Hyaluronic Acid Loaded with Cerium Oxide Nanoparticles as Antioxidant in Hydrogen Peroxide Induced Chondrocytes Injury: An In Vitro Osteoarthritis Model
Abstract
1. Introduction
2. Results
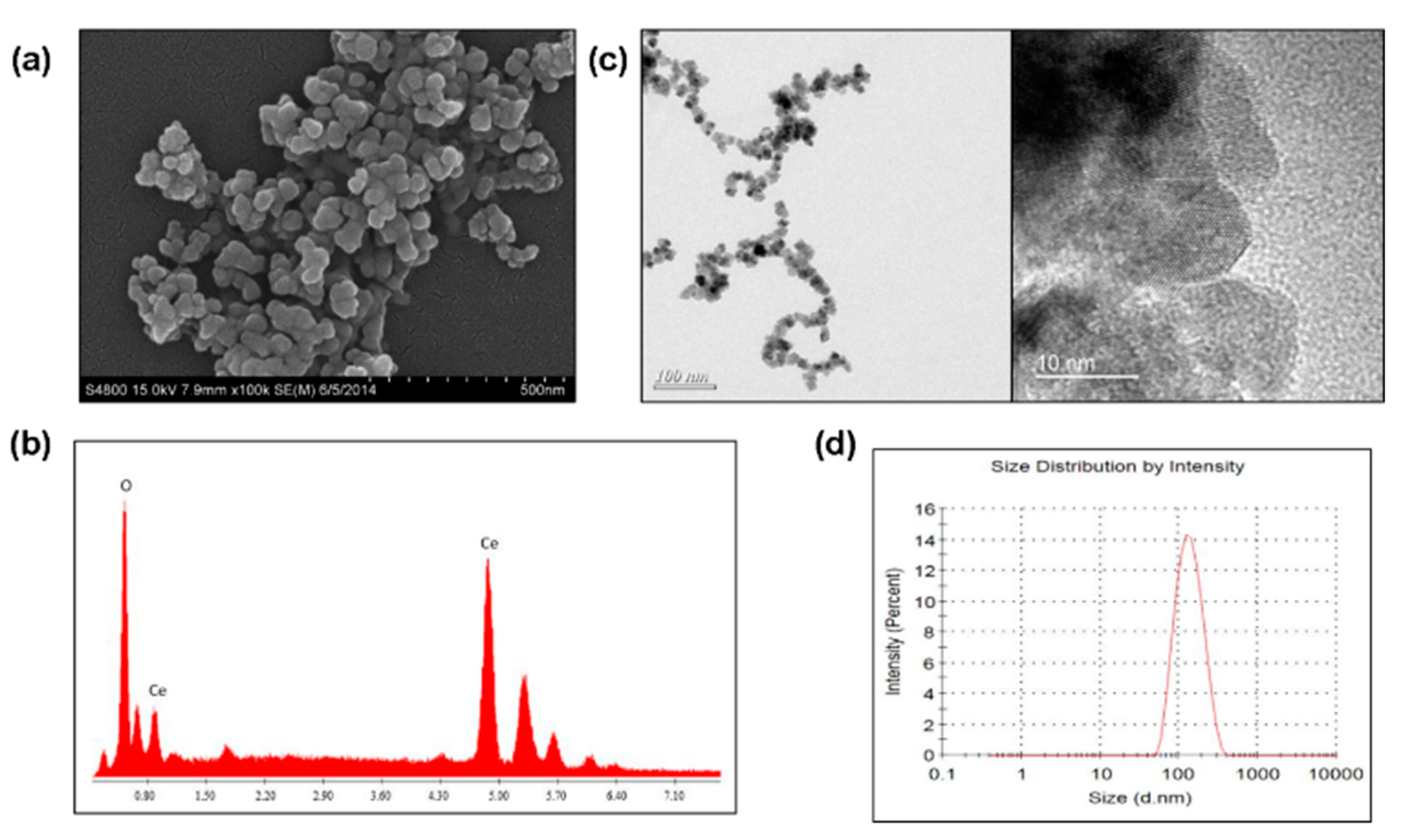
2.1. Morphology and Composition of CeO2 Nanoparticles
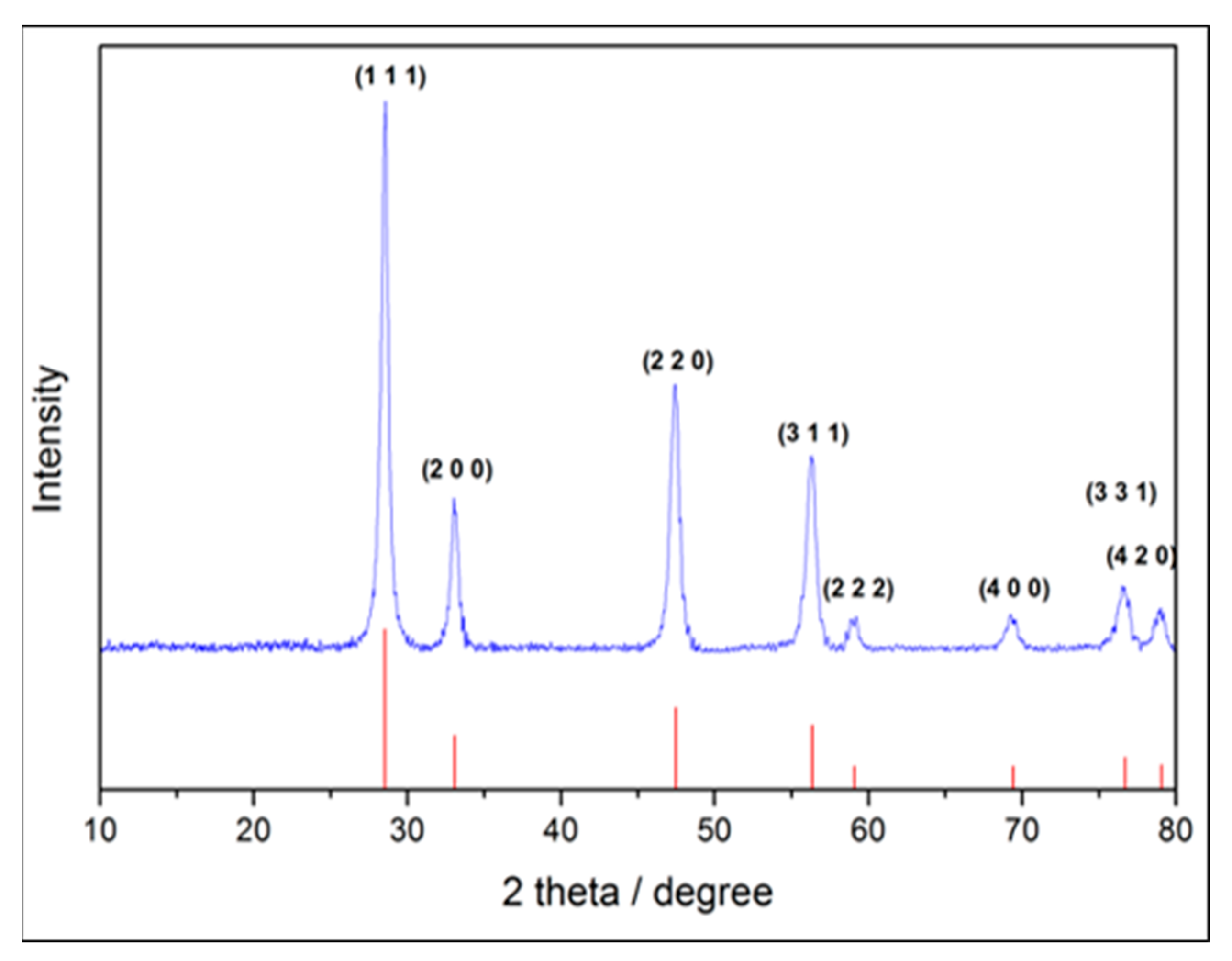
2.2. Crystal Phase Identification
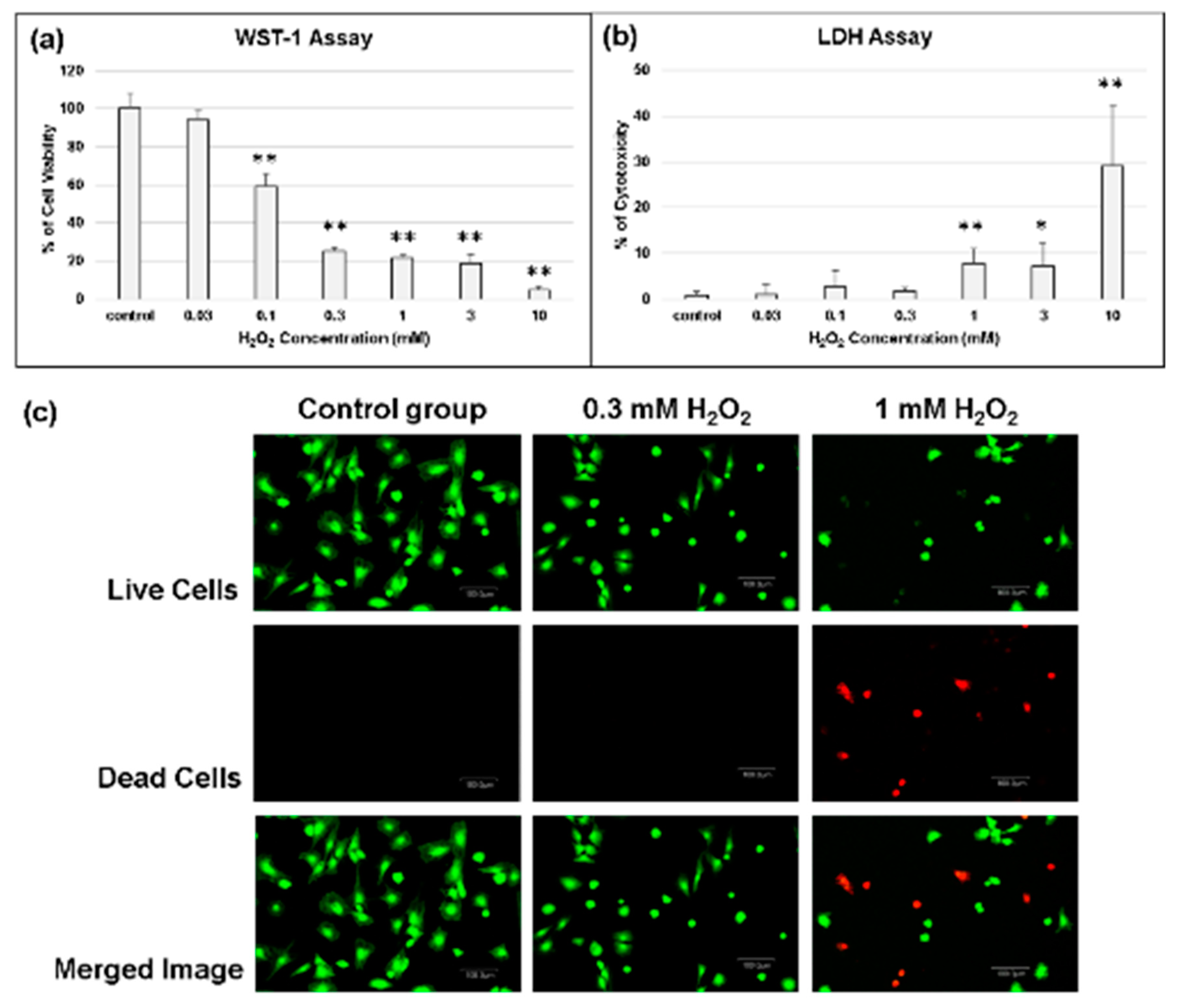
2.3. Determination of the Effect of Oxidative Stress on Chondrocytes
2.4. Biocompatibility of Cerium Oxide Nanoparticles
2.5. Cell Apoptosis and Gene Assay
2.6. Effect of CeO2 Nanoparticles-Loaded Hyaluronic Acid Treatment When Chondrocytes under Oxidative Stress
2.7. Effect of CeO2 Nanoparticles-Loaded Hyaluronic Acid Treatment Glycosaminoglycan (GAG) Synthesis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesize of Cerium Oxide Nanoparticles
4.3. Characterization
4.3.1. Morphology and Composition of Cerium Oxide Nanoparticles
4.3.2. Particle Size Identification
4.3.3. Crystal Structure Identification
4.4. In Vitro Study
4.4.1. Isolation of Chondrocytes
4.4.2. Determination of Experimental Concentrations of H2O2
4.4.3. Cell Viability
4.4.4. Cytotoxicity
4.4.5. Live/Dead Assay
4.4.6. Detection of Cell Apoptosis Rate by Flow Cytometry
4.4.7. Gene Expression
4.4.8. Alcian Blue Staining for Mucopolysaccharides
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, Z.W.; Zhou, M.; Wang, Q.; Zhu, M.J.; Chen, S.; Li, H. Mechanical and hypoxia stress can cause chondrocytes apoptosis through over-activation of endoplasmic reticulum stress. Arch. Oral Biol. 2017, 84, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Helmick, C.G.; Felson, D.T.; Lawrence, R.C.; Gabriel, S.; Hirsch, R.; Kwoh, C.K.; Liang, M.H.; Kremers, H.M.; Mayes, M.D.; Merkel, P.A.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum-Us 2008, 58, 15–25. [Google Scholar] [CrossRef]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, R.W. The burden of osteoarthritis: Clinical and quality-of-life issues. Am. J. Manag. Care 2009, 15, 223–229. [Google Scholar]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.M.; Bathon, J.M. Osteoarthritis in older adults. J. Am. Geriatr. Soc. 1998, 46, 216–225. [Google Scholar] [CrossRef]
- Sperati, A.; Picconi, O.; Tancioni, G.; Agabiti, N. Outcomes of hip replacement: A hospital-based longitudinal study in Lazio region (Italy). Ann. Ig. 2008, 20, 141–157. [Google Scholar]
- Lo, G.H.; LaValley, M.; McAlindon, T.; Felson, D.T. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: A meta-analysis. JAMA 2003, 290, 3115–3121. [Google Scholar] [CrossRef]
- Moreland, L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthritis Res. Ther. 2003, 5, 54–67. [Google Scholar] [CrossRef]
- Goldberg, V.M.; Buckwalter, J.A. Hyaluronans in the treatment of osteoarthritis of the knee: Evidence for disease-modifying activity. Osteoarthritis Cartilage 2005, 13, 216–224. [Google Scholar] [CrossRef]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar] [PubMed]
- Goldstein, I.M.; Roos, D.; Kaplan, H.B.; Weissmann, G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J. Clin. Investig. 1975, 56, 1155–1163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnston, R.B., Jr.; Lehmeyer, J.E.; Guthrie, L.A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J. Exp. Med. 1976, 143, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. Free radicals and inflammation: Protection of synovial fluid by superoxide dismutase. Science 1974, 185, 529–531. [Google Scholar] [CrossRef]
- Greenwald, R.A.; Moy, W.W.; Lazarus, D. Degradation of Cartilage Proteoglycans and Collagen by Superoxide Radical. Arthritis Rheum. 1976, 19, 799. [Google Scholar]
- Greenwald, R.A.; Moy, W.W. Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheum. 1979, 22, 251–259. [Google Scholar] [CrossRef]
- Esch, F.; Fabris, S.; Zhou, L.; Montini, T.; Africh, C.; Fornasiero, P.; Comelli, G.; Rosei, R. Electron localization determines defect formation on ceria substrates. Science 2005, 309, 752–755. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.; Sims, C.M. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants (Basel) 2016, 5, 15. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Monteiro-Riviere, N.A.; Aggarwal, R.; Davis, J.P.; Self, W.T.; McGinnis, J.; Seal, S. Nanoceria as antioxidant: Synthesis and biomedical applications. Jom 2008, 60, 33–37. [Google Scholar] [CrossRef]
- Reed, K.; Cormack, A.; Kulkarni, A.; Mayton, M.; Sayle, D.; Klaessig, F.; Stadler, B. Exploring the properties and applications of nanoceria: Is there still plenty of room at the bottom? Environ. Sci. Nano 2014, 1, 390–405. [Google Scholar] [CrossRef]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Park, H.H. Pleiotropic functions of antioxidant nanoparticles for longevity and medicine. Adv. Colloid Interface Sci. 2013, 201, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.; Das, S.; Seal, S.; Erlichman, J.; Heckman, K.; Ghibelli, L.; Traversa, E.; McGinnis, J.F.; Self, W.T. Catalytic properties and biomedical applications of cerium oxide nanoparticles. Environ. Sci. Nano 2015, 2, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Cha, M.Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease. Acs Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef]
- Zhuang, C.P.; Wang, X.P.; Chen, T.S. H2O2 Induces Apoptosis of Rabbit Chondrocytes Via Both the Extrinsic and the Caspase-Independent Intrinsic Pathways. J. Innov. Opt. Health Sci. 2013, 6, 1350022. [Google Scholar] [CrossRef]
- Pan, Y.T.; Chen, D.; Lu, Q.Y.; Liu, L.F.; Li, X.; Li, Z.C. Baicalin prevents the apoptosis of endplate chondrocytes by inhibiting the oxidative stress induced by H2O2. Mol. Med. Rep. 2017, 16, 2985–2991. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef]
- Cheung, D.L. Aggregation of nanoparticles on one and two-component bilayer membranes. J. Chem. Phys. 2014, 141, 194908. [Google Scholar] [CrossRef]
- Li, B.; Jiang, T.M.; Liu, H.; Miao, Z.K.; Fang, D.P.; Zheng, L.; Zhao, J.M. Andrographolide protects chondrocytes from oxidative stress injury by activation of the Keap1-Nrf2-Are signaling pathway J. Chem. Phys. 2019, 234, 561–571. [Google Scholar] [CrossRef]
- Sun, J.; Wei, X.L.; Lu, Y.D.; Cui, M.; Li, F.G.; Lu, J.; Liu, Y.J.; Zhang, X. Glutaredoxin 1 (GRX1) inhibits oxidative stress and apoptosis of chondrocytes by regulating CREB/HO-1 in osteoarthritis. Mol. Immunol. 2017, 90, 211–218. [Google Scholar] [CrossRef]
- Sakata, S.; Hayashi, S.; Fujishiro, T.; Kawakita, K.; Kanzaki, N.; Hashimoto, S.; Lwasa, K.; Chinzei, N.; Kihara, S.; Haneda, M.; et al. Oxidative Stress-induced Apoptosis and Matrix Loss of Chondrocytes Is Inhibited by Eicosapentaenoic Acid. J. Orthop. Res. 2015, 33, 359–365. [Google Scholar] [CrossRef]
- Karakurum, G.; Karakok, M.; Tarakcioglu, M.; Kocer, N.E.; Kocabas, R.; Bagci, C. Comparative effect of intra-articular administration of hyaluronan and/or cortisone with evaluation of malondialdehyde on degenerative osteoarthritis of the rabbit’s knee. Tohoku J. Exp. Med. 2003, 199, 127–134. [Google Scholar] [CrossRef]
- Abusarah, J.; Bentz, M.; Benabdoune, H.; Rondon, P.E.; Shi, Q.; Fernandes, J.C.; Fahmi, H.; Benderdour, M. An overview of the role of lipid peroxidation-derived 4-hydroxynonenal in osteoarthritis. Inflamm. Res. 2017, 66, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, N.H.; Baydar, M.; Atay, T.; Perktas, I.; Baykal, B.; Ozmeric, A. The effect of arthroscopic surgery and intraarticular drug injection to the antioxidation system and lipid peroxidation at osteoarthritis of knee. Saudi Med. J. 2008, 29, 397–402. [Google Scholar] [PubMed]
- Ostalowska, A.; Birkner, E.; Wiecha, M.A.; Kasperczyk, S.; Kasperczyk, A.; Kapolka, D.; Zon-Giebel, A. Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. Osteoarthritis Cartilage 2006, 14, 139–145. [Google Scholar] [CrossRef]
- Partsch, G.; Schwarzer, C.; Neumuller, J.; Dunky, A.; Petera, P.; Broll, H.; Ittner, G.; Jantsch, S. Modulation of the Migration and Chemotaxis of Pmn Cells by Hyaluronic-Acid. Z. Rheumatol. 1989, 48, 123–128. [Google Scholar] [PubMed]
- Li, Y.; Li, P.; Yu, H.; Bian, Y. Recent advances (2010–2015) in studies of cerium oxide nanoparticles’ health effects. Environ. Toxicol. Pharmacol. 2016, 44, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef]
- Cordoba-Jover, B.; Arce-Cerezo, A.; Ribera, J.; Pauta, M.; Oro, D.; Casals, G.; Fernandez-Varo, G.; Casals, E.; Puntes, V.; Jimenez, W. Cerium oxide nanoparticles improve liver regeneration after acetaminophen-induced liver injury and partial hepatectomy in rats. J. Nanobiotechnol. 2019, 17, 1–12. [Google Scholar] [CrossRef]
- Tatar, T.; Polat, Y.; Comu, F.M.; Kartal, H.; Arslan, M.; Kucku, A. Effect of cerium oxide on erythrocyte deformability in rat lower extremity ischemia reperfusion injury. Bratisl. Med. J. 2018, 119, 441–443. [Google Scholar] [CrossRef]
- Turin-Moleavin, I.A.; Fifere, A.; Lungoci, A.-L.; Rosca, I.; Coroaba, A.; Peptanariu, D.; Nastasa, V.; Pasca, S.-A.; Bostanaru, A.-C.; Mares, M.; et al. In Vitro and In Vivo Antioxidant Activity of the New Magnetic-Cerium Oxide Nanoconjugates. Nanomaterials 2019, 9, 1565. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Inbaraj, B.S. Various physicochemical and surface properties controlling the bioactivity of cerium oxide nanoparticles. Crit. Rev. Biotechnol. 2018, 38, 1003–1024. [Google Scholar] [CrossRef]
- Hu, J.Z.; Cui, W.; Ding, W.; Gu, Y.; Wang, Z.; Fan, W. Globular Adiponectin Attenuated H2O2-Induced Apoptosis in Rat Chondrocytes by Inducing Autophagy Through the AMPK/mTOR Pathway. Cell. Physiol. Biochem. 2017, 43, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.L.; Azfer, A.; Rogers, L.M.; Wang, X.H.; Kolattukudy, E.K. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc. Res. 2007, 73, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Marcos, R.; Hernandez, A. Nanoceria acts as antioxidant in tumoral and transformed cells. Chem.-Biol. Interact. 2018, 291, 7–15. [Google Scholar] [CrossRef]
- Fang, C.H.; Lin, Y.W.; Lin, F.H.; Sun, J.S.; Chao, Y.H.; Lin, H.Y.; Chang, Z.C. Biomimetic Synthesis of Nanocrystalline Hydroxyapatite Composites: Therapeutic Potential and Effects on Bone Regeneration. Int. J. Mol. Sci. 2019, 20, 6002. [Google Scholar] [CrossRef]
- Wallin, R.F.; Arscott, E. A practical guide to ISO 10993-5: Cytotoxicity. Med. Dev. Diagnostic Ind. 1998, 20, 96–98. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


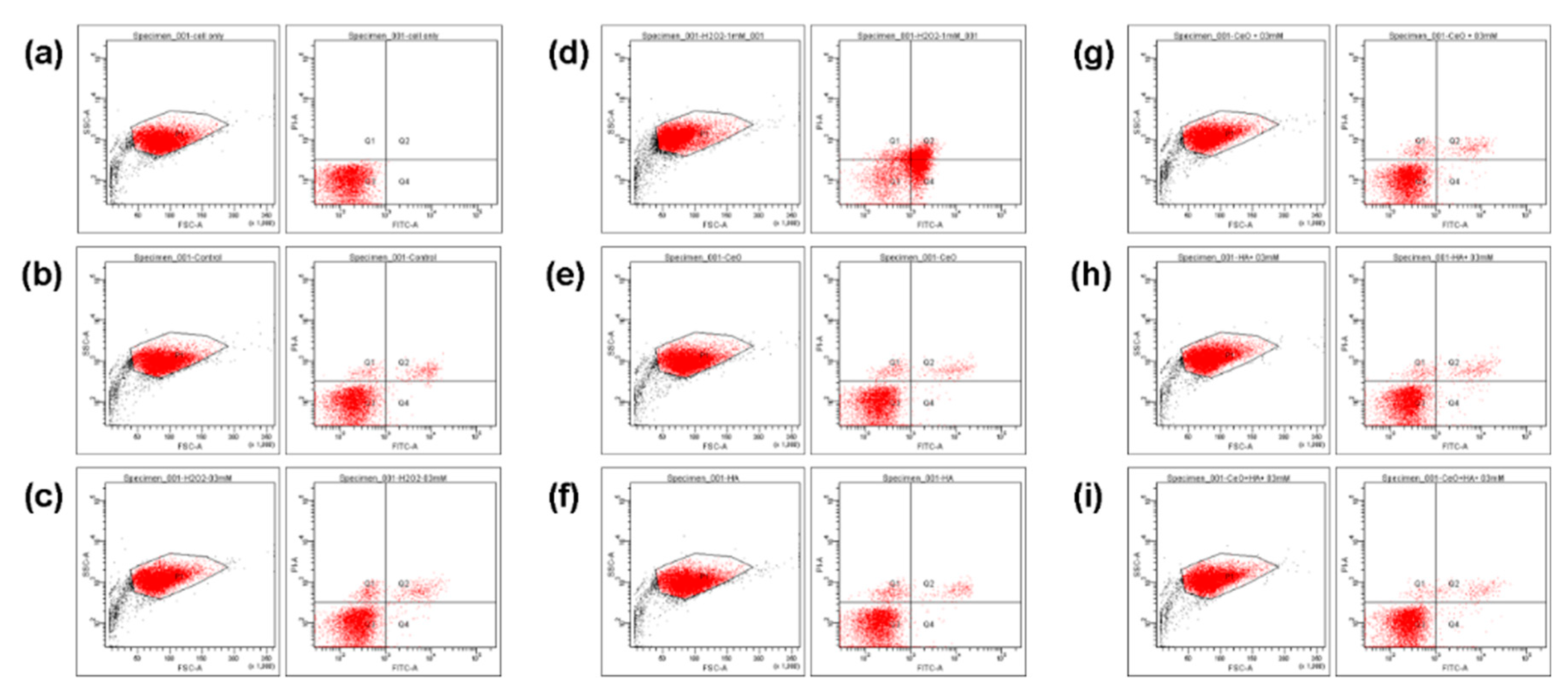


| Q1/% | Q2/% | Q3/% | Q4/% | ||
|---|---|---|---|---|---|
| a. | Normal cell | 0 | 0 | 100 | 0 |
| b. | Control group | 1.7 | 2.9 | 94.8 | 0.6 |
| c. | 0.3 mM H2O2 | 3.1 | 3.3 | 93 | 0.6 |
| d. | 1 mM H2O2 | 7.4 | 26.9 | 29.6 | 36.2 |
| e. | 0.02 μg/mL CeO2 | 2.6 | 2.4 | 94.6 | 0.5 |
| f. | 1% HA | 3.3 | 2.2 | 94.1 | 0.4 |
| g. | 0.02 μg/mL CeO2 + 0.3 mM H2O2 | 2.5 | 2.7 | 94.2 | 0.6 |
| h. | 1% HA + 0.3 mM H2O2 | 1.9 | 2.9 | 94.6 | 0.7 |
| i. | 0.02 μg/mL CeO2/1% HA + 0.3 mM H2O2 | 1.1 | 2.2 | 96 | 0.7 |
| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| ACAN | AGATGGCACCCTCCGATAC | ACACACCTCGGAAGCAGAAG |
| COL 1A1 | AGAGGTCGCCCTGGAGC | CAGGAACACCCTGTTCACCA |
| COL 2A1 | GGAGGGAACGGTCCACGAT | AGTCCGCGTATCCACAA |
| GAPDH | GCATTGTGGAAGGGCTCA | GGGTAGGAACACGGAAGG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-W.; Fang, C.-H.; Meng, F.-Q.; Ke, C.-J.; Lin, F.-H. Hyaluronic Acid Loaded with Cerium Oxide Nanoparticles as Antioxidant in Hydrogen Peroxide Induced Chondrocytes Injury: An In Vitro Osteoarthritis Model. Molecules 2020, 25, 4407. https://doi.org/10.3390/molecules25194407
Lin Y-W, Fang C-H, Meng F-Q, Ke C-J, Lin F-H. Hyaluronic Acid Loaded with Cerium Oxide Nanoparticles as Antioxidant in Hydrogen Peroxide Induced Chondrocytes Injury: An In Vitro Osteoarthritis Model. Molecules. 2020; 25(19):4407. https://doi.org/10.3390/molecules25194407
Chicago/Turabian StyleLin, Yi-Wen, Chih-Hsiang Fang, Fan-Qi Meng, Cherng-Jyh Ke, and Feng-Huei Lin. 2020. "Hyaluronic Acid Loaded with Cerium Oxide Nanoparticles as Antioxidant in Hydrogen Peroxide Induced Chondrocytes Injury: An In Vitro Osteoarthritis Model" Molecules 25, no. 19: 4407. https://doi.org/10.3390/molecules25194407
APA StyleLin, Y.-W., Fang, C.-H., Meng, F.-Q., Ke, C.-J., & Lin, F.-H. (2020). Hyaluronic Acid Loaded with Cerium Oxide Nanoparticles as Antioxidant in Hydrogen Peroxide Induced Chondrocytes Injury: An In Vitro Osteoarthritis Model. Molecules, 25(19), 4407. https://doi.org/10.3390/molecules25194407







