High Efficacy of Ozonated Oils on the Removal of Biofilms Produced by Methicillin-Resistant Staphylococcus aureus (MRSA) from Infected Diabetic Foot Ulcers
Abstract
1. Introduction
2. Results
2.1. Determination of Peroxide Value
2.2. Fatty Acid Content Analysis
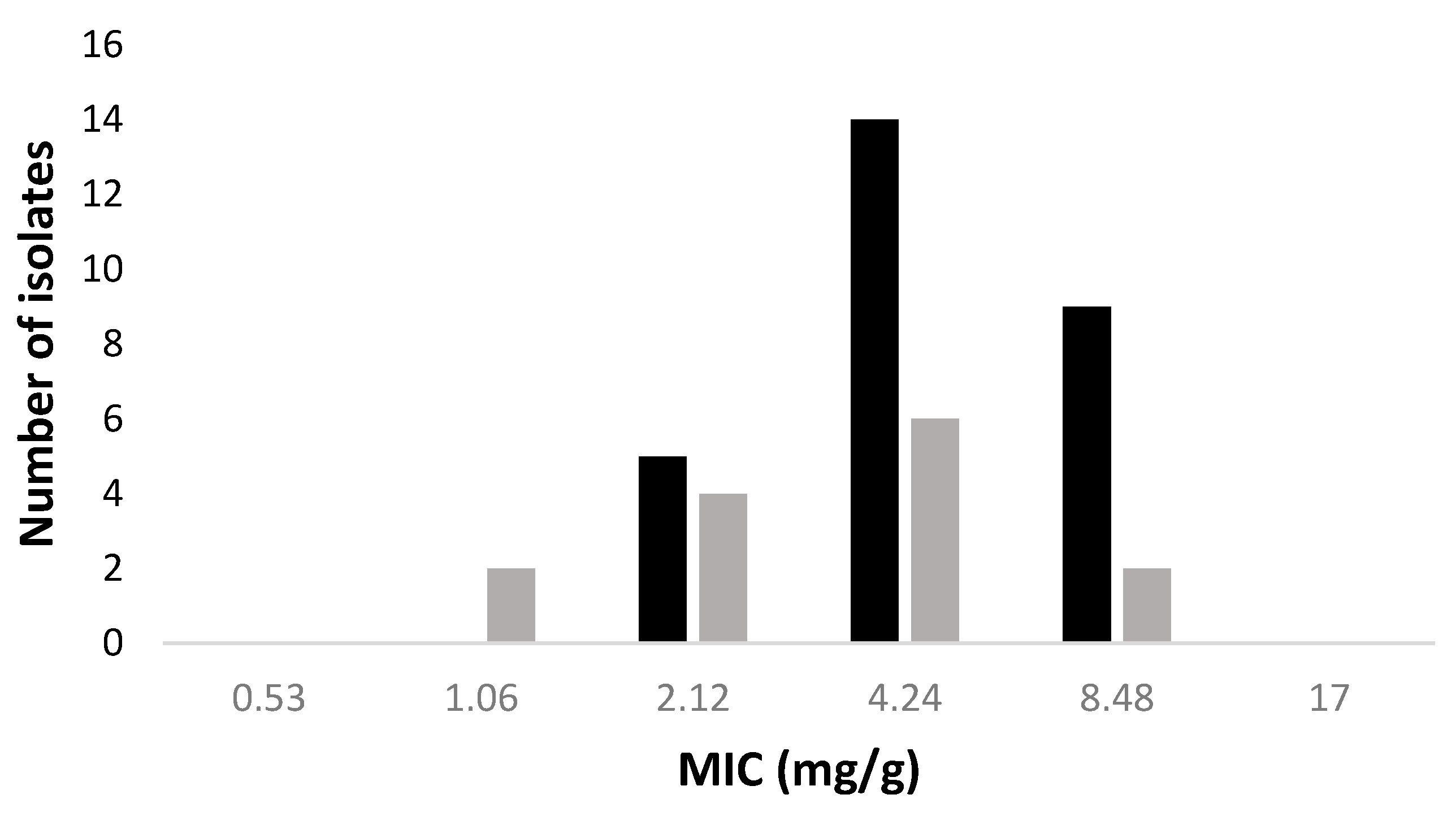
2.3. Antimicrobial and Antibiofilm Activities
3. Discussion
4. Material and Methods
4.1. Oil Ozonisation Procedure
4.2. Determination of Peroxide Value
4.3. Fatty Acid Methyl Esters Analysis
4.4. Bacterial Isolates
Antibacterial Susceptibility Test
4.5. Antibiofilm Effect of Ozonated Oils Using Crystal Violet (CV) Assay
4.5.1. Effect on Biofilm Formation Ability
4.5.2. Effect on Established Biofilms
4.5.3. Assessment of Biofilm Biomass
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, T.; Arora, N.; Puri, G.; Aravinda, K.; Dixit, A.; Jatti, D. Efficacy of ozonized olive oil in the management of oral lesions and conditions: A clinical trial. Contemp. Clin. Dent. 2016, 7, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Grootveld, M.; Baysan, A.; Sidiiqui, N.; Sim, J.; Silwood, C.; Lynch, E. History of the clinical applications of ozone. In Ozone: The Revolution in Dentistry; Quintessence Publishing Co.: London, UK, 2004; pp. 23–30. [Google Scholar]
- Guzzon, R.; Nardin, T.; Micheletti, O.; Nicolini, G.; Larcher, R. Antimicrobial activity of ozone. Effectiveness against the main wine spoilage microorganisms and evaluation of impact on simple phenols in wine. Aust. J. Grape Wine Res. 2013, 19, 180–188. [Google Scholar] [CrossRef]
- Monzillo, V.; Lallitto, F.; Russo, A.; Poggio, C.; Scribante, A.; Arciola, C.R.; Bertuccio, F.R.; Colombo, M. Ozonized Gel Against Four Candida Species: A Pilot Study and Clinical Perspectives. Materials 2020, 13, 1731. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.Á.; Elias, S.T.; da Silva, S.M.M.; Magalhães, P.O.; Macedo, S.B.; Ribeiro, A.P.D.; Guerra, E.N.S. In vitro evaluation of wound healing and antimicrobial potential of ozone therapy. J. Cranio-Maxillofac. Surg. 2017, 45, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Su, C.; Zhou, J.; Xu, L.; Qian, Y.; Chen, H. Effects and mechanisms of ultraviolet, chlorination, and ozone disinfection on antibiotic resistance genes in secondary effluents of municipal wastewater treatment plants. Chem. Eng. J. 2017, 317, 309–316. [Google Scholar] [CrossRef]
- Stoker, G. The surgical uses of ozone. Lancet 1916, 188, 712. [Google Scholar] [CrossRef]
- Smith, N.L.; Wilson, A.L.; Gandhi, J.; Vatsia, S.; Khan, S.A. Ozone therapy: An overview of pharmacodynamics, current research, and clinical utility. Med. Gas Res. 2017, 7, 212. [Google Scholar]
- Elvis, A.M.; Ekta, J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011, 2, 66. [Google Scholar] [CrossRef]
- Izadi, M.; Kheirjou, R.; Mohammadpour, R.; Aliyoldashi, M.H.; Moghadam, S.J.; Khorvash, F.; Jafari, N.J.; Shirvani, S.; Khalili, N. Efficacy of comprehensive ozone therapy in diabetic foot ulcer healing. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 822–825. [Google Scholar] [CrossRef]
- Silva, V.; Almeida, F.; Carvalho, J.A.; Castro, A.P.; Ferreira, E.; Manageiro, V.; Tejedor-Junco, M.T.; Caniça, M.; Igrejas, G.; Poeta, P. Emergence of community-acquired methicillin-resistant Staphylococcus aureus EMRSA-15 clone as the predominant cause of diabetic foot ulcer infections in Portugal. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 179–186. [Google Scholar] [CrossRef]
- Cervantes-García, E.; García-González, R.; Reséndiz-Albor, A.; Salazar-Schettino, P.M. Infections of Diabetic Foot Ulcers with Methicillin-Resistant Staphylococcus aureus. Int. J. Low. Extrem. Wounds 2015, 14, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Capelo, J.L.; Igrejas, G.; Poeta, P. Molecular Epidemiology of Staphylococcus aureus Lineages in Wild Animals in Europe: A Review. Antibiotics 2020, 9, 122. [Google Scholar] [CrossRef]
- Silva, V.; Hermenegildo, S.; Ferreira, C.; Manaia, C.M.; Capita, R.; Alonso-Calleja, C.; Carvalho, I.; Pereira, J.E.; Maltez, L.; Capelo, J.L. Genetic Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Human Bloodstream Infections: Detection of MLSB Resistance. Antibiotics 2020, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Dakheel, K.H.; Abdul Rahim, R.; Neela, V.K.; Al-Obaidi, J.R.; Hun, T.G.; Yusoff, K. Methicillin-resistant Staphylococcus aureus biofilms and their influence on bacterial adhesion and cohesion. Biomed Res. Int. 2016, 2016, 4708425. [Google Scholar] [CrossRef]
- Perez, A.P.; Perez, N.; Lozano, C.M.S.; Altube, M.J.; de Farias, M.A.; Portugal, R.V.; Buzzola, F.; Morilla, M.J.; Romero, E.L. The anti MRSA biofilm activity of Thymus vulgaris essential oil in nanovesicles. Phytomedicine 2019, 57, 339–351. [Google Scholar] [CrossRef]
- Hasan, N.; Cao, J.; Lee, J.; Naeem, M.; Hlaing, S.P.; Kim, J.; Jung, Y.; Lee, B.-L.; Yoo, J.-W. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater. Sci. Eng. C 2019, 103, 109741. [Google Scholar] [CrossRef]
- Choi, M.; Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Yoo, J.-W. Chitosan-based nitric oxide-releasing dressing for anti-biofilm and in vivo healing activities in MRSA biofilm-infected wounds. Int. J. Biol. Macromol. 2020, 142, 680–692. [Google Scholar] [CrossRef]
- Uysal, B. Ozonated olive oils and the troubles. J. Intercult. Ethnopharmacol. 2014, 3, 49–50. [Google Scholar] [CrossRef]
- Sega, A.; Zanardi, I.; Chiasserini, L.; Gabbrielli, A.; Bocci, V.; Travagli, V. Properties of sesame oil by detailed 1H and 13C NMR assignments before and after ozonation and their correlation with iodine value, peroxide value, and viscosity measurements. Chem. Phys. Lipids 2010, 163, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, J.; Johansson, B.; Johannessen, E.; Friman, R.; Broniarz-Press, L.; Rosenholm, J.B. Characterization of ozonated vegetable oils by spectroscopic and chromatographic methods. Chem. Phys. Lipids 2008, 151, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Günaydın, Y.; Sevim, H.; Tanyolaç, D.; Gürpınar, Ö.A. Ozonated Olive Oil with a High Peroxide Value for Topical Applications: In-Vitro Cytotoxicity Analysis with L929 Cells. Ozone Sci. Eng. 2018, 40, 37–43. [Google Scholar] [CrossRef]
- Pai, S.A.; Gagangras, S.A.; Kulkarni, S.S.; Majumdar, A.S. Potential of ozonated sesame oil to augment wound healing in rats. Indian J. Pharm. Sci. 2014, 76, 87. [Google Scholar]
- De Almeida, N.R.; Beatriz, A.; Micheletti, A.C.; de Arruda, E.J. Ozonized vegetable oils and therapeutic properties: A review. Orbital Electron. J. Chem. 2012, 4, 313–326. [Google Scholar] [CrossRef]
- Dourou, M.; Mizerakis, P.; Papanikolaou, S.; Aggelis, G. Storage lipid and polysaccharide metabolism in Yarrowia lipolytica and Umbelopsis isabellina. Appl. Microbiol. Biotechnol. 2017, 101, 7213–7226. [Google Scholar] [CrossRef]
- Beltrán, G.; del Rio, C.; Sánchez, S.; Martínez, L. Influence of Harvest Date and Crop Yield on the Fatty Acid Composition of Virgin Olive Oils from Cv. Picual. J. Agric. Food Chem. 2004, 52, 3434–3440. [Google Scholar] [CrossRef]
- Kieliszek, M.; Dourou, M. Effect of Selenium on the Growth and Lipid Accumulation of Yarrowia lipolytica Yeast. Biol. Trace Elem. Res. 2020, 1–12. [Google Scholar] [CrossRef]
- Sadras, V.O.; Villalobos, F.J. Physiological characteristics related to yield improvement in sunflower (Helianthus annuus L.). In Genetic Improvement of Field Crops: Current Status and Development; Slafer, G., Ed.; Marcel Dekker: New York, NY, USA, 1994; pp. 287–320. [Google Scholar]
- Díaz, M.F.; Sánchez, Y.; Gómez, M.; Hernández, F.; Veloso, M.C.d.C.; Pereira, P.A.d.P.; Mangrich, A.S.; de Andrade, J.B. Physicochemical characteristics of ozonated sunflower oils obtained by different procedures. Grasas y Aceites 2012, 63, 466–474. [Google Scholar] [CrossRef]
- Uzun, H.; Kaynak, E.G.; Ibanoglu, E.; Ibanoglu, S. Chemical and structural variations in hazelnut and soybean oils after ozone treatments. Grasas y Aceites 2018, 69, 253. [Google Scholar] [CrossRef]
- Liu, H.-R.; White, P.J. Oxidative stability of soybean oils with altered fatty acid compositions. J. Am. Oil Chem. Soc. 1992, 69, 528–532. [Google Scholar] [CrossRef]
- Díaz, M.F.; Hernández, R.; Martínez, G.; Vidal, G.; Gómez, M.; Fernández, H.; Garcés, R. Comparative study of ozonized olive oil and ozonized sunflower oil. J. Braz. Chem. Soc. 2006, 17, 403–407. [Google Scholar] [CrossRef]
- Zahardis, J.; LaFranchi, B.W.; Petrucci, G.A. The heterogeneous reaction of particle-phase methyl esters and ozone elucidated by photoelectron resonance capture ionization: Direct products of ozonolysis and secondary reactions leading to the formation of ketones. Int. J. Mass Spectrom. 2006, 253, 38–47. [Google Scholar] [CrossRef]
- Price, L.B.; Stegger, M.; Hasman, H.; Aziz, M.; Larsen, J.; Andersen, P.S.; Pearson, T.; Waters, A.E.; Foster, J.T.; Schupp, J.; et al. Staphylococcus aureus CC398: Host adaptation and emergence of methicillin resistance in livestock. MBio 2012, 3, e00305–e00311. [Google Scholar] [CrossRef] [PubMed]
- Ugazio, E.; Tullio, V.; Binello, A.; Tagliapietra, S.; Dosio, F. Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques. Molecules 2020, 25, 334. [Google Scholar] [CrossRef]
- Sechi, L.A.; Lezcano, I.; Nunez, N.; Espim, M.; Duprè, I.; Pinna, A.; Molicotti, P.; Fadda, G.; Zanetti, S. Antibacterial activity of ozonized sunflower oil (Oleozon). J. Appl. Microbiol. 2001, 90, 279–284. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Cardoso, C.C.; Caputo, L.R.; Carvalho, J.C.T.; Fiorini, J.E.; Schneedorf, J.M. Cicatrizing and antimicrobial properties of an ozonised oil from sunflower seeds. Inflammopharmacology 2004, 12, 261–270. [Google Scholar] [CrossRef]
- Skalska, K.; Ledakowicz, S.; Perkowski, J.; Sencio, B. Germicidal Properties of Ozonated Sunflower Oil. Ozone Sci. Eng. 2009, 31, 232–237. [Google Scholar] [CrossRef]
- Akdeniz, S.S.; Beyler, E.; Korkmaz, Y.; Yurtcu, E.; Ates, U.; Araz, K.; Sahin, F.I.; Torun, O.Y. The effects of ozone application on genotoxic damage and wound healing in bisphosphonate-applied human gingival fibroblast cells. Clin. Oral Investig. 2018, 22, 867–873. [Google Scholar] [CrossRef]
- Serio, F.; Pizzolante, G.; Cozzolino, G.; D’Alba, M.; Bagordo, F.; De Giorgi, M.; Grassi, T.; Idolo, A.; Guido, M.; De Donno, A. A New Formulation Based on Ozonated Sunflower Seed Oil: In Vitro Antibacterial and Safety Evaluation. Ozone Sci. Eng. 2017, 39, 139–147. [Google Scholar] [CrossRef]
- Carata, E. Powerful Properties of Ozonated Extra Virgin Olive Oil. In Herbal Medicine; Tenuzzo, B.A., Ed.; IntechOpen: Rijeka, Croatia, 2019; p. 12. ISBN 978-1-78984-783-3. [Google Scholar]
- Kanaan, M.H.G. Antibacterial effect of ozonated water against methicillin-resistant Staphylococcus aureus contaminating chicken meat in Wasit Province, Iraq. Vet. World 2018, 11, 1445–1453. [Google Scholar] [CrossRef]
- Restaino, L.; Frampton, E.W.; Hemphill, J.B.; Palnikar, P. Efficacy of ozonated water against various food-related microorganisms. Appl. Environ. Microbiol. 1995, 61, 3471–3475. [Google Scholar] [CrossRef] [PubMed]
- Cihalova, K.; Chudobova, D.; Michalek, P.; Moulick, A.; Guran, R.; Kopel, P.; Adam, V.; Kizek, R. Staphylococcus aureus and MRSA Growth and Biofilm Formation after Treatment with Antibiotics and SeNPs. Int. J. Mol. Sci. 2015, 16, 24656–24672. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.; Rudkin, J.K.; Black, N.S.; Gallagher, L.; O’Neill, E.; O’Gara, J.P. Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; De Meyer, L.; Nelis, H.J.; Coenye, T. Biofilm inhibitory and eradicating activity of wound care products against Staphylococcus aureus and Staphylococcus epidermidis biofilms in an in vitro chronic wound model. J. Appl. Microbiol. 2013, 114, 1833–1842. [Google Scholar] [CrossRef]
- Bialoszewski, D.; Pietruczuk-Padzik, A.; Kalicinska, A.; Bocian, E.; Czajkowska, M.; Bukowska, B.; Tyski, S. Activity of ozonated water and ozone against Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Med. Sci. Monit. 2011, 17, BR339–BR344. [Google Scholar] [CrossRef]
- Koyama, R.; Okuda, K.; Matsushita, K.; Beppu, M.; Mizunoe, Y. Antimicrobial and Antibiofilm Effects of Ozonated Water for Prevention and Treatment of Bone and Joint Infections. J. St. Marian. Univ. 2015, 6, 1–7. [Google Scholar] [CrossRef][Green Version]
- Al-Saadi, H.; Potapova, I.; Rochford, E.T.J.; Moriarty, T.F.; Messmer, P. Ozonated saline shows activity against planktonic and biofilm growing Staphylococcus aureus in vitro: A potential irrigant for infected wounds. Int. Wound J. 2016, 13, 936–942. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Di Ilio, C.; De Laurenzi, V. Die for the community: An overview of programmed cell death in bacteria. Cell Death Dis. 2015, 6, e1609. [Google Scholar] [CrossRef]
- Kuvshinov, D.; Siswanto, A.; Lozano-Parada, J.; Zimmerman, W.B. Efficient compact micro DBD plasma reactor for ozone generation for industrial application in liquid and gas phase systems. World Acad. Sci. Eng. Technol. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2014, 8, 80–83. [Google Scholar]
- Bicalho, B.; David, F.; Rumplel, K.; Kindt, E.; Sandra, P. Creating a fatty acid methyl ester database for lipid profiling in a single drop of human blood using high resolution capillary gas chromatography and mass spectrometry. J. Chromatogr. A 2008, 1211, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.S.; Casal, S.; Pereira, J.A.; Seabra, R.M.; Oliveira, B.P.P. Determination of Sterol and Fatty Acid Compositions, Oxidative Stability, and Nutritional Value of Six Walnut (Juglans regia L.) Cultivars Grown in Portugal. J. Agric. Food Chem. 2003, 51, 7698–7702. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Almeida, F.; Silva, A.; Correia, S.; Carvalho, J.A.; Castro, A.P.; Ferreira, E.; Manageiro, V.; Caniça, M.; Igrejas, G.; et al. First report of linezolid-resistant cfr-positive methicillin-resistant Staphylococcus aureus in humans in Portugal. J. Glob. Antimicrob. Resist. 2019, 17, 323–325. [Google Scholar] [CrossRef]
Sample Availability: Not available from the authors. |

| Sample | Ozonation time | Peroxide Value 1 |
|---|---|---|
| CTR | 0 | 3.9 ± 0.0 a |
| 1 | 10 min | 44.4 ± 3.4 b |
| 2 | 20 min | 69.5 ± 4.6 c |
| 3 | 40 min | 83.4 ± 5.3 c |
| 4 | 80 min | 101.7 ± 4.7 d |
| 5 | 160 min | 113.5 ± 3.7 d |
| 6 | 320 min | 220.7 ± 5.6 e |
| Fatty acid | Sample CTR | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 |
|---|---|---|---|---|---|---|---|
| C14:0 | 0.05 ± 0.00 a | 0.04 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.33 ± 0.02 b |
| C16:0 | 8.45 ± 0.25 a | 8.65 ± 0.11 a | 8.91 ± 0.08 a | 9.23 ± 0.08 a,b | 9.94 ± 0.05 b | 11.53 ± 0.18 c | 14.67 ± 0.53 d |
| C16:1 | 0.41 ±0.00 a | 0.42 ± 0.00 a | 0.40 ± 0.03 a | 0.06 ± 0.03 b,c | 0.06 ± 0.00 b,c | 0.04 ± 0.00 b | 0.12 ± 0.03 c |
| C17:0 | 0.24 ±0,00 a | 0.24 ± 0.00 a | 0.41 ± 0.00 a,b | 0.78 ± 0.05 b | 1.57 ± 0.02 c | 2.72 ± 0.07 d | 6.58 ± 0.37 e |
| C17:1 | 0.06 ±0.00 a | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.05 ± 0.00 a | 0.03 ± 0.03 a | 0.05 ± 0.01 a | 0.65 ± 0.03 b |
| C18:0 | 3.24 ± 0.14 a | 3.21 ± 0.00 a | 3.41 ± 0.01 a | 3.82 ± 0.08 a | 4.68 ± 0.02 b | 6.52 ± 0.17 c | 8.58 ± 0.40 d |
| C18:1n9c | 53.19 ± 0.68 a | 52.11 ± 0.68 a,b | 51.02 ± 0.07 b,c | 50.19 ± 0.38 c,d | 46.56 ± 0.16 e | 40.07 ± 0.51 f | 17.50 ± 0.60 g |
| C18:1n7 | 2.29 ± 0.00 a | 2.18 ± 0.04 a,b | 2.16 ± 0.01 a,b | 2.07 ± 0.09 b,c | 1.98 ± 0.02 c | 1.76 ± 0.04 d | 0.49 ± 0.02 e |
| C18:2n6c | 30.70 ± 1.13 a | 29.72 ± 0.39 a | 28.89 ± 0.05 a,b | 27.35 ± 0.42 b | 23.24 ± 0.13 c | 17.05 ± 0.48 d | 5.87 ± 0.28 e |
| C18:3n3 | 0.17 ± 0.02 a | 0.16 ± 0.01 a | 0.18 ± 0.01 a | 0.16 ± 0.02 a | 0.15 ± 0.01 a,b | 0.10 ± 0.02 b | n.d. |
| C20:0 | 0.31 ± 0.01 a | 0.31 ± 0.02 a | 0.31 ± 0.00 a | 0.30 ± 0.03 a | 0.34 ± 0.07 a | 0.42 ± 0.03 a | 0.39 ± 0.02 a |
| C20:1 | 0.27 ± 0.01 a | 0.27 ± 0.01 a | 0.27 ± 0.00 a | 0.24 ± 0.03 a,b | 0.19 ± 0.02 b,c | 0.12 ± 0.02 c | 0.14 ± 0.03 c |
| C22:0 | 0.50 ± 0.05 a | 0.47 ± 0.08 a | 0.60 ± 0.01 a,b | 0.84 ± 0.08 b | 1.27 ± 0.01 c | 1.93 ± 0.09 d | 2.29 ± 0.13 e |
| C24:0 | 0.13 ± 0.00 a | 0.12 ± 0.00 a | 0.13 ± 0.02 a | 0.13 ± 0.03 a | 0.21 ± 0.06 a | 0.39 ± 0.07 b | 0.51 ± 0.01 b |
| Total ID | 100.00 | 97.99 | 96.79 | 95.26 | 90.09 | 82.76 | 58.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.; Peirone, C.; Amaral, J.S.; Capita, R.; Alonso-Calleja, C.; Marques-Magallanes, J.A.; Martins, Â.; Carvalho, Á.; Maltez, L.; Pereira, J.E.; et al. High Efficacy of Ozonated Oils on the Removal of Biofilms Produced by Methicillin-Resistant Staphylococcus aureus (MRSA) from Infected Diabetic Foot Ulcers. Molecules 2020, 25, 3601. https://doi.org/10.3390/molecules25163601
Silva V, Peirone C, Amaral JS, Capita R, Alonso-Calleja C, Marques-Magallanes JA, Martins Â, Carvalho Á, Maltez L, Pereira JE, et al. High Efficacy of Ozonated Oils on the Removal of Biofilms Produced by Methicillin-Resistant Staphylococcus aureus (MRSA) from Infected Diabetic Foot Ulcers. Molecules. 2020; 25(16):3601. https://doi.org/10.3390/molecules25163601
Chicago/Turabian StyleSilva, Vanessa, Cecília Peirone, Joana S. Amaral, Rosa Capita, Carlos Alonso-Calleja, José A. Marques-Magallanes, Ângela Martins, Águeda Carvalho, Luís Maltez, José Eduardo Pereira, and et al. 2020. "High Efficacy of Ozonated Oils on the Removal of Biofilms Produced by Methicillin-Resistant Staphylococcus aureus (MRSA) from Infected Diabetic Foot Ulcers" Molecules 25, no. 16: 3601. https://doi.org/10.3390/molecules25163601
APA StyleSilva, V., Peirone, C., Amaral, J. S., Capita, R., Alonso-Calleja, C., Marques-Magallanes, J. A., Martins, Â., Carvalho, Á., Maltez, L., Pereira, J. E., Capelo, J. L., Igrejas, G., & Poeta, P. (2020). High Efficacy of Ozonated Oils on the Removal of Biofilms Produced by Methicillin-Resistant Staphylococcus aureus (MRSA) from Infected Diabetic Foot Ulcers. Molecules, 25(16), 3601. https://doi.org/10.3390/molecules25163601









