Properties of Biomimetic Artificial Spider Silk Fibers Tuned by PostSpin Bath Incubation
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of the Spinning Dope
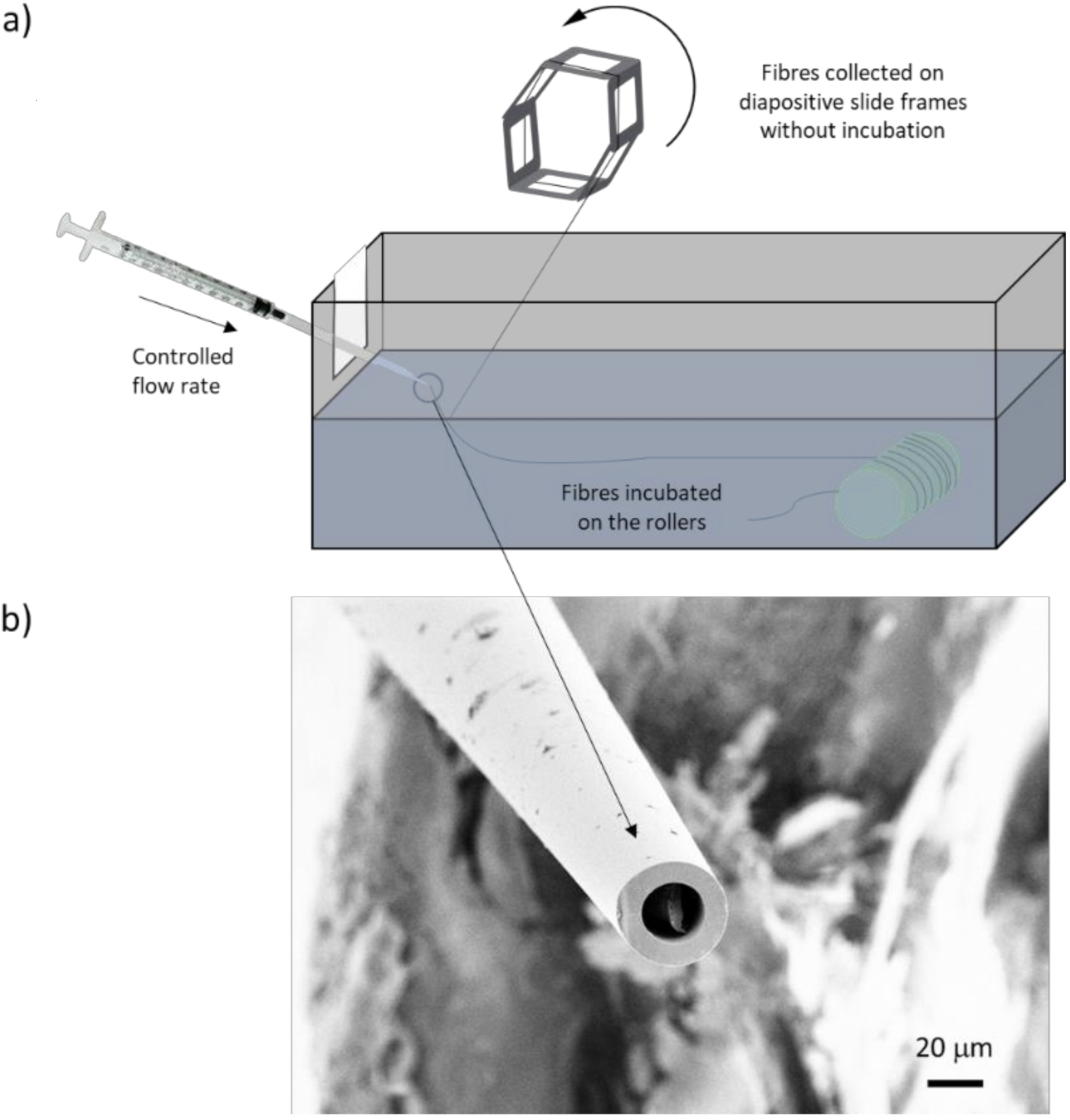
5.2. Artificial Spider Silk Spinning and Incubation
5.3. Solubility Assay
5.4. Fourier Transform Infrared (FTIR) Spectroscopy
5.5. Tensile Tests
5.6. Statistical Analysis
5.6.1. ANOVA Analysis
5.6.2. Light Microscopy
5.6.3. Scanning Electron Microscopy
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Tang, X.; Feng, S.; Zhong, Z.; Yao, J.; Zhong, Y. ZIF-8@SiO2 composite nanofiber membrane with bioinspired spider web-like structure for efficient air pollution control. J. Memb. Sci. 2019, 581, 252–261. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Yan, Y.; Zhang, B. Bionics design and dynamics analysis of space webs based on spider predation. Acta Astronaut. 2019, 159, 294–307. [Google Scholar] [CrossRef]
- Yang, Y.; Greco, G.; Maniglio, D.; Mazzolai, B.; Migliaresi, C.; Pugno, N.M.; Motta, A. Spider (Linothele megatheloides) and silkworm (Bombyx mori) silks: Comparative physical and biological evaluation. Mater. Sci. Eng. C 2020, 107, 110197. [Google Scholar] [CrossRef]
- Dellaquila, A.; Greco, G.; Campodoni, E.; Mazzocchi, M.; Mazzolai, B.; Tampieri, A.; Pugno, N.M.; Sandri, M. Optimized production of a high-performance hybrid biomaterial: Biomineralized spider silk for bone tissue engineering. J. Appl. Polym. Sci. 2019, 137, 48739. [Google Scholar] [CrossRef]
- Allmeling, C.; Jokuszies, A.; Reimers, K.; Kall, S.; Choi, C.Y.; Brandes, G.; Kasper, C.; Scheper, T.; Guggenheim, M.; Vogt, P.M. Spider silk fibres in artificial nerve constructs promote peripheral nerve regeneration. Cell Prolif. 2008, 41, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Widhe, M.; Bysell, H.; Nystedt, S.; Schenning, I.; Malmsten, M.; Johansson, J.; Rising, A.; Hedhammar, M. Recombinant spider silk as matrices for cell culture. Biomaterials 2010, 31, 9575–9585. [Google Scholar] [CrossRef]
- Salehi, S.; Koeck, K.; Scheibel, T. Spider Silk for Tissue Engineering Applications. Molecules 2020, 25, 737. [Google Scholar] [CrossRef] [Green Version]
- Schacht, K.; Vogt, J.; Scheibel, T. Foams Made of Engineered Recombinant Spider Silk Proteins as 3D Scaffolds for Cell Growth. ACS Biomater. Sci. Eng. 2016, 2, 517–525. [Google Scholar] [CrossRef]
- Vepari, C.; David, L.K. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Jokisch, S.; Scheibel, T. Spider silk foam coating of fabric. Pure Appl. Chem. 2017, 89, 1769–1776. [Google Scholar] [CrossRef]
- Arindam, B. Advances in Silk Science and Technology, 1st ed.; The Textile Institute: Boston, BSN, USA, 2015. [Google Scholar]
- Shah, D.U.; Porter, D.; Vollrath, F. Can silk become an effective reinforcing fibre? A property comparison with flax and glass reinforced composites. Compos. Sci. Technol. 2014, 101, 173–183. [Google Scholar] [CrossRef]
- Rainer, F. Biology of Spiders, 3rd ed.; Oxford University Press: London, UK, 2011. [Google Scholar]
- Rising, A.; Johansson, J. Toward spinning artificial spider silk. Nat. Chem. Biol. 2015, 11, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Edlund, A.M.; Jones, J.; Lewis, R.; Quinn, J.C. Economic feasibility and environmental impact of synthetic spider silk production from escherichia coli. N. Biotechnol. 2018, 42, 12–18. [Google Scholar] [CrossRef]
- Fahnestock, S.R.; Bedzyk, L.A. Production of synthetic spider dragline silk protein in Pichia pastoris. Appl. Microbiol. Biotechnol. 1997, 47, 33–39. [Google Scholar] [CrossRef]
- Scheller, J.; Henggeler, D.; Viviani, A.; Conrad, U. Purification of spider silk-elastin from transgenic plants and application for human chondrocyte proliferation. Transgenic Res. 2004, 13, 51–57. [Google Scholar] [CrossRef]
- Menassa, R.; Zhu, H.; Karatzas, C.N.; Lazaris, A.; Richman, A.; Brandle, J. Spider dragline silk proteins in transgenic tobacco leaves: Accumulation and field production. Plant. Biotechnol. J. 2004, 2, 431–438. [Google Scholar] [CrossRef]
- Scheller, J.; Conrad, U. Production of Spider Silk Proteins in Transgenic Tobacco and Potato. Mol. Farming 2005, 19, 171–181. [Google Scholar]
- Fahnestock, S.R.; Irwin, S.L. Synthetic spider dragline silk proteins and their production in Escherichia coli. Appl. Microbiol. Biotechnol. 1997, 47, 23–32. [Google Scholar] [CrossRef]
- Koeppel, A.; Holland, C. Progress and Trends in Artificial Silk Spinning: A Systematic Review. ACS Biomater. Sci. Eng. 2017, 3, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Rising, A.; Widhe, M.; Johansson, J.; Hedhammar, M. Spider silk proteins: Recent advances in recombinant production, structure-function relationships and biomedical applications. Cell. Mol. Life Sci. 2011, 68, 169–184. [Google Scholar] [CrossRef]
- Lazaris, A.; Arcidiacono, S.; Huang, Y.; Zhou, J.F.; Duguay, F.; Chretien, N.; Welsh, E.A.; Soares, J.W.; Karatzas, C.N. Spider silk fibers spun from soluble recombinant silk produced in mammalian cells. Science 2002, 295, 472–476. [Google Scholar] [CrossRef] [Green Version]
- An, B.; Tang-Schomer, M.D.; Huang, W.; He, J.; Jones, J.A.; Lewis, R.V.; Kaplan, D.L. Physical and biological regulation of neuron regenerative growth andnetwork formation on recombinant dragline silks. Biomaterials 2015, 48, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.T.; Fan, B.L.; Yu, S.Y.; Huang, Y.H.; Zhao, Z.H.; Lian, Z.X.; Dai, Y.P.; Wang, L.L.; Liu, Z.L.; Fei, J.; et al. Construct synthetic gene encoding artificial spider dragline silk protein and its expression in milk of transgenic mice. Anim. Biotechnol. 2007, 18, 1–12. [Google Scholar] [CrossRef]
- Hinman, M.B.; Jones, J.A.; Lewis, R.V. Synthetic spider silk: A modular fiber. Trends Biotechnol. 2000, 18, 374–379. [Google Scholar] [CrossRef]
- Hijirida, D.H.; Do, K.G.; Michal, C.; Wong, S.; Zax, D.; Jelinski, L.W. 13C NMR of Nephila clavipes major ampullate silk gland. Biophys. J. 1996, 71, 3442–3447. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.; Chen, G.; Otikovs, M.; Landreh, M.; Nordling, K.; Kronqvist, N.; Westermark, P.; Jörnvall, H.; Knight, S.; Ridderstråle, Y.; et al. Carbonic Anhydrase Generates CO2and H+That Drive Spider Silk Formation Via Opposite Effects on the Terminal Domains. PLOS Biol. 2014, 12, 8. [Google Scholar] [CrossRef]
- Andersson, M.; Jia, Q.; Abella, A.; Lee, X.Y.; Landreh, M.; Purhonen, P.; Hebert, H.; Tenje, M.; Robinson, C.V.; Meng, Q.; et al. Biomimetic spinning of artificial spider silk from a chimeric minispidroin. Nat. Chem. Biol. 2017, 13, 262–264. [Google Scholar] [CrossRef]
- Gonska, N.; Lopez, P.A.; Lozano-picazo, P.; Thorpe, M.; Gustavo, V.; Johansson, J.; Barth, A.; Rising, A. Structure-function relationship of artificial spider silk fibers produced by straining flow spinning. Biomacromolecules 2020, 21, 2116–2124. [Google Scholar] [CrossRef]
- Venkatesan, H.; Chen, J.; Liu, H.; Kim, Y.; Na, S.; Wei, L.; Hu, J. Artificial spider silk is smart like natural one: Having humidity-sensitive shape memory with superior recovery stress. Mater. Chem. Front. 2019, 3, 2472–2482. [Google Scholar] [CrossRef]
- Andreas, B. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar]
- Chirgadze, Y.N.; Nevskaya, N.A. Infrared spectra and resonance interaction of amide-I vibration of the antiparallel-chain pleated sheet. Biopolymers 1976, 15, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Kubelka, J.; Keiderling, T.A. Differentiation of β-sheet-forming structures: Ab initio-based simulations of IR absorption and vibrational CD for model peptide and protein β-sheets. J. Am. Chem. Soc. 2001, 123, 12048–12058. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Pugno, N.M. Mechanical Properties and Weibull Scaling Laws of Unknown Spider Silks. Molecules 2020, 25, 2938. [Google Scholar] [CrossRef] [PubMed]
- Elices, M.; Plaza, G.R.; Pérez-Rigueiro, J.; Guinea, G.V. The hidden link between supercontraction and mechanical behavior of spider silks. J. Mech. Behav. Biomed. Mater. 2011, 4, 658–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagn, F.; Eisoldt, L.; Hardy, J.G.; Vendrely, C.; Coles, M.; Scheibel, T.; Kessler, H. A conserved spider silk domain acts as a molecular switch that controls fibre assembly. Nature 2010, 465, 239–242. [Google Scholar] [CrossRef] [Green Version]
- Kronqvist, N.; Otikovs, M.; Chmyrov, V.; Chen, G.; Andersson, M.; Nordling, K.; Jo, H.; Wennmalm, S.; Widengren, J.; Meng, Q.; et al. Sequential pH-driven dimerization and stabilization of the N-terminal domain enables rapid spider silk formation. Nat. Commun. 2014, 5, 3254. [Google Scholar] [CrossRef] [Green Version]
- Cranford, S.W.; Tarakanova, A.; Pugno, N.M.; Buehler, M.J. Nonlinear material behaviour of spider silk yields robust webs. Nature 2012, 482, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of Β-sheet crystals in silk. Nat. Mater. 2010, 9, 359–367. [Google Scholar] [CrossRef]
- Nova, A.; Keten, S.; Pugno, N.M.; Redaelli, A.; Buehler, M.J. Molecular and nanostructural mechanisms of deformation, strength and toughness of spider silk fibrils. Nano Lett. 2010, 10, 2626–2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Bowen, C.H.; Dai, B.; Sargent, C.J.; Bai, W.; Ladiwala, P.; Feng, H.; Huang, W.; Kaplan, D.L.; Galazka, J.M.; Zhang, F. Recombinant Spidroins Fully Replicate Primary Mechanical Properties of Natural Spider Silk. Biomacromolecules 2018, 19, 3853–3860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollrath, F.; Knight, D.P. Liquid crystal spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef]
- Koeppel, A.; Laity, P.R.; Holland, C. Extensional flow behaviour and spinnability of native silk. Soft Matter 2018, 14, 8838–8845. [Google Scholar] [CrossRef] [Green Version]
- Greco, G.; Pantano, M.F.; Mazzolai, B.; Pugno, N.M. Imaging and mechanical characterization of different junctions in spider orb webs. Sci. Rep. 2019, 9, 5776. [Google Scholar] [CrossRef]
- Blackledge, T.A.; Cardullo, R.A.; Hayashi, C.Y. Polarized light microscopy, variability in spider silk diameters, and the mechanical characterization of spider silk. Invertebr. Biol. 2005, 124, 165–173. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, G.; Francis, J.; Arndt, T.; Schmuck, B.; G. Bäcklund, F.; Barth, A.; Johansson, J.; M. Pugno, N.; Rising, A. Properties of Biomimetic Artificial Spider Silk Fibers Tuned by PostSpin Bath Incubation. Molecules 2020, 25, 3248. https://doi.org/10.3390/molecules25143248
Greco G, Francis J, Arndt T, Schmuck B, G. Bäcklund F, Barth A, Johansson J, M. Pugno N, Rising A. Properties of Biomimetic Artificial Spider Silk Fibers Tuned by PostSpin Bath Incubation. Molecules. 2020; 25(14):3248. https://doi.org/10.3390/molecules25143248
Chicago/Turabian StyleGreco, Gabriele, Juanita Francis, Tina Arndt, Benjamin Schmuck, Fredrik G. Bäcklund, Andreas Barth, Jan Johansson, Nicola M. Pugno, and Anna Rising. 2020. "Properties of Biomimetic Artificial Spider Silk Fibers Tuned by PostSpin Bath Incubation" Molecules 25, no. 14: 3248. https://doi.org/10.3390/molecules25143248
APA StyleGreco, G., Francis, J., Arndt, T., Schmuck, B., G. Bäcklund, F., Barth, A., Johansson, J., M. Pugno, N., & Rising, A. (2020). Properties of Biomimetic Artificial Spider Silk Fibers Tuned by PostSpin Bath Incubation. Molecules, 25(14), 3248. https://doi.org/10.3390/molecules25143248







