacetone dimer tms | 1857 | nd | 0.52 ± 0.03 | 0.61 ± 0.01 |
| 28. | Sclareol oxide | 1876 | nd | 3.67 ± 0.04 | nd |
| 29. | Isopimara-9(11),15-diene | 1905 | 2.60 ± 0.02 | nd | nd |
| 30. | Dibutyl phthalate | 1912 | 3.46 ± 0.02 | nd | 0.90 ± 0.01 |
| 31. | Farnesyl acetone | 1912 | nd | 0.40 ± 0.03 | nd |
| 32. | Cembrene (Thunbergen) | 1937 | 5.58 ± 0.04 | nd | nd |
| 33. | Sclarene | 1973 | 8.42 ± 0.07 | nd | nd |
| 34. | Manoyl oxide | 1994 | 8.11 ± 0.06 | nd | nd |
| 35. | Hexadecanoic acid tms | 2039 | nd | 1.25 ± 0.02 | nd |
| 36. | Manool | 2056 | 14.23 ± 0.08 | 0.50 ± 0.01 | nd |
| 37. | Verticillol | 2090 | 4.82 ± 0.04 | nd | nd |
| 38. | Podocarp-7-en-3-one, 13β-methyl-13-vinyl- | 2100 | 4.43 ± 0.03 | nd | nd |
| 39. | Pimara-7,15-dien-3-one | 2104 | 3.43 ± 0.02 | nd | nd |
| 40. | Podocarp-7-en-3β-ol, 13β-methyl-13-vinyl- | 2127 | 4.68 ± 0.03 | nd | nd |
| 41. | Phytol tms | 2163 | nd | 1.76 ± 0.01 | 1.09 ± 0.01 |
| 42. | α-Linolenic acid | 2168 | nd | 0.62 ± 0.01 | nd |
| 43. | Sclareol | 2222 | 3.55 ± 0.03 | 14.20 ± 0.09 | 6.85 ± 0.04 |
| 44. | 3-α-acetoxy-Manool | 2236 | nd | 0.78 ± 0.06 | nd |
| 45. | 3-α-hydroxy-Manool | 2286 | nd | 0.35 ± 0.03 | nd |
| 46. | Octadecanoic acid tms | 2340 | nd | 1.44 ± 0.02 | nd |
| 47. | n-Tetraicosane | 2400 | nd | 1.85 ± 0.02 | 0.67 ± 0.01 |
| 48. | n-Pentacosane | 2500 | nd | 1.34 ± 0.02 | 0.42 ± 0.01 |
| 49. | n-Hexacosane | 2600 | nd | 1.50 ± 0.02 | 0.92 ± 0.01 |
| 50. | n-Heptacosane | 2700 | nd | 1.27 ± 0.02 | 1.44 ± 0.01 |
| 51. | Diacylglycerol | 2780 | nd | nd | 0.52 ± 0.01 |
| 52. | n-Octacosane | 2800 | nd | 5.10 ± 0.04 | 0.41 ± 0.01 |
| 53. | Squalene | 2812 | nd | 1.63 ± 0.02 | 2.92 ± 0.02 |
| 54. | n-Nonacosane | 2900 | nd | 6.96 ± 0.05 | 0.91 ± 0.01 |
| 55. | n-Triacontane | 3000 | nd | 10.65 ± 0.09 | 0.87 ± 0.01 |
| 56. | n-Hentriacontane | 3100 | nd | 3.88 ± 0.02 | 3.07 ± 0.02 |
| 57. | α-Tocopherol | 3136 | nd | nd | 8.20 ± 0.03 |
| 58. | n-Dotriacontane | 3200 | nd | 5.48 ± 0.06 | 0.72 ± 0.01 |
| 59. | β-Stigmasterol | 3226 | nd | nd | 2.08 ± 0.01 |
| 60. | n-Tritriacontane | 3300 | nd | 6.17 ± 0.05 | 0.63 ± 0.01 |
| 61. | n-Tetratriacontane | 3400 | nd | 4.09 ± 0.03 | 1.56 ± 0.01 |
| 62. | n-Pentatriacontane | 3500 | nd | 3.15 ± 0.02 | 1.71 ± 0.01 |
| 63. | n-Hexatriacontane | 3600 | nd | 6.52 ± 0.03 | 1.37 ± 0.01 |
| 64. | n-Heptatriacontane | 3700 | nd | 0.94 ± 0.01 | 2.73 ± 0.02 |
| 65. | Tridecanoin | 3744 | nd | nd | 6.90 ± 0.05 |
| 66. | n-Octatriacontane | 3800 | nd | 1.48 ± 0.02 | 0.35 ± 0.00 |
| Sum of the identified | 97.28 | 95.49 | 95.07 | ||
| Compounds | Content, % of the identified | ||
|---|---|---|---|
| EO | Concrete | Resinoid | |
| Hydrocarbons | nd | 63.24 | 18.7 |
| Oxygenated hydrocarbons | 4.26 | 11.58 | 15.65 |
| Oxygenated monoterpenes | 9.49 | 0.31 | 7.51 |
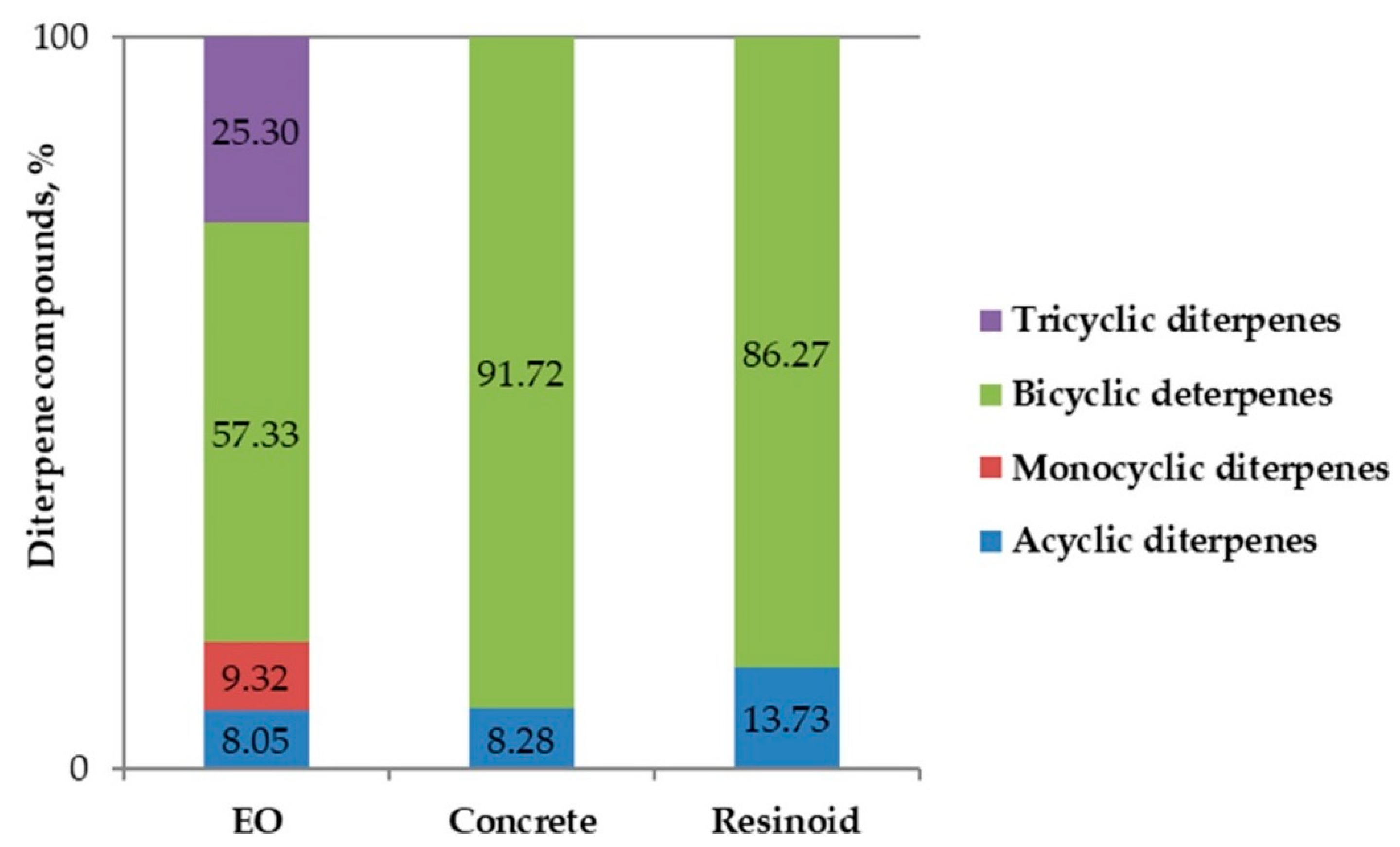
| Diterpenes | 61.52 | 22.68 | 8.35 |
| Triterpenes | nd | 1.71 | 3.07 |
| Phenyl propanoids | 24.55 | nd | 9.9 |
| Alkaloids | 0.18 | 0.48 | 34.63 |
| Total | 100 | 100 | 100 |
| Test Microorganism | Sclareol | Positive Control 4 | |||
|---|---|---|---|---|---|
| IZ ± SD 1 | MIC 2 | MBC/MFC 3 | IZ ± SD | MIC | |
| mm | µg/mL | µg/mL | mm | µg/mL | |
| Bacillus cereus ATCC 11778 | 11.2 ± 0.25 | 1024 | 1024 | 28.3 ± 0.30 | 0.125 |
| Escherichia coli ATCC 8739 | 10.1 ± 0.11 | 1024 | 1024 | 21.0 ± 0.28 | 0.25 |
| Salmonella abony ATCC 6017 | 9.5 ± 0.10 | 1024 | 1024 | 21.0 ± 0.28 | 0.25 |
| Pseudomonas putida ATCC 12633 | nd 5 | nd | nd | 10.3 ± 0.29 | 1.0 |
| Pseudomonas aeruginosa ATCC 9027 | nd | nd | nd | 9.6 ± 0.17 | 1.0 |
| Staphylococcus aureus ATCC 6538 | 14.2 ± 0.06 | 512 | 512 | 31.3 ± 0.29 | 0.125 |
| Proteus mirabilis ATCC 14153 | 10.5 ± 0.11 | 1024 | 1024 | 19.6 ± 0.17 | 0.25 |
| Proteus vulgaris ATCC 13315 | 11.1 ± 0.11 | 1024 | 1024 | 19.6 ± 0.17 | 0.25 |
| Candida albicans АТСС 10231 | 16.5 ± 0.11 | 256 | 512 | 16.6 ± 0.29 | 0.25 |
| Candida glabrata ATCC 90030 | 14.5 ± 0.30 | 256 | 512 | 15.0 ± 3.30 | 4.0 |
| Candida parapsilosis clinical isolate | 14.2 ± 0.10 | 256 | 512 | 15.7 ± 2.90 | 2.0 |
| Candida tropicalis NBIMCC 23 | 15.2 ± 0.10 | 256 | 512 | 14.7 ± 3.90 | 4.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, V.; Ivanova, T.; Stoyanova, A.; Nikolova, V.; Hristeva, T.; Gochev, V.; Yonchev, Y.; Nikolov, N.; Zheljazkov, V.D. Terpenoids in the Essential Oil and Concentrated Aromatic Products Obtained from Nicotiana glutinosa L. Leaves. Molecules 2020, 25, 30. https://doi.org/10.3390/molecules25010030
Popova V, Ivanova T, Stoyanova A, Nikolova V, Hristeva T, Gochev V, Yonchev Y, Nikolov N, Zheljazkov VD. Terpenoids in the Essential Oil and Concentrated Aromatic Products Obtained from Nicotiana glutinosa L. Leaves. Molecules. 2020; 25(1):30. https://doi.org/10.3390/molecules25010030
Chicago/Turabian StylePopova, Venelina, Tanya Ivanova, Albena Stoyanova, Violeta Nikolova, Tsveta Hristeva, Velizar Gochev, Yonko Yonchev, Nikolay Nikolov, and Valtcho D. Zheljazkov. 2020. "Terpenoids in the Essential Oil and Concentrated Aromatic Products Obtained from Nicotiana glutinosa L. Leaves" Molecules 25, no. 1: 30. https://doi.org/10.3390/molecules25010030





