Rhodium-Catalyzed Synthesis of Organosulfur Compounds Involving S-S Bond Cleavage of Disulfides and Sulfur
Abstract
:1. Introduction
1.1. Structure and Reactivity of Organic Disulfides
1.2. Synthesis of Organosulfur Compounds Using Disulfides
1.3. Rhodium-Catalyzed Synthesis of Organosulfur Compounds Using Disulfides
1.4. Reversible Nature of Rhodium-Catalyzed Reactions of Disulfides
2. Rhodium-Catalyzed Exchange Reactions of Disulfides
3. Rhodium-Catalyzed Substitution Reactions Using Disulfides
3.1. Substitution Reactions of Thioesters
3.2. Substitution Reactions of 1-Alkynes
3.3. Substitution Reactions of Active Methylene Compounds
3.4. Substitution Reactions of Ketones and Heteroarenes
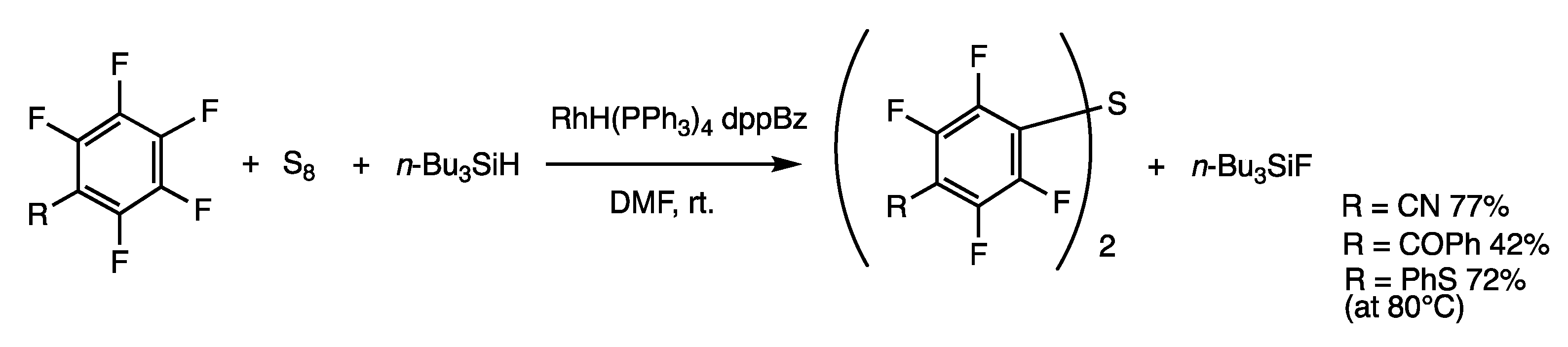
3.5. Substitution Reactions of Aromatic Fluorides
3.6. Substitution Reactions of Organophosphorus Compounds
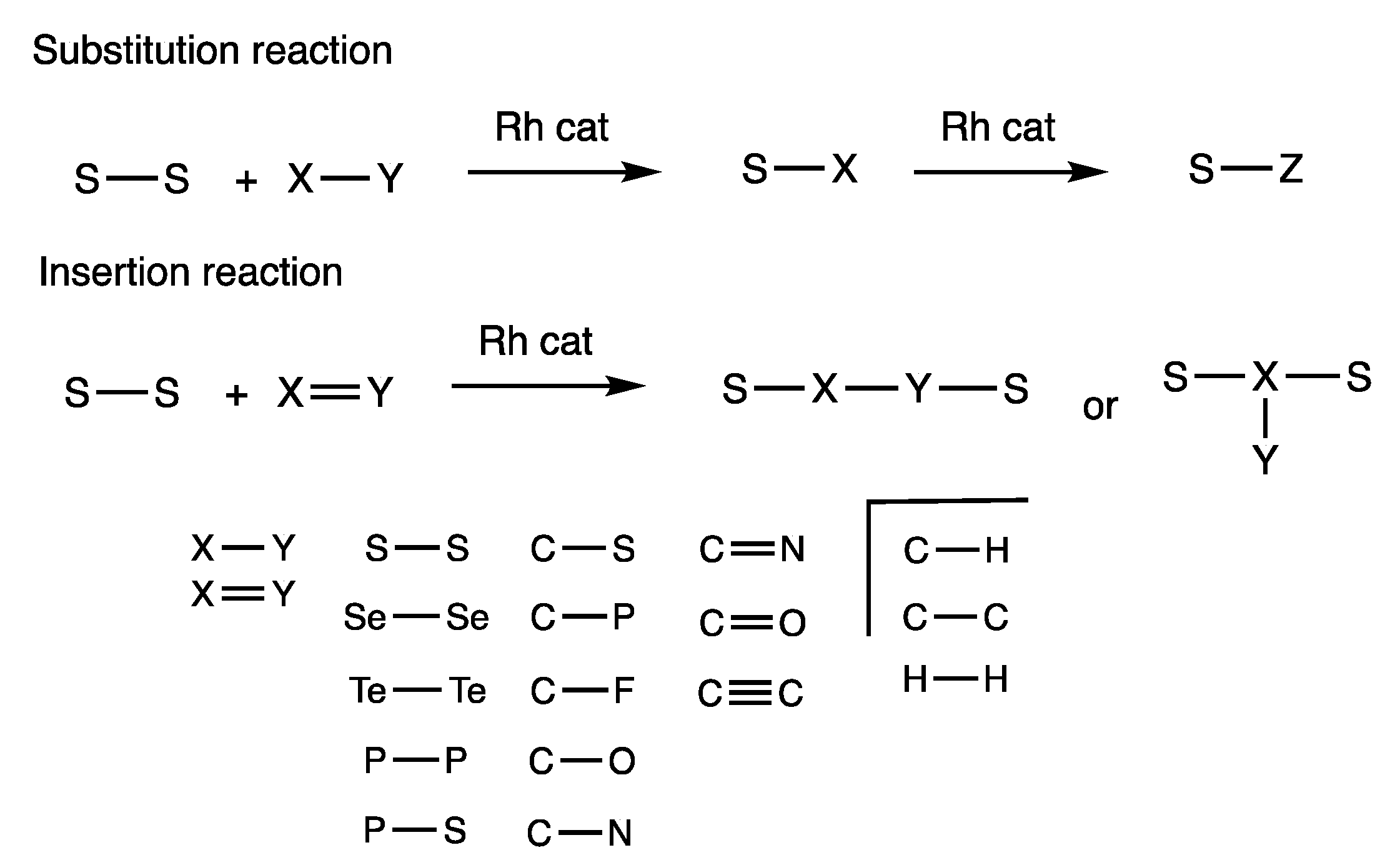
4. Rhodium-Catalyzed Insertion Reactions Using Disulfides
5. Rhodium-Catalyzed Reduction/Oxidation Reactions of Disulfides
6. Rhodium-Catalyzed Reactions of Sulfur
7. Reversible Nature of Rhodium-Catalyzed C-S Bond Formation
7.1. Reactions of 1-Thioalkynes
7.2. Reactions of Thioesters
7.3. Reactions of α-Thioketone
7.4. Reactions of Aryl/Heteroaryl Sulfides
8. Conclusions
8.1. Mechanisms of Rhodium-Catalyzed Substitution and Insertion Reactions of Disulfides
8.2. Rhodium-Catalyzed Synthesis of Diverse Organosulfur Compounds
8.3. Chemical Reaction Network System
Author Contributions
Funding
Conflicts of Interest
References
- Kennepohl, D.; Farmer, S.; Reusch, W.; Soderberg, T.; Schaller, C.P. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Thiols_and_Sulfides/Thiols_and_Sulfides (accessed on 5 August 2020).
- Schaeffer, C.D., Jr.; Strausser, C.A.; Thomsen, M.W.; Yoder, C.H. Data for General, Organic, and Physical Chemistry. Available online: http://chembook.weebly.com/uploads/2/5/7/7/257728/schaeffer_cd_data_for_general_organic_and_physical_chemistry.pdf (accessed on 5 August 2020).
- Benson, S.W. Thermochemistry and Kinetics of Sulfur-Containing Molecules and Radicals. Chem. Rev. 1978, 78, 23–35. [Google Scholar] [CrossRef]
- Koval’, I.V. The Chemistry of Disulfides. Russ. Chem. Rev. 1994, 63, 735–750. [Google Scholar]
- Voronkov, M.G.; Deryagina, E.N. Thermal Reactions of Thiyl Radicals. Russ. Chem. Rev. 1990, 59, 778–791. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Robert, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2014. [Google Scholar]
- Lodish, H.; Berk, A.; Kaiser, C.A.; Krieger, M.; Scott, M.P.; Bretscher, A.; Ploegh, H.; Matsudaira, P. Molecular Cell Biology, 6th ed.; W.H. Freeman and Company: New York, NY, USA, 2008. [Google Scholar]
- Fass, D.; Thorpe, C. Chemistry and Enzymology of Disulfide Cross-Linking in Proteins. Chem. Rev. 2018, 118, 1169–1198. [Google Scholar] [CrossRef]
- Raina, S.; Missiakas, D. Making and Breaking Disulfide Bonds. Ann. Rev. Microbiol. 1997, 51, 179–202. [Google Scholar] [CrossRef]
- Chiu, J.; Hogg, P.J. Allosteric Disulfides: Sophisticated Molecular Structures Enabling Flexible Protein Regulation. J. Biol. Chem. 2019, 294, 2949–2960. [Google Scholar] [CrossRef] [Green Version]
- Hogg, P.J. Disulfide Bonds as Switches for Protein Function. Trends Biochem. Sci. 2003, 28, 210–214. [Google Scholar] [CrossRef]
- Wang, N.; Saidhareddy, P.; Jiang, X. Construction of Sulfur-containing Moieties in the Total Synthesis of Natural Products. Nat. Prod. Rep. 2020, 37, 246–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Glass, R.S.; Char, K.; Pyun, J. Recent Advances in the Polymerization of Elemental Sulphur, Inverse Vulcanization and Methods to Obtain Functional Chalcogenide Hybrid Inorganic/Organic Polymers (CHIPs). Polym. Chem. 2019, 10, 4078–4105. [Google Scholar]
- Bang, E.-K.; Lista, M.; Sforazzini, G.; Sakai, N.; Matile, S. Poly(disulfide)s. Chem. Sci. 2012, 3, 1752–1763. [Google Scholar] [CrossRef]
- Mandal, B.; Basu, B. Recent Advances in S–S Bond Formation. RSC Adv. 2014, 4, 1385–13881. [Google Scholar] [CrossRef]
- Witt, D. Recent Developments in Disulfide Bond Formation. Synthesis 2008, 2491–2509. [Google Scholar] [CrossRef]
- Carey, F.; Sundberg, R.J. Advanced Organic Chemistry: Part A: Structure and Mechanisms, 15th ed.; Springer: Berlin, Germany, 2007. [Google Scholar]
- Otocka, S.; Kwiatkowska, M.; Madalińsska, L.; Kiełbasiński, P. Chiral Organosulfur Ligands/Catalysts with a Stereogenic Sulfur Atom: Applications in Asymmetric Synthesis. Chem. Rev. 2017, 117, 4147–4181. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Klose, I.; Oost, R.; Neuhaus, J.; Maulide, N. Bond-Forming and -Breaking Reactions at Sulfur(IV): Sulfoxides, Sulfonium Salts, Sulfur Ylides, and Sulfinate Salts. Chem. Rev. 2019, 119, 8701–8780. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Feng, B.; Yang, Y. Rh(III)-Catalyzed Direct ortho-Chalcogenation of Phenols and Anilines. J. Org. Chem. 2017, 82, 12430–12438. [Google Scholar] [CrossRef]
- Mukaiyama, T. Oxidation-Reduction Condensation. Angew. Chem. Int. Ed. 1976, 15, 94–103. [Google Scholar] [CrossRef]
- Kuniyasu, H.; Ogawa, A.; Miyazaki, S.; Ryu, I.; Kambe, N.; Sonoda, N. Palladium-Catalyzed Addition and Carbonylative Addition of Diaryl Disulfides and Diselenides to Terminal Acetylenes. J. Am. Chem. Soc. 1991, 113, 9796–9803. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Ananikov, V.P. Transition-Metal-Catalyzed C−S, C−Se, and C−Te Bond Formation via Cross-Coupling and Atom-Economic Addition Reactions. Chem. Rev. 2011, 111, 1596–1636. [Google Scholar] [CrossRef]
- Kondo, T.; Uenoyama, S.; Fujita, K.; Mitsudo, T. First Transition-Metal Complex Catalyzed Addition of Organic Disulfides to Alkenes Enables the Rapid Synthesis of Vicinal-Dithioethers. J. Am. Chem. Soc. 1999, 121, 482–483. [Google Scholar] [CrossRef]
- Qi, X.; Li, Y.; Bai, R.; Lan, Y. Mechanism of Rhodium-Catalyzed C−H Functionalization: Advances in Theoretical Investigation. Acc. Chem. Res. 2017, 50, 2799–2808. [Google Scholar] [CrossRef]
- Arisawa, M.; Tanii, S.; Tazawa, T.; Yamaguchi, M. Synthesis of Unsymmetric HetAr–X–HetAr’ Compounds by Rhodium-Catalyzed Heteroaryl Exchange Reactions. Heterocycles 2017, 94, 2179–2207. [Google Scholar] [CrossRef] [Green Version]
- Arisawa, M. Rhodium-catalyzed Synthesis of Unsymmetric Di(heteroaryl) Compounds via Heteroaryl Exchange Reactions. Phosphorus Sulfur Silicon Rel. Elem. 2019, 194, 643–648. [Google Scholar] [CrossRef]
- Arisawa, M.; Yamaguchi, M. Transition-metal-catalyzed Synthesis of Organosulfur Compounds. Pure Appl. Chem. 2008, 80, 993–1003. [Google Scholar] [CrossRef]
- Arisawa, M. Synthesis of Organosulfides Using Transition-metal-catalyzed Substitution Reactions: To Construct Exergonic Reactions Employing Metal Inorganic and Organic Co-substrate/co-product Methods. Tetrahedron Lett. 2014, 55, 3391–3399. [Google Scholar] [CrossRef] [Green Version]
- Arisawa, M.; Yamaguchi, M. Rhodium-Catalyzed Synthesis of Organosulfur Compounds using Sulfur. Synlett 2019, 30, 1621–1631. [Google Scholar]
- Arisawa, M. Transition-metal-catalyzed Synthesis of Organophosphorus Compounds Involving P-P Bond Cleavage. Synthesis 2020. [Google Scholar] [CrossRef]
- Arisawa, M.; Yamaguchi, M. Rhodium-Catalyzed Disulfide Exchange Reaction. J. Am. Chem. Soc. 2003, 125, 6624–6625. [Google Scholar] [CrossRef]
- Miyagawa, M.; Arisawa, M.; Yamaguchi, M. Equilibrium Shift Induced by Chiral Nanoparticle Precipitation in Rhodium-Catalized Disulfide Exchange Reaction. Tetrahedron 2015, 71, 4920–4926. [Google Scholar] [CrossRef]
- Arisawa, M.; Suwa, A.; Yamaguchi, M. RhCl3-catalyzed Disulfide Exchange Reaction Using Water Solvent in Homogeneous and Heterogeneous Systems. J. Organomet. Chem. 2006, 691, 1159–1168. [Google Scholar] [CrossRef]
- Arisawa, M.; Kuwajima, M.; Suwa, A.; Yamaguchi, M. RhCl3-Catalyzed Disulfide Exchange Reaction of Insulfin and Dithiodiglycolic Acid. Heterocycles 2010, 80, 1239–1248. [Google Scholar]
- Arisawa, M.; Kubota, T.; Yamaguchi, M. Rhodium-Catalyzed Alkylthio Exchange Reaction of Thioester and Disulfide. Tetrahedron Lett. 2008, 49, 1975–1978. [Google Scholar] [CrossRef]
- Arisawa, M.; Yamada, T.; Yamaguchi, M. Rhodium-Catalyzed Interconversion between Acid Fluorides and Thioesters Controlled using Heteroatom Acceptors. Tetrahedron Lett. 2010, 51, 6090–6092. [Google Scholar] [CrossRef]
- Arisawa, M.; Suzuki, R.; Ohashi, K.; Yamaguchi, M. Rhodium-Catalyzed Synthesis of Heteroarylselenyl Esters from Diheteroaryl Diselenides and Acid Fluorides. Asian J. Org. Chem. 2020, 9, 553–556. [Google Scholar] [CrossRef]
- Arisawa, M.; Fujimoto, K.; Morinaka, S.; Yamaguchi, M. Equilibrating C-S Bond Formation by C-H and S-S Bond Metathesis. Rhodium-Catalyzed Alkylthiolation Reaction of 1-Alkynes with Disulfides. J. Am. Chem. Soc. 2005, 127, 12226–12227. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, M.; Nihei, Y.; Yamaguchi, M. Rhodium-Catalyzed Arylthiolation Reaction of Nitroalkanes, Diethyl Malonate, and 1,2-Diphenylethanone with Diaryl Disulfides: Control of Disfavored Equilibrium Reaction. Tetrahedron Lett. 2012, 53, 5729–5732. [Google Scholar] [CrossRef]
- Arisawa, M.; Toriyama, F.; Yamaguchi, M. Rhodium-Catalyzed Organothio Exchange Reaction of α-Organothioketones with Disulfides. Chem. Pharm. Bull. 2010, 58, 1349–1352. [Google Scholar] [CrossRef] [Green Version]
- Arisawa, M.; Toriyama, F.; Yamaguchi, M. An Activated Catalyst RhH(PPh3)4-dppe-Me2S2 for α-Methylthiolation of α-Phenyl Ketones. Heteroatom Chem. 2011, 22, 18–23. [Google Scholar] [CrossRef]
- Arisawa, M.; Suwa, K.; Yamaguchi, M. Rhodium-Catalyzed Methylthio Transfer Reaction between Ketone α-Positions: Reversible Single-Bond Metathesis of C-S and C-H Bonds. Org. Lett. 2009, 11, 625–627. [Google Scholar] [CrossRef]
- Arisawa, M.; Toriyama, F.; Yamaguchi, M. Rhodium-catalyzed α-Methylthiolation Reaction of Unactivated Ketones Using 1,2-Diphenyl−2-Methylthio−1-Ethanone for the Methylthio Transfer Reagent. Tetrahedron 2011, 67, 2305–2312. [Google Scholar] [CrossRef]
- Arisawa, M.; Toriyama, F.; Yamaguchi, M. Rhodium-catalyzed Phenylthiolation Reaction of Heteroaromatic Compounds using α-(Phenylthio) isobutyrophenone. Tetrahedron Lett. 2011, 52, 2344–2347. [Google Scholar] [CrossRef]
- Macgregor, S.A.; Roe, D.C.; Marshall, W.J.; Bloch, K.M.; Bakhmutov, V.I.; Grushin, V.V. The F/Ph Rearrangement Reaction of [(Ph3P)3RhF], the Fluoride Congener of Wilkinson’s Catalyst. J. Am. Chem. Soc. 2005, 127, 15304–15321. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, M.; Suzuki, T.; Ishikawa, T.; Yamaguchi, M. Rhodium-Catalyzed Substitution Reaction of Aryl Fluorides with Disulfides: p-Orientation in the Polyarylthiolation of Polyfluorobenzenes. J. Am. Chem. Soc. 2008, 130, 12214–12215. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, M.; Ono, T.; Yamaguchi, M. Rhodium-Catalyzed Thiophosphinylation and Phosphinylation Reactions of Disulfides and Diselenides. Tetrahedron Lett. 2005, 46, 5669–5671. [Google Scholar] [CrossRef]
- Arisawa, M.; Igarashi, Y.; Kobayashi, H.; Yamada, T.; Bando, K.; Ichikawa, T.; Yamaguchi, M. Equilibrium Shift in the Rhodium-catalyzed Acyl Transfer Reactions. Tetrahedron 2011, 67, 7846–7859. [Google Scholar] [CrossRef]
- Arisawa, M.; Fukumoto, K.; Yamaguchi, M. Rhodium-Catalyzed Phophorylation Reaction of Water-Soluble Disulfides Using Hypodiphosphoric Acid Tetraalkyl Esters in Water. RSC Adv. 2020, 10, 13820–13823. [Google Scholar] [CrossRef]
- Arisawa, M.; Sawahata, K.; Yamada, T.; Sarkar, D.; Yamaguchi, Y. Rhodium-Catalyzed Insertion Reaction of PhP Group of Pentaphenylcyclopentaphosphine with Acyclic and Cyclic Disulfides. Org. Lett. 2018, 20, 938–941. [Google Scholar] [CrossRef]
- Arisawa, M.; Tazawa, T.; Ichinose, W.; Kobayashi, H.; Yamaguchi, M. Rhodium-Catalyzed Synthesis of Dialkyl(Heteroaryl)Phosphine Sulfides by Phosphinylation of Heteroaryl Sulfides. Adv. Synth. Catal. 2018, 360, 3488–3491. [Google Scholar] [CrossRef]
- Arisawa, M.; Watanabe, T.; Yamaguchi, M. Direct Transformation of Organosulfur Compounds to Organophosphorus Compounds: Rhodium-catalyzed Synthesis of 1-Alkynylphosphine Sulfides and Acylphosphine Sulfides. Tetrahedron Lett. 2011, 52, 2410–2412. [Google Scholar] [CrossRef]
- Arisawa, M.; Yamaguchi, M. Addition Reaction of Dialkyl Disulfides to Terminal Alkynes Catalyzed by a Rhodium Complex and Trifluoromethanesulfonic Acid. Org. Lett. 2001, 3, 763–764. [Google Scholar] [CrossRef]
- Arisawa, M.; Kozuki, Y.; Yamaguchi, M. Rhodium-Catalyzed Regio-and Stereoselective 1-Seleno−2-thiolation of 1-Alkynes. J. Org. Chem. 2003, 68, 8964–8967. [Google Scholar] [CrossRef]
- Arisawa, M.; Suwa, A.; Fujimoto, K.; Yamaguchi, M. Transition Metal-Catalyzed Synthesis of (E)−2-(Alkylthio)-alka−1,3-dienes from Allenes and Dialkyl Disulfides with Concomitant Hydride Transfer. Adv. Synth. Catal. 2003, 345, 560–563. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Yang, H.; Fu, H. Rhodium-catalyzed Denitrogenative Thioacetalization of N-Sulfonyl−1,2,3-triazoles with Disulfides: An Entry to Diverse Transformation of Terminal Alkynes. Org. Biomol. Chem 2015, 13, 6149–6153. [Google Scholar] [CrossRef] [PubMed]
- Khanal, H.D.; Kimb, S.H.; Lee, Y.R. Rhodium(II)-catalyzed Direct Sulfenylation of Diazooxindoles with Disulfides. RSC Adv. 2016, 6, 58501–58510. [Google Scholar] [CrossRef]
- Arisawa, M.; Sugata, C.; Yamaguchi, M. Oxidation/Reduction Interconversion of Thiols and Disulfides Using Hydrogen and Oxygen Catalyzed by a Rhodium Complex. Tetrahedron Lett. 2005, 46, 6097–6099. [Google Scholar] [CrossRef]
- Arisawa, M.; Tanaka, K.; Yamaguchi, M. Rhodium-Catalyzed Sulfur Atom Exchange Reaction between Organic Polysulfides and Sulfur. Tetrahedron Lett. 2005, 46, 4797–4800. [Google Scholar] [CrossRef]
- Arisawa, M.; Ashikawa, M.; Suwa, A.; Yamaguchi, M. Rhodium-catalyzed Synthesis of Isothiocyanate from Isonitrile and Sulfur. Tetrahedron Lett. 2005, 46, 1727–1729. [Google Scholar] [CrossRef]
- Adam, W.; Bargon, R.M.; Bosio, S.G.; Schenk, W.A.; Stalke, D. Direct Synthesis of Isothiocyanates from Isonitriles by Molybdenum-Catalyzed Sulfur Transfer with Elemental Sulfur. J. Org. Chem. 2002, 67, 7037–7041. [Google Scholar] [CrossRef]
- Arisawa, M.; Ichikawa, T.; Yamaguchi, M. Rhodium-Catalyzed Synthesis of Diaryl Sulfides Using Aryl Fluorides and Sulfur/Organopolysulfides. Org. Lett. 2012, 14, 5318–5321. [Google Scholar] [CrossRef]
- Adam, W.; Bargon, R.M.; Schenk, W.A. Direct Episulfidation of Alkenes and Allenes with Elemental Sulfur and Thiiranes as Sulfur Sources, Catalyzed by Molybdenum Oxo Complexes. J. Am. Chem. Soc. 2003, 125, 3871–3876. [Google Scholar] [CrossRef]
- Arisawa, M.; Ichikawa, T.; Yamaguchi, M. Synthesis of Thiiranes by Rhodium-Catalyzed Sulfur Addition Reaction to Reactive Alkenes. Chem. Commun. 2015, 51, 8821–8824. [Google Scholar] [CrossRef]
- Arisawa, M.; Ichikawa, T.; Tanii, S.; Yamaguchi, M. Synthesis of Symmetrical and Unsymmetrical 1,4-Dithiins by Rhodium-Catalyzed Sulfur Addition Reaction to Alkynes. Synthesis 2016, 48, 3107–3119. [Google Scholar] [CrossRef] [Green Version]
- Arisawa, M.; Tagami, Y.; Yamaguchi, M. Two Types of Rhodium-Catalyzed CS/CS Metathesis Reactions: Formation of CS/CS Bonds and CC/SS Bonds. Tetrahedron Lett. 2008, 49, 1593–1597. [Google Scholar] [CrossRef]
- Arisawa, M.; Igarashi, Y.; Tagami, Y.; Yamaguchi, M.; Kabuto, C. Rhodium-catalyzed Carbothiolation Reaction of 1-Alkylthio−1-Alkynes. Tetrahedron Lett. 2011, 52, 920–922. [Google Scholar] [CrossRef]
- Li, G.; Arisawa, M.; Yamaguchi, M. Rhodium-Catalyzed Synthesis and Reactions of N-Acylphthalimides. Asian J. Org. Chem. 2013, 2, 983–988. [Google Scholar] [CrossRef]
- Arisawa, M.; Nihei, Y.; Suzuki, T.; Yamaguchi, M. Rhodium-Catalyzed Cleavage Reaction of Aryl Methyl Ethers with Thioesters. Org. Lett. 2012, 14, 855–857. [Google Scholar] [CrossRef]
- Arisawa, M.; Tazawa, T.; Tanii, S.; Horiuchi, K.; Yamaguchi, M. Rhodium-Catalyzed Synthesis of Unsymmetric Di(heteroaryl) Sulfides Using Heteroaryl Ethers and S-Heteroaryl Thioesters via Heteroarylthio Exchange. J. Org. Chem 2017, 82, 804–810. [Google Scholar] [CrossRef]
- Arisawa, M.; Nakai, K.; Yamada, T.; Suzuki, R.; Yamaguchi, M. Synthesis of Cycloalkyl/Steroidal Heteroaryl Sulfides Using Rhodium-Catalyzed Heteroaryl Exchange Reaction. Heterocycles 2020, 100, 104–118. [Google Scholar] [CrossRef]
- Arisawa, M.; Kuwajima, M.; Toriyama, F.; Li, G.; Yamaguchi, M. Rhodium-Catalyzed Acyl-Transfer Reaction between Benzyl Ketones and Thioesters: Synthesis of Unsymmetric Ketones by Ketone CO-C Bond Cleavage and Intermolecular Rearrangement. Org. Lett. 2012, 14, 3804–3807. [Google Scholar] [CrossRef]
- Li, G.; Arisawa, M.; Yamaguchi, M. Rhodium-catalyzed Synthesis of Unsymmetrical Di (Aryl/Heteroaryl) methanes Using Aryl/Heteroarylmethyl Ketons via CO-C. Bond Cleavage. Chem. Commun. 2014, 50, 4328–4330. [Google Scholar] [CrossRef]
- Arisawa, M.; Tanii, S.; Yamada, T.; Yamaguchi, M. Palladium-catalyzed Addition Reaction of Thioesters to Norbornenes. Tetrahedron 2015, 71, 6449–6458. [Google Scholar] [CrossRef]
- Arisawa, M.; Li, G.; Yamaguchi, M. Rhodium-Catalyzed Synthesis of 2,3-Diaryl-1,4-Diketones via Oxidative Coupling of Benzyl Ketones Using α-Thioketone Oxidizing Reagent. Tetrahedron Lett. 2013, 54, 1298–1301. [Google Scholar] [CrossRef]
- Arisawa, M.; Ichikawa, T.; Yamaguchi, M. Synthesis of Unsymmetrical Polyfluorinated Diaryl Dulfides by Rhodium-Catalyzed Aryl Exchange Reaction. Tetrahedron Lett. 2013, 54, 4327–4329. [Google Scholar] [CrossRef]
- Arisawa, M.; Nihei, Y.; Yamaguchi, M. Rhodium-Catalyzed 2-Methylthiolation Reaction of Thiazoles/Oxazoles Using 2-(Methylthio) Thizole. Heterocycles 2015, 90, 939–949. [Google Scholar] [CrossRef]
- Shi, G.; Khan, R.; Zhang, X.; Yang, Y.; Zhan, Y.; Li, J.; Luo, Y.; Fan, B. Rhodium-Catalyzed Direct ortho C–H Thiolation of Cyclic N-Sulfonyl Ketimines. Asian J. Org. Chem. 2020, 9, 788–792. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Zhang, P.; Li, M.-Y.; Chen, Y.-K.; Xu, H.-J.; Zhao, J.; Sun, W.-Y.; Yu, J.-Q.; Lu, Y. Ligand-Promoted RhIII-Catalyzed Thiolation of Benzamides with a Broad Disulfide Scope. Angew. Chem. Int. Ed. 2019, 58, 9099–9103. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fang, Y.; Wang, S.-Y.; Ji, S.-J. RhCl3·3H2O-Catalyzed Ligand-Enabled Highly Regioselective Thiolation of Acrylic Acids. Acs. Catal. 2019, 9, 8910–8915. [Google Scholar] [CrossRef]
- Liu, C.; Fang, Y.; Wang, S.-Y.; Ji, S.-J. Highly Regioselective RhIII-Catalyzed Thiolation of N-Tosyl Acrylamides: General Access to (Z)-β-Alkenyl Sulfides. Org. Lett. 2018, 20, 6112–6116. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Deng, B.; Guo, X.; Li, Z.; Xiang, H.; Zhou, X. Rhodium(III)-Catalyzed Thiolation of Azobenzenes. Asian J. Org. Chem. 2018, 7, 439–443. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, X.-F. Carbonylative Synthesis of 3-Substituted Thiochromenones via Rhodium-Catalyzed [3 + 2 + 1] Cyclization of Different Aromatic Sulfides, Alkynes, and Carbon Monoxide. J. Org. Chem. 2018, 83, 13612–13617. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B. Recent Advances in Organic Reactions Involving Elemental Sulfur. Adv. Synth. Cat. 2017, 359, 1066–1130. [Google Scholar] [CrossRef]
- Maity, S.; Karmakar, U.; Samanta, R. Regiocontrolled Direct C4 and C2-Methylthiolation of Indoles under Rhodium-catalyzed Mild Conditions. Chem. Commun. 2017, 53, 12197–12200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Li, B.; Wang, B. Rh(III)-Catalyzed C7-Thiolation and Selenation of Indolines. J. Org. Chem. 2016, 81, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wu, A.; Wang, M.; Zhu, J. Rhodium(III)-Catalyzed Directed ortho-C−H Bond Functionalization of Aromatic Ketazines via C−S and C−C Coupling. J. Org. Chem. 2015, 80, 10457–10463. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hou, W.; Qin, L.; Du, J.; Feng, H.; Zhou, B.; Li, Y. Rhodium-Catalyzed Directed Sulfenylation of Arene C–H Bonds. Chem. Eur. J. 2014, 20, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Kato, M.; Nishii, Y.; Miura, M. Synthesis of Benzo[b]thiophenes through Rhodium-Catalyzed Three-Component Reaction using Elemental Sulfur. Adv. Synth. Catal. 2020, 362, 1669–1673. [Google Scholar] [CrossRef]
- Arisawa, M.; Sawahata, K.; Ichikawa, T.; Yamaguchi, M. Rhodium-Catalyzed Isomerization and Alkyne Exchange Reactions of 1,4-Dithiins via the 1,2-Ethenedithiolato Rhodium Complex. Organometallics 2018, 37, 3174–3180. [Google Scholar] [CrossRef]
- Gal, A.W.; Gosselink, J.W.; Vollenbroek, F.A. The Oxidative Addition of Unsaturated Cyclic Five-membered Disulphides to RhCl(PPh3)3 and Pt(PPh3)4. Inorg. Chim. Acta. 1979, 32, 235–241. [Google Scholar] [CrossRef]
- Seino, H.; Yoshikawa, T.; Hidai, M.; Mizobe, Y. Preparation of Mononuclear and Dinuclear Rh Hydrotris(pyrazolyl)borato Complexes Containing Arenethiolato Ligands and Conversion of the Mononuclear Complexes into Dinuclear Rh-Rh and Rh-Ir Complexes with Bridging Arenethiolato Ligands. Dalton Trans. 2004, 3593–3600. [Google Scholar] [CrossRef]
- De Croon, M.H.J.M.; van Gaal, H.L.M. Rhodium(l)- and Iridium-(1)-diethyldithiocarbamatoalkene Complexes and their Use in the Preparation of Rhodium(III)- and Iridium(III)-diethyldithiocarbamato Complexes. Inorg. Nucl. Chem. Lett. 1974, 10, 1081–1086. [Google Scholar] [CrossRef]
- Ginsberg, A.P.; Lindsell, W.E.; Sprinkle, C.R.; West, K.W.; Cohen, R.L. Disulfur and Diselenium Complexes of Rhodium and Iridium. Inorg. Chem. 1982, 21, 3666–3681. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Basak, P.; Drew, M.; Gangopadhyay, P.K. In Situ Formation of Ligand 2,2’-[(E)-diazene−1,2-diyldicarbonothioyl] diphenol and Structural Characterization of its Binuclear Rhodium(V) Complex Containing RhO2 +. Chem. Commun. 2010, 46, 7436–7438. [Google Scholar] [CrossRef] [PubMed]
- Duckett, S.B.; Haddleton, D.M.; Jackson, S.A.; Perutz, R.N.; Poliakoff, M.; Upmacis, R.K. Photochemical Oxidative Addition Reactions of (η5-Cyclopentadienyl)-bis(ethene)rhodium with Dihydrogen and Trialkylsilanes: Formation and Isolation of Rhodlum(III) and Rhodium(V) Hydrides. Organometallics 1988, 7, 1526–1532. [Google Scholar] [CrossRef]
- Ruiz, J.; Spencer, C.M.; Mann, B.E.; Taylor, B.F.; Maitlis, P.M. The Synthesis and Characterisation of Dihydridobis(trialkyltin)(pentamethylcyclopentadienyl)-rhodium(V) and -iridium(V) Complexes and Related Reactions. J. Organomet. Chem. 1987, 325, 253–260. [Google Scholar] [CrossRef]
- Fernandez, M.-J.; Bailey, P.M.; Bentz, P.O.; Ricci, J.S.; Koetzle, T.F.; Maitlis, P.M. Synthesis, X-ray, and Low-Temperature Neutron Diffraction Study of a Rhodium(V) Complex: Dihydridobis(triethylsilyl)- pentamethylcyclopentadienylrhodium. J. Am. Chem. Soc. 1984, 106, 5458–5463. [Google Scholar] [CrossRef]
- Tanaka, K.; Ajiki, K. Rhodium-Catalyzed Reaction of Thiols with Polychloroalkanes in the Presence of Triethylamine. Org. Lett. 2005, 7, 1537–1539. [Google Scholar] [CrossRef]
- Japan Soda Industry Association. Available online: https://www.jsia.gr.jp/description/ (accessed on 5 August 2020).
- The Federation of Electric Power Companies of Japan. Available online: https://www.fepc.or.jp/library/data/demand/__icsFiles/afieldfile/2016/04/28/juyou_k_fy2015.pdf (accessed on 5 August 2020).
- Trost, B.M. The Atom Economy-A Search for Synthetic Efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]























































© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arisawa, M.; Yamaguchi, M. Rhodium-Catalyzed Synthesis of Organosulfur Compounds Involving S-S Bond Cleavage of Disulfides and Sulfur. Molecules 2020, 25, 3595. https://doi.org/10.3390/molecules25163595
Arisawa M, Yamaguchi M. Rhodium-Catalyzed Synthesis of Organosulfur Compounds Involving S-S Bond Cleavage of Disulfides and Sulfur. Molecules. 2020; 25(16):3595. https://doi.org/10.3390/molecules25163595
Chicago/Turabian StyleArisawa, Mieko, and Masahiko Yamaguchi. 2020. "Rhodium-Catalyzed Synthesis of Organosulfur Compounds Involving S-S Bond Cleavage of Disulfides and Sulfur" Molecules 25, no. 16: 3595. https://doi.org/10.3390/molecules25163595
APA StyleArisawa, M., & Yamaguchi, M. (2020). Rhodium-Catalyzed Synthesis of Organosulfur Compounds Involving S-S Bond Cleavage of Disulfides and Sulfur. Molecules, 25(16), 3595. https://doi.org/10.3390/molecules25163595





