Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering
Abstract
1. Introduction
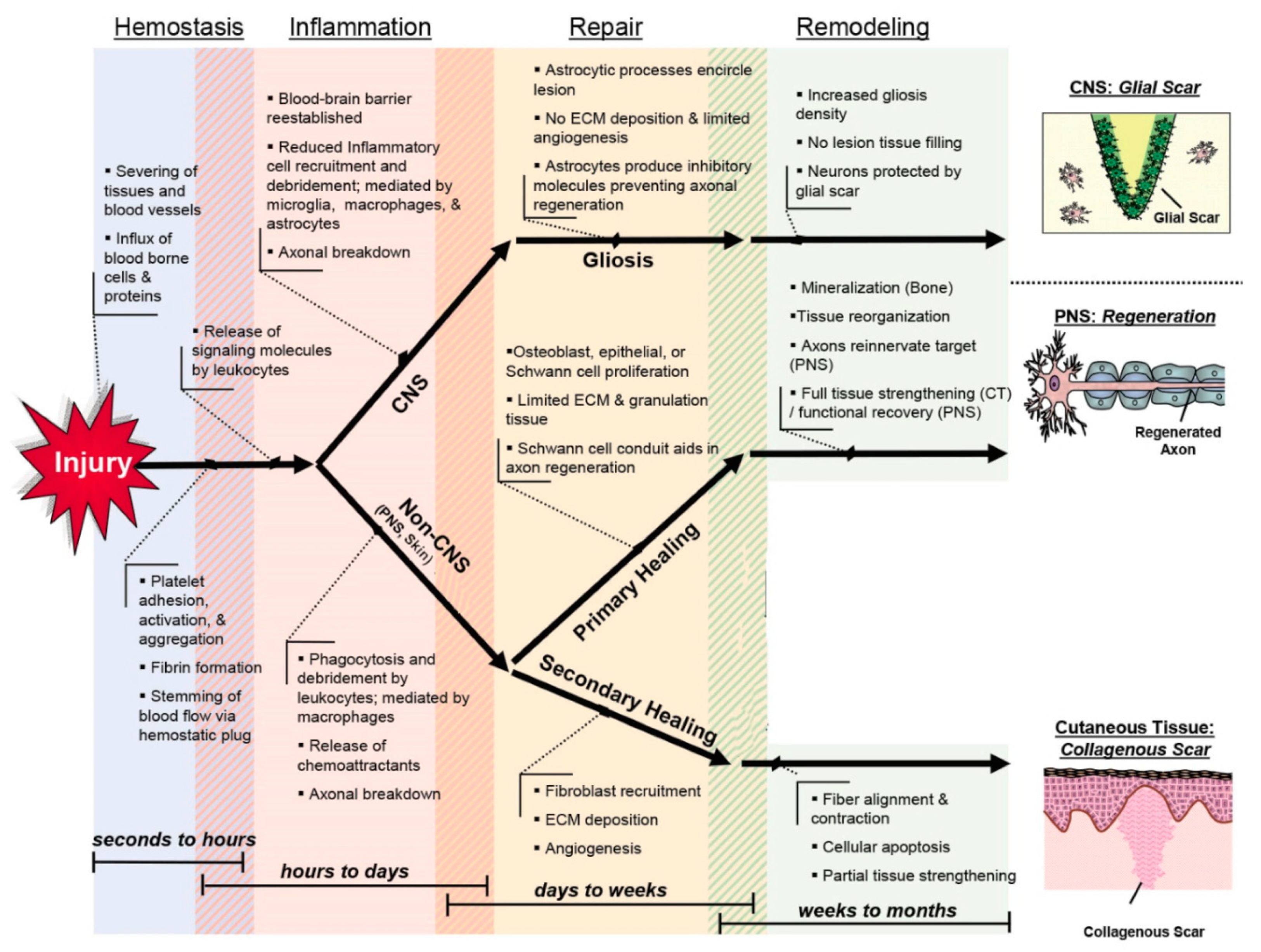
2. Wound Healing: What We Know and Need
3. Nanoceria: Biological Superiorities for Soft-Tissue Healing
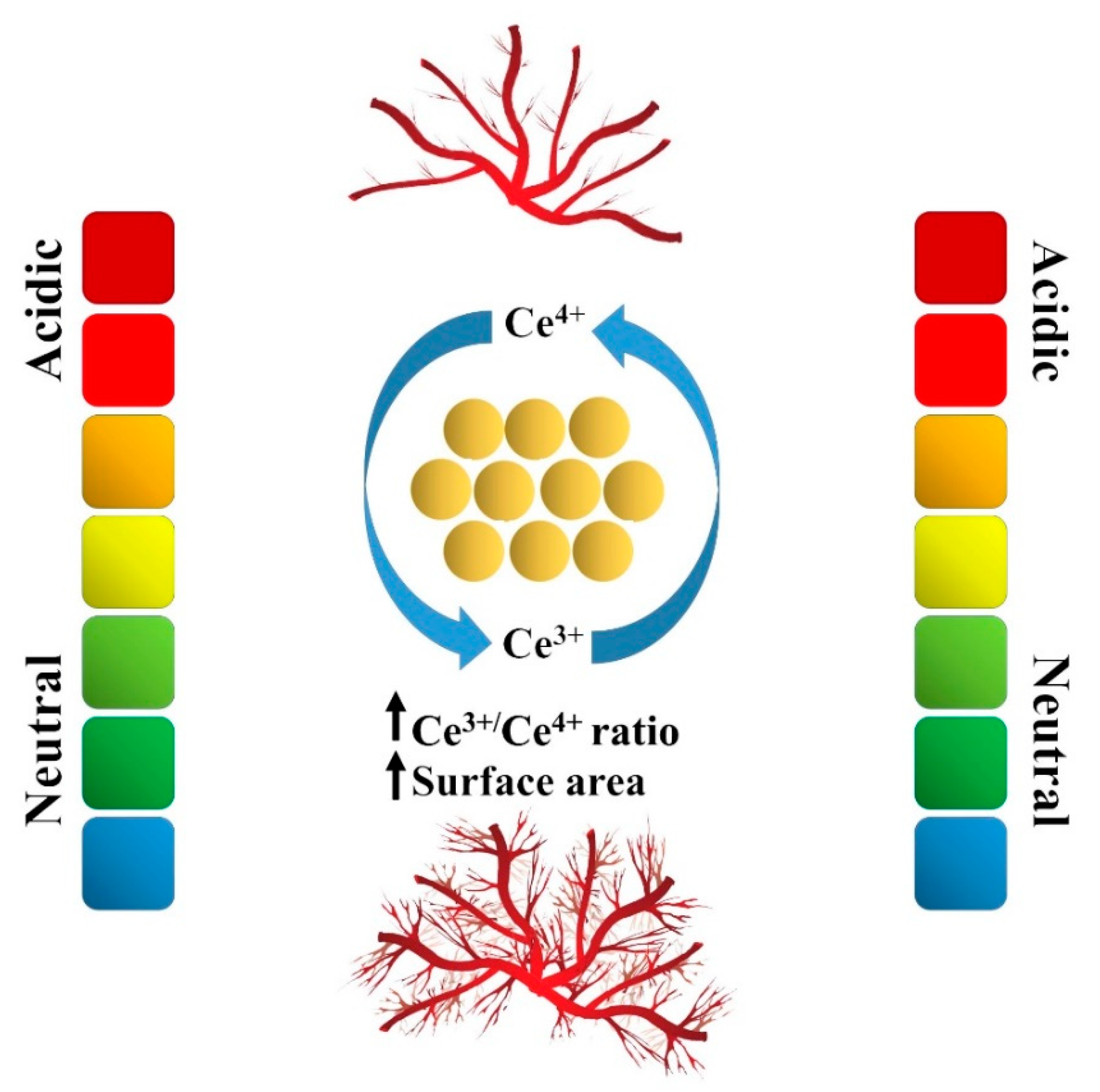
3.1. Cell and Tissue Compatibility
(II) Ce3+ + O2 → Ce4+ + O2−
(III) O2−+ O2− → O2 + H2O2
(IV) H2O2 + Ce3+ → Ce4+ + OH− + OH•
(V) LOOH + Ce3+ → Ce4++LO• + OH−
3.2. Antioxidant, Antiapoptosis, and Anti-Inflammatory Activities
(II) O2•− + Ce4+ → O2 + Ce3+ (Ce4+ is reduced)
(III) H2O2 + 2Ce4+ + 2OH− → 2H2O + O2 + 2Ce3+ (CNPs with low Ce3+/Ce4+ surface ratios)
(IV) Ce2O3 + 2[•OH] → 2CeO2 + H2O
(V) 2CeO2 (in presence of aqueous H+) → Ce2O3 + ½O2
(VI) Ce4+ + •NO → [Ce4+ + NO ↔ Ce3+ + NO+] (CNPs with low Ce3+/Ce4+ surface ratios)
3.3. Angiogenic Activity
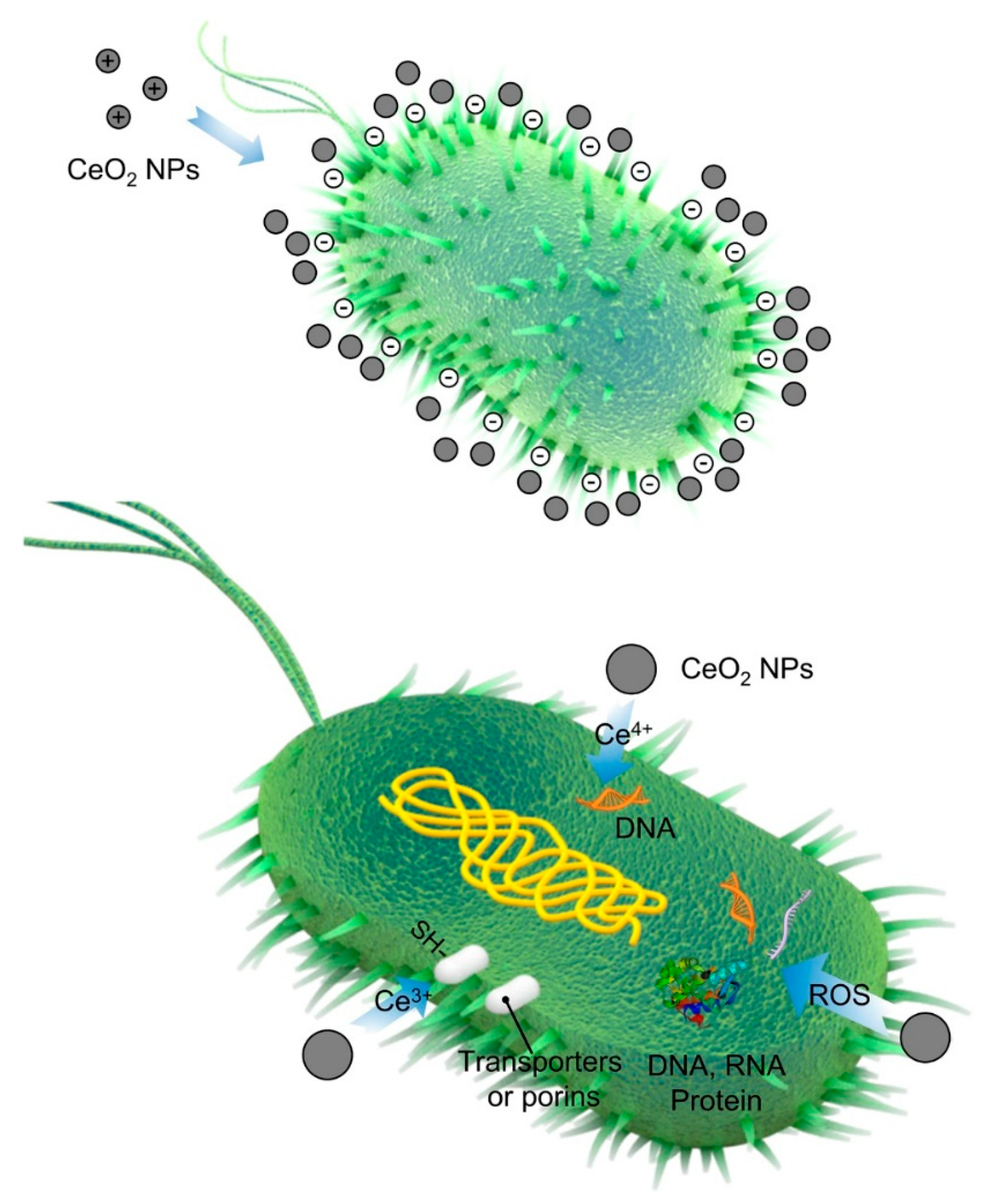
3.4. Antibacterial Properties
4. Nanoceria for Soft-Tissue Engineering
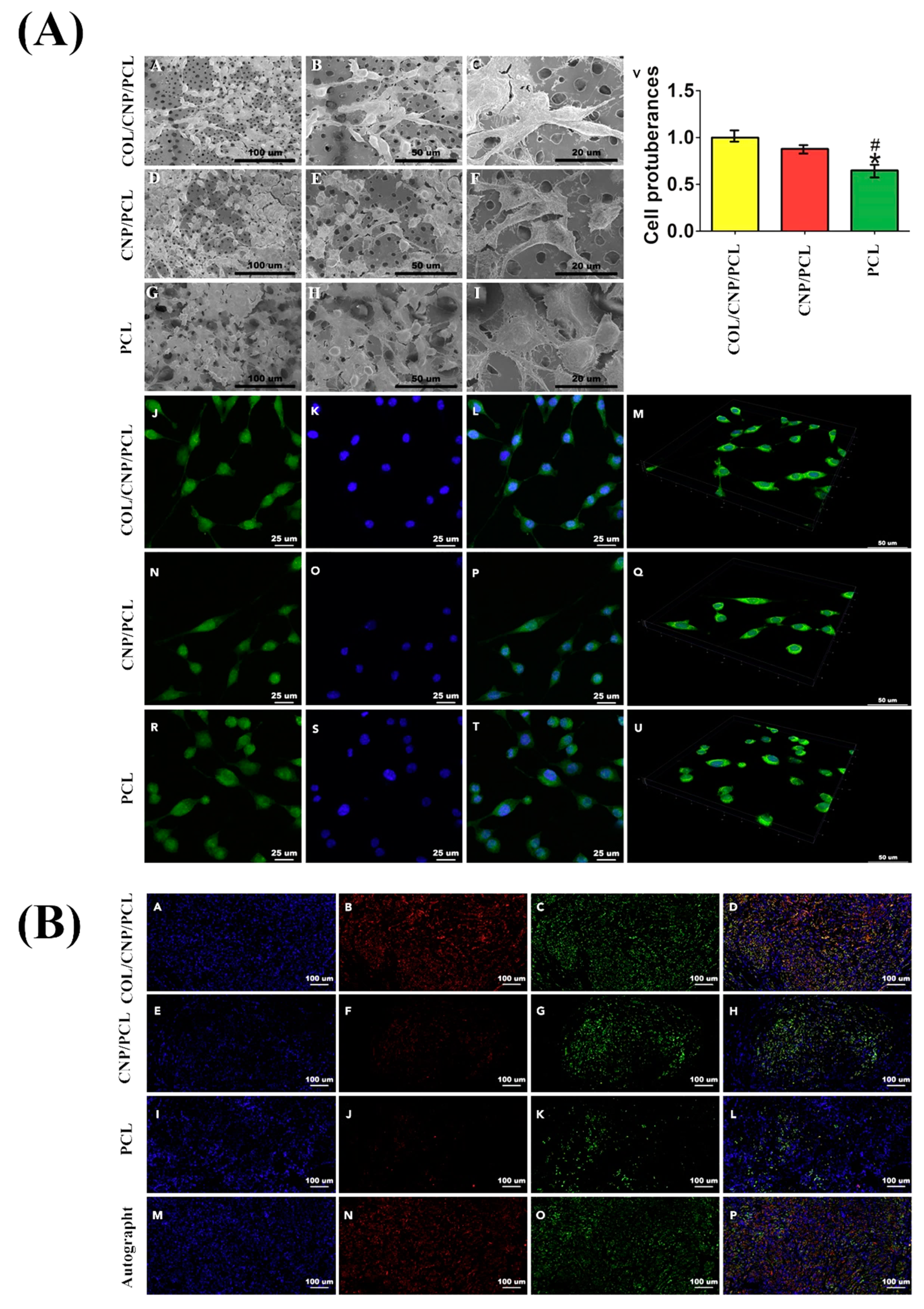
4.1. Skin Wound Healing
4.2. Regeneration of the Nervous System
4.3. Cardiac Regeneration
4.4. Ophthalmic Applications
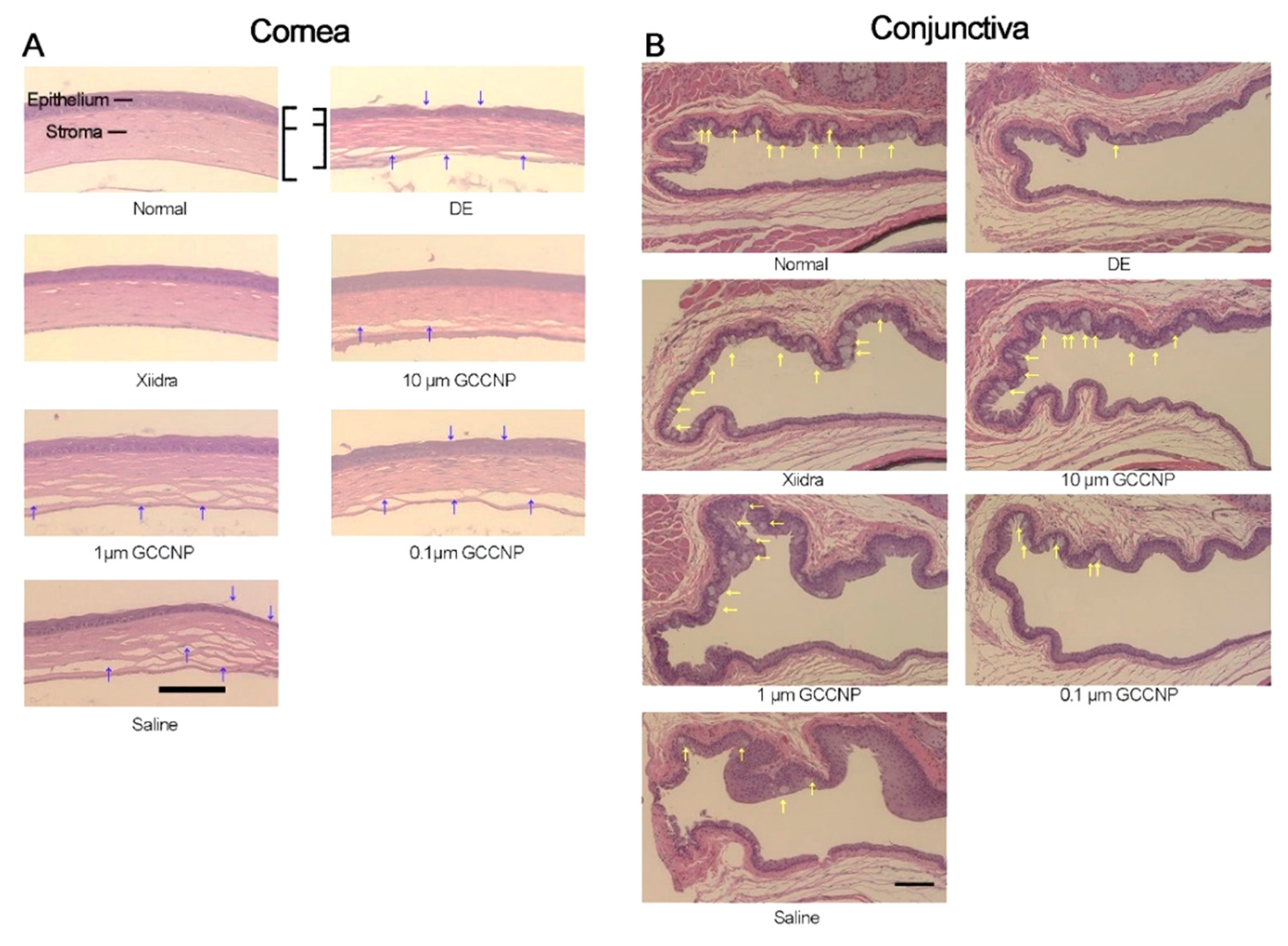
4.4.1. Ocular Surface Applications
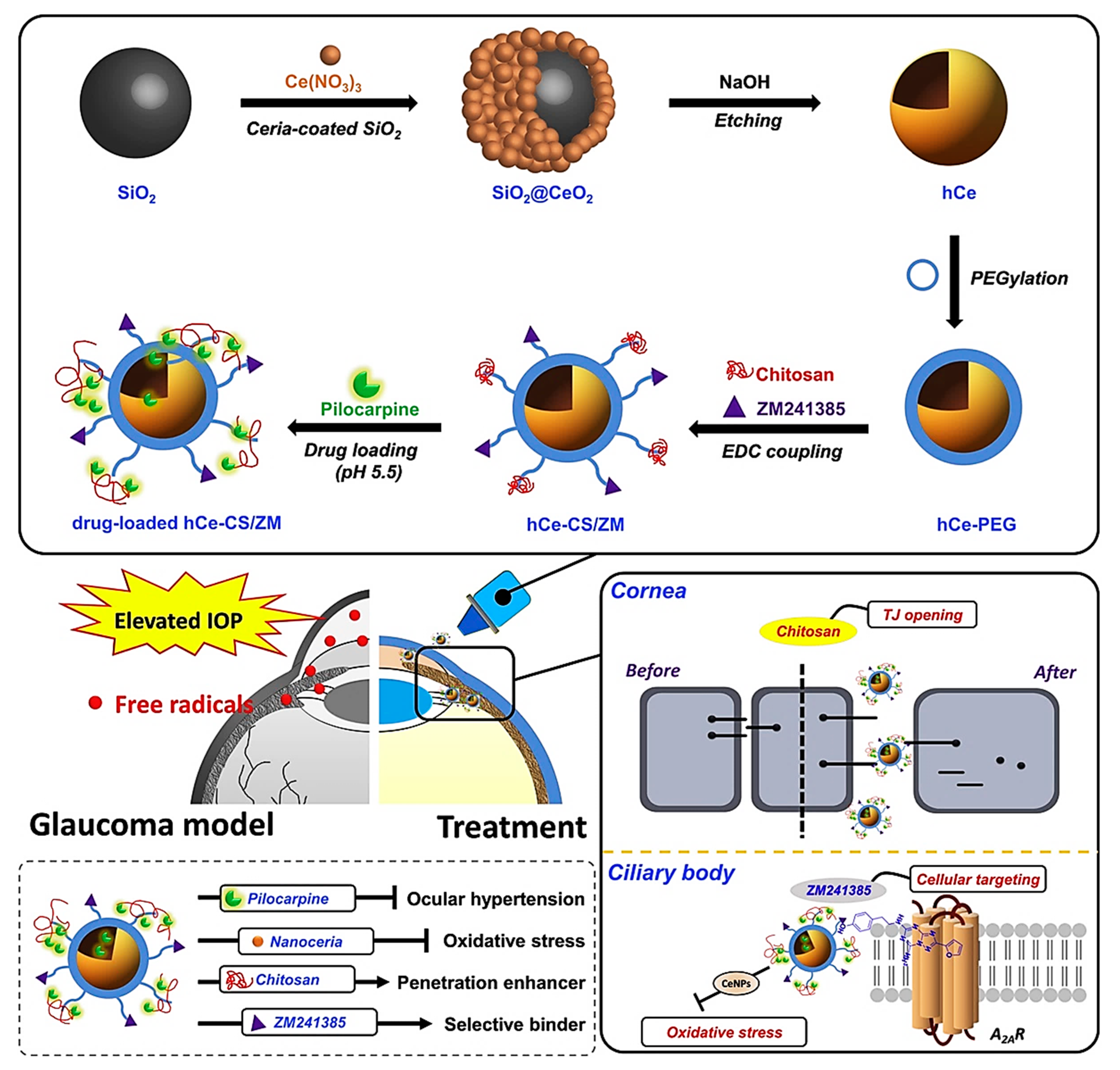
4.4.2. Glaucoma Treatment
4.4.3. Retinal Applications
4.4.4. Contact Lenses
4.4.5. Crystalline Lens Applications
5. Nanoceria: Remaining Issues before Clinical Trials
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Ko, C.-N.; Li, G.; Leung, C.-H.; Ma, D.-L. Dual function luminescent transition metal complexes for cancer theranostics: The combination of diagnosis and therapy. Coord. Chem. Rev. 2019, 381, 79–103. [Google Scholar] [CrossRef]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and promising applications of Sr-containing bioactive glasses in bone tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef]
- Landsiedel, R.; Ma-Hock, L.; Kroll, A.; Hahn, D.; Schnekenburger, J.; Wiench, K.; Wohlleben, W. Testing metal-oxide nanomaterials for human safety. Adv. Mater. 2010, 22, 2601–2627. [Google Scholar] [CrossRef]
- Kargozar, S.; Ramakrishna, S.; Mozafari, M. Chemistry of biomaterials: Future prospects. Curr. Opin. Biomed. Eng. 2019, 10, 181–190. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hoseini, S.J.; Hamzehlou, S.; Darroudi, M.; Verdi, J.; Hasanzadeh, L.; Kim, H.-W.; Mozafari, M. Biomedical applications of nanoceria: New roles for an old player. Nanomedicine 2018, 13, 3051–3069. [Google Scholar] [CrossRef] [PubMed]
- Cheisson, T.; Kersey, K.D.; Mahieu, N.; McSkimming, A.; Gau, M.R.; Carroll, P.J.; Schelter, E.J. Multiple Bonding in Lanthanides and Actinides: Direct Comparison of Covalency in Thorium (IV)-and Cerium (IV)-Imido Complexes. J. Am. Chem. Soc. 2019, 141, 9185–9190. [Google Scholar] [CrossRef] [PubMed]
- Scirè, S.; Palmisano, L. 1 - Cerium and cerium oxide: A brief introduction. In Cerium Oxide (CeO2): Synthesis, Properties and Applications; Scirè, S., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–12. [Google Scholar]
- Dhall, A.; Self, W. Cerium oxide nanoparticles: A brief review of their synthesis methods and biomedical applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Tsunekawa, S.; Sivamohan, R.; Ito, S.; Kasuya, A.; Fukuda, T. Structural study on monosize CeO2-x nano-particles. Nanostructured Mater. 1999, 11, 141–147. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Sivamohan, R.; Ohsuna, T.; Takahashi, H.; Tohji, K. Ultraviolet absorption spectra of CeO2 nano-particles. Mater. Sci. Forum 1999, 315–317, 439–445. [Google Scholar]
- Li, C.; Shi, X.; Shen, Q.; Guo, C.; Hou, Z.; Zhang, J. Hot Topics and Challenges of Regenerative Nanoceria in Application of Antioxidant Therapy. J. Nanomater. 2018, 2018, 4857461. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Li, B.; Li, W.; Qiao, W.; Shen, J.; Jin, W.; Jiang, X.; Yeung, K.W.; Chu, P.K. Valence state manipulation of cerium oxide nanoparticles on a titanium surface for modulating cell fate and bone formation. Adv. Sci. 2018, 5, 1700678. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, C.; Lord, M.S.; Lovell, E.; Wong, R.J.; Jung, M.S.; Oscar, D.; Mann, R.; Amal, R. Oxygen-vacancy engineering of cerium-oxide nanoparticles for antioxidant activity. ACS Omega 2019, 4, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- Pesaraklou, A.; Mahdavi-Shahri, N.; Hassanzadeh, H.; Ghasemi, M.; Kazemi, M.; Mousavi, N.S.; Matin, M.M. Use of cerium oxide nanoparticles: A good candidate to improve skin tissue engineering. Biomed. Mater. 2019, 14, 035008. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Al-Nsour, F.; Rice, A.B.; Marshburn, J.; Yingling, B.; Ji, Z.; Zink, J.I.; Walker, N.J.; Garantziotis, S. Cerium dioxide nanoparticles induce apoptosis and autophagy in human peripheral blood monocytes. ACS Nano 2012, 6, 5820–5829. [Google Scholar] [CrossRef] [PubMed]
- Peloi, K.E.; Lancheros, C.A.C.; Nakamura, C.V.; Singh, S.; Sakthivel, T.S.; Seal, S.; Lautenschlager, S.D.O.S. Antioxidative Photochemoprotector Effects of Cerium Oxide Nanoparticles on UVB Irradiated Fibroblast cells. Colloids Surf. B Biointerfaces 2020, 111013. [Google Scholar] [CrossRef]
- Adebayo, O.A.; Akinloye, O.; Adaramoye, O.A. Cerium oxide nanoparticles attenuate oxidative stress and inflammation in the liver of Diethylnitrosamine-treated mice. Biol. Trace Elem. Res. 2020, 193, 214–225. [Google Scholar] [CrossRef]
- Li, X.; Qi, M.; Li, C.; Dong, B.; Wang, J.; Weir, M.D.; Imazato, S.; Du, L.; Lynch, C.D.; Xu, L. Novel nanoparticles of cerium-doped zeolitic imidazolate frameworks with dual benefits of antibacterial and anti-inflammatory functions against periodontitis. J. Mater. Chem. B 2019, 7, 6955–6971. [Google Scholar] [CrossRef]
- Park, I.-S.; Mahapatra, C.; Park, J.S.; Dashnyam, K.; Kim, J.-W.; Ahn, J.C.; Chung, P.-S.; Yoon, D.S.; Mandakhbayar, N.; Singh, R.K. Revascularization and limb salvage following critical limb ischemia by nanoceria-induced Ref-1/APE1-dependent angiogenesis. Biomaterials 2020, 119919. [Google Scholar] [CrossRef]
- Yu, H.; Jin, F.; Di Liu, G.S.; Wang, X.; Qi, J.; Sun, M.; Yang, P.; Jiang, S.; Ying, X.; Du, Y. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics 2020, 10, 2342. [Google Scholar] [CrossRef]
- Nethi, S.K.; Nanda, H.S.; Steele, T.W.; Patra, C.R. Functionalized nanoceria exhibit improved angiogenic properties. J. Mater. Chem. B 2017, 5, 9371–9383. [Google Scholar] [CrossRef]
- Raja, I.S.; Fathima, N.N. Gelatin–cerium oxide nanocomposite for enhanced excisional wound healing. ACS Appl. Bio Mater. 2018, 1, 487–495. [Google Scholar] [CrossRef]
- Augustine, R.; Dalvi, Y.B.; Dan, P.; George, N.; Helle, D.; Varghese, R.; Thomas, S.; Menu, P.; Sandhyarani, N. Nanoceria can act as the cues for angiogenesis in tissue-engineering scaffolds: Toward next-generation in situ tissue engineering. ACS Biomater. Sci. Eng. 2018, 4, 4338–4353. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, Y.; Chang, H.; Yang, S.; Tu, M.; Zhang, X.; Yu, B. Cerium-containing α-calcium sulfate hemihydrate bone substitute promotes osteogenesis. J. Biomater. Appl. 2019, 34, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Singh, R.K.; Kim, H.-W.; Baino, F. “Hard” ceramics for “Soft” tissue engineering: Paradox or opportunity? Acta Biomater. 2020, 115, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Stroncek, J.D.; Reichert, W.M. Overview of wound healing in different tissue types. Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment 2008, 1, 3–41. [Google Scholar]
- Guo, S.a.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Tardáguila-García, A.; García-Morales, E.; García-Alamino, J.M.; Álvaro-Afonso, F.J.; Molines-Barroso, R.J.; Lázaro-Martínez, J.L. Metalloproteinases in chronic and acute wounds: A systematic review and meta-analysis. Wound Repair Regen. 2019, 27, 415–420. [Google Scholar] [CrossRef]
- Kim, J.H.; Yang, B.; Tedesco, A.; Lebig, E.G.D.; Ruegger, P.M.; Xu, K.; Borneman, J.; Martins-Green, M. High Levels of Oxidative Stress and Skin Microbiome are Critical for Initiation and Development of Chronic Wounds in Diabetic Mice. Sci. Rep. 2019, 9, 19318. [Google Scholar] [CrossRef]
- Wu, Y.-K.; Cheng, N.-C.; Cheng, C.-M. Biofilms in chronic wounds: Pathogenesis and diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Seth, A.K.; Hong, S.J.; Geringer, M.R.; Xie, P.; Leung, K.P.; Mustoe, T.A.; Galiano, R.D. Deficient cytokine expression and neutrophil oxidative burst contribute to impaired cutaneous wound healing in diabetic, biofilm-containing chronic wounds. Wound Repair Regen. 2013, 21, 833–841. [Google Scholar] [CrossRef]
- Madhusudhan, V. Efficacy of 1% acetic acid in the treatment of chronic wounds infected with Pseudomonas aeruginosa: Prospective randomised controlled clinical trial. Int. Wound J. 2016, 13, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Lam, G.; Fontaine, R.; Ross, F.L.; Chiu, E.S. Hyperbaric oxygen therapy: Exploring the clinical evidence. Adv. Ski. Wound Care 2017, 30, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M. Nanotechnology in wound care: One step closer to the clinic. Mol. Ther. 2018, 26, 2085–2086. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Mostafavi, E.; Saleh, B.; Soltantabar, P.; Webster, T.J. Chitosan/PVA Hydrogels Incorporated with Green Synthesized Cerium Oxide Nanoparticles for Wound Healing Applications. Eur. Polym. J. 2020, 109853. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Majumder, S.; Gupta, K.C.; Kumar, A. Dextran based amphiphilic nano-hybrid hydrogel system incorporated with curcumin and cerium oxide nanoparticles for wound healing. Colloids Surf. B: Biointerfaces 2020, 195, 111263. [Google Scholar] [CrossRef]
- Wei, R.; Nie, S.; Ma, J.; Feng, C.; Kuang, H. A Novel Functionalization of Bioactive Antimicrobial Peptide onto the Nano-CeO2/Reduced Graphene Oxide Cluster Type Biocomposite Wound Dressings for Diabetic Wound Care Management: In Vitro and In Vivo Evaluations. J. Biomater. Tissue Eng. 2020, 10, 37–45. [Google Scholar] [CrossRef]
- Kalyanaraman, V.; Naveen, S.V.; Mohana, N.; Balaje, R.M.; Navaneethakrishnan, K.R.; Brabu, B.; Murugan, S.S.; Kumaravel, T.S. Biocompatibility studies on cerium oxide nanoparticles – combined study for local effects, systemic toxicity and genotoxicity via implantation route. Toxicol. Res. 2018, 8, 25–37. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, J.-S.; Kim, S.Y.; Lee, S.-J.; Shin, Y.-J.; Im, W.-J.; Kim, S.-H.; Park, K.; Jeong, E.J.; Nam, S.-Y. Safety assessment of cerium oxide nanoparticles: Combined repeated-dose toxicity with reproductive/developmental toxicity screening and biodistribution in rats. Nanotoxicology 2020, 1–15. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Fahim, M.A.; Ali, B.H. Cerium Oxide Nanoparticles in Lung Acutely Induce Oxidative Stress, Inflammation, and DNA Damage in Various Organs of Mice. Oxidative Med. Cell. Longev. 2017, 2017, 9639035. [Google Scholar] [CrossRef]
- García, A.; Espinosa, R.; Delgado, L.; Casals, E.; González, E.; Puntes, V.; Barata, C.; Font, X.; Sánchez, A. Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests. Desalination 2011, 269, 136–141. [Google Scholar] [CrossRef]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; Shimada, M.; Kubo, M. Pulmonary toxicity of well-dispersed cerium oxide nanoparticles following intratracheal instillation and inhalation. J. Nanoparticle Res. 2015, 17, 442. [Google Scholar] [CrossRef] [PubMed]
- Arslan, K.; Akbaba, G.B. In vitro genotoxicity assessment and comparison of cerium (IV) oxide micro-and nanoparticles. Toxicol. Ind. Health 2020, 36, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.; Fromm, K.M. Toxicity and protective effects of cerium oxide nanoparticles (nanoceria) depending on their preparation method, particle size, cell type, and exposure route. Eur. J. Inorg. Chem. 2015, 2015, 4510–4517. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.; Leclerc, L.; Hochepied, J.-F.; Trouvé, A.; Sarry, G.; Pourchez, J. Impact of cerium oxide nanoparticles shape on their in vitro cellular toxicity. Toxicology in Vitro 2017, 38, 136–141. [Google Scholar] [CrossRef]
- Benameur, L.; Auffan, M.; Cassien, M.; Liu, W.; Culcasi, M.; Rahmouni, H.; Stocker, P.; Tassistro, V.; Bottero, J.-Y.; Rose, J.m. DNA damage and oxidative stress induced by CeO2 nanoparticles in human dermal fibroblasts: Evidence of a clastogenic effect as a mechanism of genotoxicity. Nanotoxicology 2015, 9, 696–705. [Google Scholar] [CrossRef]
- Yokel, R.A.; Hussain, S.; Garantziotis, S.; Demokritou, P.; Castranova, V.; Cassee, F.R. The yin: An adverse health perspective of nanoceria: Uptake, distribution, accumulation, and mechanisms of its toxicity. Environ. Sci. Nano 2014, 1, 406–428. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, X.; Zhang, H.; Lin, S.; Meng, H.; Sun, B.; George, S.; Xia, T.; Nel, A.E.; Zink, J.I. Designed synthesis of CeO2 nanorods and nanowires for studying toxicological effects of high aspect ratio nanomaterials. ACS Nano 2012, 6, 5366–5380. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Orsiere, T.; De Meo, M.; Thill, A.; Zeyons, O.; Proux, O.; Masion, A.; Chaurand, P.; Spalla, O. CeO2 nanoparticles induce DNA damage towards human dermal fibroblasts in vitro. Nanotoxicology 2009, 3, 161–171. [Google Scholar] [CrossRef]
- Fang, X.; Song, H. Synthesis of cerium oxide nanoparticles loaded on chitosan for enhanced auto-catalytic regenerative ability and biocompatibility for the spinal cord injury repair. J. Photochem. Photobiol. B Biol. 2019, 191, 83–87. [Google Scholar] [CrossRef]
- Del Turco, S.; Ciofani, G.; Cappello, V.; Parlanti, P.; Gemmi, M.; Caselli, C.; Ragusa, R.; Papa, A.; Battaglia, D.; Sabatino, L. Effects of cerium oxide nanoparticles on hemostasis: Coagulation, platelets, and vascular endothelial cells. J. Biomed. Mater. Res. Part. A 2019, 107, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.M.; Asati, A.; Nath, S.; Kaittanis, C. Synthesis of biocompatible dextran-coated nanoceria with pH-dependent antioxidant properties. Small 2008, 4, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Li, K.; Huang, L.; Zheng, X. Synthesis of carbon coated-ceria with improved cytocompatibility. Ceram. Int. 2019, 45, 19981–19990. [Google Scholar] [CrossRef]
- Pierscionek, B.K.; Li, Y.; Yasseen, A.A.; Colhoun, L.M.; Schachar, R.A.; Chen, W. Nanoceria have no genotoxic effect on human lens epithelial cells. Nanotechnology 2009, 21, 035102. [Google Scholar] [CrossRef]
- Chigurupati, S.; Mughal, M.R.; Okun, E.; Das, S.; Kumar, A.; McCaffery, M.; Seal, S.; Mattson, M.P. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials 2013, 34, 2194–2201. [Google Scholar] [CrossRef]
- Sangomla, S.; Saifi, M.A.; Khurana, A.; Godugu, C. Nanoceria ameliorates doxorubicin induced cardiotoxicity: Possible mitigation via reduction of oxidative stress and inflammation. J. Trace Elem. Med. Biol. 2018, 47, 53–62. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Ludin, A.; Gur-Cohen, S.; Golan, K.; Kaufmann, K.B.; Itkin, T.; Medaglia, C.; Lu, X.-J.; Ledergor, G.; Kollet, O.; Lapidot, T. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid. Redox Signal. 2014, 21, 1605–1619. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of mitochondrial DNA damage in ROS-mediated pathogenesis of age-related macular degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [Google Scholar] [CrossRef]
- Estevez, A.Y.; Ganesana, M.; Trentini, J.F.; Olson, J.E.; Li, G.; Boateng, Y.O.; Lipps, J.M.; Yablonski, S.E.; Donnelly, W.T.; Leiter, J.C. Antioxidant Enzyme-Mimetic Activity and Neuroprotective Effects of Cerium Oxide Nanoparticles Stabilized with Various Ratios of Citric Acid and EDTA. Biomolecules 2019, 9, 562. [Google Scholar] [CrossRef]
- Dowding, J.M.; Dosani, T.; Kumar, A.; Seal, S.; Self, W.T. Cerium oxide nanoparticles scavenge nitric oxide radical (·NO). Chem. Commun. 2012, 48, 4896–4898. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, Y.; Wang, Y.; Jiang, M.; Yao, X. Insight into Several Factors that Affect the Conversion between Antioxidant and Oxidant Activities of Nanoceria. ACS Appl. Mater. Interfaces 2016, 8, 23580–23590. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Pesaraklou, A.; Matin, M.M. Cerium oxide nanoparticles and their importance in cell signaling pathways for predicting cellular behavior. Nanomedicine 2020, 15, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-X.; Zhu, Y.-F.; Chang, H.-F.; Liang, Y. Nanoceria restrains PM2. 5-induced metabolic disorder and hypothalamus inflammation by inhibition of astrocytes activation related NF-κB pathway in Nrf2 deficient mice. Free Radic. Biol. Med. 2016, 99, 259–272. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhong, M.; Wang, J.; Xia, D.; Bao, J. Synthesis of Baicalein Modified Cerium Oxide Nanoparticles for Inhibitory Activation of NF-κB and Mitogen-Activated Protein Kinase Signals in Rotenone-Induced Parkinsonian Rats. Sci. Adv. Mater. 2020, 12, 93–100. [Google Scholar] [CrossRef]
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018, 3, 201–211. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Godugu, C. Nanoceria suppresses multiple low doses of streptozotocin-induced Type 1 diabetes by inhibition of Nrf2/NF-κB pathway and reduction of apoptosis. Nanomedicine 2018, 13, 1905–1922. [Google Scholar] [CrossRef]
- Chen, S.; Hou, Y.; Cheng, G.; Zhang, C.; Wang, S.; Zhang, J. Cerium Oxide Nanoparticles Protect Endothelial Cells from Apoptosis Induced by Oxidative Stress. Biol. Trace Elem. Res. 2013, 154, 156–166. [Google Scholar] [CrossRef]
- Clark, A.; Zhu, A.; Sun, K.; Petty, H.R. Cerium oxide and platinum nanoparticles protect cells from oxidant-mediated apoptosis. J. Nanoparticle Res. 2011, 13, 5547. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, X.; Xing, Z.; Xu, P.; Sun, J. Nano-Cerium Oxide Promotes Proliferation of Hepatoma Cells and Regulates mRNA Expression of Apoptosis-Related Genes Bcl-2 and Bax, as Detected Through Real-Time Fluorescent Quantitative Polymerase Chain Reaction. J. Nanosci. Nanotechnol. 2020, 20, 7457–7463. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; De Nicola, M.; Mandoli, C.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Ce3+ Ions Determine Redox-Dependent Anti-apoptotic Effect of Cerium Oxide Nanoparticles. ACS Nano 2011, 5, 4537–4549. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Sethy, N.K.; Das, M.; Singh, S.K.; Das, A.; Ujjain, S.K.; Sharma, R.K.; Sharma, M.; Bhargava, K. Cerium oxide nanoparticles prevent apoptosis in primary cortical culture by stabilizing mitochondrial membrane potential. Free Radic. Res. 2014, 48, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Gojova, A.; Lee, J.-T.; Jung, H.S.; Guo, B.; Barakat, A.I.; Kennedy, I.M. Effect of cerium oxide nanoparticles on inflammation in vascular endothelial cells. Inhal. Toxicol. 2009, 21, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hirst, S.M.; Karakoti, A.S.; Tyler, R.D.; Sriranganathan, N.; Seal, S.; Reilly, C.M. Anti-inflammatory properties of cerium oxide nanoparticles. Small 2009, 5, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.; Nassar, A.M.; Noreldin, A.E.; Samak, D.; Elshony, N.; Wasef, L.; Elewa, Y.H.; Hassan, S.; Saati, A.A.; Hetta, H.F. Chemo-Protective Potential of Cerium Oxide Nanoparticles against Fipronil-Induced Oxidative Stress, Apoptosis, Inflammation and Reproductive Dysfunction in Male White Albino Rats. Molecules 2020, 25, 3479. [Google Scholar] [CrossRef]
- Kyosseva, S.V.; Chen, L.; Seal, S.; McGinnis, J.F. Nanoceria inhibit expression of genes associated with inflammation and angiogenesis in the retina of Vldlr null mice. Exp. Eye Res. 2013, 116, 63–74. [Google Scholar] [CrossRef]
- Wingard, C.J.; Walters, D.M.; Cathey, B.L.; Hilderbrand, S.C.; Katwa, P.; Lin, S.; Ke, P.C.; Podila, R.; Rao, A.; Lust, R.M. Mast cells contribute to altered vascular reactivity and ischemia-reperfusion injury following cerium oxide nanoparticle instillation. Nanotoxicology 2011, 5, 531–545. [Google Scholar] [CrossRef]
- Lord, M.S.; Jung, M.; Teoh, W.Y.; Gunawan, C.; Vassie, J.A.; Amal, R.; Whitelock, J.M. Cellular uptake and reactive oxygen species modulation of cerium oxide nanoparticles in human monocyte cell line U937. Biomaterials 2012, 33, 7915–7924. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Karakoti, A.; Graham, R.P.; Maguire, J.L.; Reilly, C.M.; Seal, S.; Rattan, R.; Shridhar, V. Nanoceria: A rare-earth nanoparticle as a novel anti-angiogenic therapeutic agent in ovarian cancer. PloS ONE 2013, 8, e54578. [Google Scholar] [CrossRef] [PubMed]
- Dowding, J.M.; Das, S.; Kumar, A.; Dosani, T.; McCormack, R.; Gupta, A.; Sayle, T.X.T.; Sayle, D.C.; von Kalm, L.; Seal, S.; et al. Cellular Interaction and Toxicity Depend on Physicochemical Properties and Surface Modification of Redox-Active Nanomaterials. ACS Nano 2013, 7, 4855–4868. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hamblin, M.R.; Mozafari, M. Nanotechnology for angiogenesis: Opportunities and challenges. Chem. Soc. Rev. 2020, 49, 5008–5057. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, S.; Dowding, J.M.; Oommen, S.; Kumar, A.; Sayle, T.X.; Saraf, S.; Patra, C.R.; Vlahakis, N.E.; Sayle, D.C. The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments. Biomaterials 2012, 33, 7746–7755. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Li, W.; Zheng, X.; Li, X.; Sun, Y.; Wang, Y.; Li, C.; Wang, L. Cerium and Its Oxidant-Based Nanomaterials for Antibacterial Applications: A State-of-the-Art Review. Front. Mater. 2020, 7. [Google Scholar] [CrossRef]
- Thill, A.; Zeyons, O.; Spalla, O.; Chauvat, F.; Rose, J.; Auffan, M.; Flank, A.M. Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ. Sci. Technol. 2006, 40, 6151–6156. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Pelletier, D.A.; Suresh, A.K.; Holton, G.A.; McKeown, C.K.; Wang, W.; Gu, B.; Mortensen, N.P.; Allison, D.P.; Joy, D.C.; Allison, M.R.; et al. Effects of Engineered Cerium Oxide Nanoparticles on Bacterial Growth and Viability. Appl. Environ. Microbiol. 2010, 76, 7981. [Google Scholar] [CrossRef]
- Zeyons, O.; Thill, A.; Chauvat, F.; Menguy, N.; Cassier-Chauvat, C.; Oréar, C.; Daraspe, J.; Auffan, M.; Rose, J.; Spalla, O. Direct and indirect CeO2 nanoparticles toxicity for Escherichia coli and Synechocystis. Nanotoxicology 2009, 3, 284–295. [Google Scholar] [CrossRef]
- Bellio, P.; Luzi, C.; Mancini, A.; Cracchiolo, S.; Passacantando, M.; Di Pietro, L.; Perilli, M.; Amicosante, G.; Santucci, S.; Celenza, G. Cerium oxide nanoparticles as potential antibiotic adjuvant. Effects of CeO2 nanoparticles on bacterial outer membrane permeability. Biochim. Et Biophys. Acta (BBA) Biomembr. 2018, 1860, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Suganya Bharathi, B.; Stalin, T. Cerium oxide and peppermint oil loaded polyethylene oxide/graphene oxide electrospun nanofibrous mats as antibacterial wound dressings. Mater. Today Commun. 2019, 21, 100664. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Ramachandra Kurup Sasikala, A.; Sathishkumar, Y.; Lee, Y.S.; Park, C.H.; Kim, C.S. Nanoceria doped electrospun antibacterial composite mats for potential biomedical applications. Ceram. Int. 2014, 40, 12003–12012. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Kahya, N.; Adımcılar, V.; Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Erim, F.B. Antibacterial nano cerium oxide/chitosan/cellulose acetate composite films as potential wound dressing. Eur. Polym. J. 2020, 133, 109777. [Google Scholar] [CrossRef]
- Davan, R.; Prasad, R.; Jakka, V.S.; Aparna, R.; Phani, A.; Jacob, B.; Salins, P.C.; Raju, D. Cerium oxide nanoparticles promotes wound healing activity in in-vivo animal model. J. Bionanoscience 2012, 6, 78–83. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Baino, F. Using bioactive glasses in the management of burns. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef]
- Wang, C.; Blough, E.; Dai, X.; Olajide, O.; Driscoll, H.; Leidy, J.W.; July, M.; Triest, W.E.; Wu, M. Protective effects of cerium oxide nanoparticles on MC3T3-E1 osteoblastic cells exposed to X-ray irradiation. Cell. Physiol. Biochem. 2016, 38, 1510–1519. [Google Scholar] [CrossRef]
- Hao, D.; Zhang, G.; Gong, Y.; Ma, Z. Development and biological evaluation of cerium oxide loaded polycaprolactone dressing on cutaneous wound healing in nursing care. Mater. Lett. 2020, 265, 127401. [Google Scholar] [CrossRef]
- Genier, F.S.; Bizanek, M.; Webster, T.J.; Roy, A.K. Increased viability of fibroblasts when pretreated with ceria nanoparticles during serum deprivation. Int. J. Nanomed. 2018, 13, 895. [Google Scholar] [CrossRef]
- Popov, A.L.; Popova, N.R.; Selezneva, I.I.; Akkizov, A.Y.; Ivanov, V.K. Cerium oxide nanoparticles stimulate proliferation of primary mouse embryonic fibroblasts in vitro. Mater. Sci. Eng. C 2016, 68, 406–413. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef] [PubMed]
- Legon’kova, O.; Ushakova, T.; Savchenkova, I.; Perova, N.; Belova, M.; Torkova, A.; Baranchikov, A.; Ivanova, O.; Korotaeva, A.; Ivanov, V. Experimental Study of the Effects of Nanodispersed Ceria on Wound Repair. Bull. Exp. Biol. Med. 2017, 162, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Hilton, S.A.; Osmond, M.J.; Zgheib, C.; Newsom, J.P.; Dewberry, L.; Singh, S.; Sakthivel, T.S.; Seal, S.; Liechty, K.W. Injectable, self-healable zwitterionic cryogels with sustained microRNA-cerium oxide nanoparticle release promote accelerated wound healing. Acta Biomater. 2020, 101, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, C.; Hilton, S.A.; Dewberry, L.C.; Hodges, M.M.; Ghatak, S.; Xu, J.; Singh, S.; Roy, S.; Sen, C.K.; Seal, S. Use of cerium oxide nanoparticles conjugated with microRNA-146a to correct the diabetic wound healing impairment. J. Am. Coll. Surg. 2019, 228, 107–115. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, Y.; Jian, H.; Feng, Y.; Chang, Y.; Zheng, R.; Wu, X.; Wang, L.; Li, X.; Zhang, H. Hollow, Rough, and Nitric Oxide-Releasing Cerium Oxide Nanoparticles for Promoting Multiple Stages of Wound Healing. Adv. Healthc. Mater. 2019, 8, 1900256. [Google Scholar] [CrossRef]
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef]
- Huang, X.; Li, L.-D.; Lyu, G.-M.; Shen, B.-Y.; Han, Y.-F.; Shi, J.-L.; Teng, J.-L.; Feng, L.; Si, S.-Y.; Wu, J.-H. Chitosan-coated cerium oxide nanocubes accelerate cutaneous wound healing by curtailing persistent inflammation. Inorg. Chem. Front. 2018, 5, 386–393. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Patan, N.K.; Dalvi, Y.B.; Varghese, R.; Antony, A.; Unni, R.N.; Sandhyarani, N.; Moustafa, A.-E.A. Cerium oxide nanoparticle incorporated electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate) membranes for diabetic wound healing applications. ACS Biomater. Sci. Eng. 2019, 6, 58–70. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Tiwari, R.; Bhatia, T.; Purohit, M.P.; Pal, A.; Jagdale, P.; Mudiam, M.K.R.; Chaudhari, B.P.; Shukla, Y.; Ansari, K.M. Accelerated and scarless wound repair by a multicomponent hydrogel through simultaneous activation of multiple pathways. Drug Deliv. Transl. Res. 2019, 9, 1143–1158. [Google Scholar] [CrossRef]
- Kobyliak, N.; Abenavoli, L.; Kononenko, L.; Kyriienko, D.; Spivak, M. Neuropathic diabetic foot ulcers treated with cerium dioxide nanoparticles: A case report. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 228–234. [Google Scholar] [CrossRef]
- Sack, M.; Alili, L.; Karaman, E.; Das, S.; Gupta, A.; Seal, S.; Brenneisen, P. Combination of conventional chemotherapeutics with redox-active cerium oxide nanoparticles—A novel aspect in cancer therapy. Mol. Cancer Ther. 2014, 13, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; De Nicola, M.; Sienkiewicz, A.; Giovanetti, A.; Bejarano, I.; Licoccia, S.; Traversa, E.; Ghibelli, L. Cerium oxide nanoparticles, combining antioxidant and UV shielding properties, prevent UV-induced cell damage and mutagenesis. Nanoscale 2015, 7, 15643–15656. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Patil, S.; Bhargava, N.; Kang, J.-F.; Riedel, L.M.; Seal, S.; Hickman, J.J. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 2007, 28, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Edoff, K.; Caputo, F.; Källman, T.; Blom, H.; Karlsson, H.L.; Ghibelli, L.; Traversa, E.; Ceccatelli, S.; Fadeel, B. Cerium oxide nanoparticles inhibit differentiation of neural stem cells. Sci. Rep. 2017, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Anitua, E.; Pedraz, J.L.; Emerich, D.F. Biomaterials for promoting brain protection, repair and regeneration. Nat. Rev. Neurosci. 2009, 10, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Kim, J. Recent Progress in Autocatalytic Ceria Nanoparticles-Based Translational Research on Brain Diseases. ACS Appl. Nano Mater. 2020, 3, 1043–1062. [Google Scholar] [CrossRef]
- Yu, D.; Ma, M.; Liu, Z.; Pi, Z.; Du, X.; Ren, J.; Qu, X. MOF-encapsulated nanozyme enhanced siRNA combo: Control neural stem cell differentiation and ameliorate cognitive impairments in Alzheimer’s disease model. Biomaterials 2020, 120160. [Google Scholar] [CrossRef]
- Kim, J.W.; Mahapatra, C.; Hong, J.Y.; Kim, M.S.; Leong, K.W.; Kim, H.W.; Hyun, J.K. Functional Recovery of Contused Spinal Cord in Rat with the Injection of Optimal-Dosed Cerium Oxide Nanoparticles. Adv. Sci. 2017, 4, 1700034. [Google Scholar] [CrossRef]
- Bailey, Z.S.; Nilson, E.; Bates, J.A.; Oyalowo, A.; Hockey, K.S.; Sajja, V.S.S.S.; Thorpe, C.; Rogers, H.; Dunn, B.; Frey, A.S. Cerium oxide nanoparticles improve outcome after in vitro and in vivo mild traumatic brain injury. J. Neurotrauma 2020, 37, 1452–1462. [Google Scholar] [CrossRef]
- Arya, A.; Gangwar, A.; Singh, S.K.; Roy, M.; Das, M.; Sethy, N.K.; Bhargava, K. Cerium oxide nanoparticles promote neurogenesis and abrogate hypoxia-induced memory impairment through AMPK–PKC–CBP signaling cascade. Int. J. Nanomed. 2016, 11, 1159. [Google Scholar]
- Lin, Y.-L.; Jen, J.-C.; Hsu, S.-h.; Chiu, M. Sciatic nerve repair by microgrooved nerve conduits made of chitosan-gold nanocomposites. Surg. Neurol. 2008, 70, S9–S18. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Ghenaatgar-Kasbi, M.; Baino, F. Bioactive Glasses and Glass/Polymer Composites for Neuroregeneration: Should We Be Hopeful? Appl. Sci. 2020, 10, 3421. [Google Scholar] [CrossRef]
- Battaglini, M.; Tapeinos, C.; Cavaliere, I.; Marino, A.; Ancona, A.; Garino, N.; Cauda, V.; Palazon, F.; Debellis, D.; Ciofani, G. Design, fabrication, and in vitro evaluation of nanoceria-loaded nanostructured lipid carriers for the treatment of neurological diseases. ACS Biomater. Sci. Eng. 2018, 5, 670–682. [Google Scholar] [CrossRef]
- Marino, A.; Tonda-Turo, C.; De Pasquale, D.; Ruini, F.; Genchi, G.; Nitti, S.; Cappello, V.; Gemmi, M.; Mattoli, V.; Ciardelli, G. Gelatin/nanoceria nanocomposite fibers as antioxidant scaffolds for neuronal regeneration. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 386–395. [Google Scholar] [CrossRef]
- Qian, Y.; Han, Q.; Zhao, X.; Li, H.; Yuan, W.-E.; Fan, C. Asymmetrical 3D Nanoceria Channel for Severe Neurological Defect Regeneration. iScience 2019, 12, 216–231. [Google Scholar] [CrossRef]
- Niu, J.; Wang, K.; Kolattukudy, P.E. Cerium oxide nanoparticles inhibits oxidative stress and nuclear factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J. Pharmacol. Exp. Ther. 2011, 338, 53–61. [Google Scholar] [CrossRef]
- Pagliari, F.; Mandoli, C.; Forte, G.; Magnani, E.; Pagliari, S.; Nardone, G.; Licoccia, S.; Minieri, M.; Di Nardo, P.; Traversa, E. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano 2012, 6, 3767–3775. [Google Scholar] [CrossRef]
- Niu, J.; Azfer, A.; Rogers, L.M.; Wang, X.; Kolattukudy, P.E. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc. Res. 2007, 73, 549–559. [Google Scholar] [CrossRef]
- Asadpour, S.; Yeganeh, H.; Ai, J.; Kargozar, S.; Rashtbar, M.; Seifalian, A.; Ghanbari, H. Polyurethane-Polycaprolactone Blend Patches: Scaffold Characterization and Cardiomyoblast Adhesion, Proliferation, and Function. ACS Biomater. Sci. Eng. 2018, 4, 4299–4310. [Google Scholar] [CrossRef]
- Gong, Y.-Y.; Luo, J.-Y.; Wang, L.; Huang, Y. MicroRNAs regulating reactive oxygen species in cardiovascular diseases. Antioxid. Redox Signal. 2018, 29, 1092–1107. [Google Scholar] [CrossRef]
- Yang, L.; Ren, Y.; Pan, W.; Yu, Z.; Tong, L.; Li, N.; Tang, B. Fluorescent nanocomposite for visualizing cross-talk between microRNA-21 and hydrogen peroxide in ischemia-reperfusion injury in live cells and in vivo. Anal. Chem. 2016, 88, 11886–11891. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Behera, M.; Mahapatra, C.; Sundaresan, N.R.; Chatterjee, K. Nanostructured Polymer Scaffold Decorated with Cerium Oxide Nanoparticles Toward Engineering an Antioxidant and Anti-hypertrophic Cardiac Patch. Mater. Sci. Eng. C 2020, 111416. [Google Scholar] [CrossRef]
- Tisi, A.; Flati, V.; Delle Monache, S.; Lozzi, L.; Passacantando, M.; Maccarone, R. Nanoceria Particles Are an Eligible Candidate to Prevent Age-Related Macular Degeneration by Inhibiting Retinal Pigment Epithelium Cell Death and Autophagy Alterations. Cells 2020, 9, 1617. [Google Scholar] [CrossRef]
- Hanafy, B.I.; Cave, G.W.; Barnett, Y.; Pierscionek, B. Ethylene glycol coated nanoceria protects against oxidative stress in human lens epithelium. RSC Adv. 2019, 9, 16596–16605. [Google Scholar] [CrossRef]
- Yu, F.; Zheng, M.; Zhang, A.Y.; Han, Z. A cerium oxide loaded glycol chitosan nano-system for the treatment of dry eye disease. J. Control. Release 2019, 315, 40–54. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Dually functional hollow ceria nanoparticle platform for intraocular drug delivery: A push beyond the limits of static and dynamic ocular barriers toward glaucoma therapy. Biomaterials 2020, 119961. [Google Scholar] [CrossRef]
- Tisi, A.; Passacantando, M.; Lozzi, L.; Riccitelli, S.; Bisti, S.; Maccarone, R. Retinal long term neuroprotection by Cerium Oxide nanoparticles after an acute damage induced by high intensity light exposure. Exp. Eye Res. 2019, 182, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wong, L.L.; Karakoti, A.S.; Seal, S.; McGinnis, J.F. Nanoceria inhibit the development and promote the regression of pathologic retinal neovascularization in the Vldlr knockout mouse. PloS ONE 2011, 6, e16733. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.L.; Hirst, S.M.; Pye, Q.N.; Reilly, C.M.; Seal, S.; McGinnis, J.F. Catalytic nanoceria are preferentially retained in the rat retina and are not cytotoxic after intravitreal injection. PloS ONE 2013, 8, e58431. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular drug delivery: Present innovations and future challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Wong, L.L.; Barkam, S.; Seal, S.; McGinnis, J.F. Temporal Distribution Patterns of Alexa Fluor 647-Conjugated CeNPs in the Mouse Retina After a Single Intravitreal Injection. In Retinal Degenerative Diseases; Springer: Berlin, Germany, 2019; pp. 125–130. [Google Scholar]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J. Therapeutic contact lenses with polymeric vehicles for ocular drug delivery: A review. Materials 2018, 11, 1125. [Google Scholar] [CrossRef]
- Choi, S.W.; Cha, B.G.; Kim, J. Therapeutic Contact Lens for Scavenging Excessive Reactive Oxygen Species on the Ocular Surface. ACS Nano 2020, 14, 2483–2496. [Google Scholar] [CrossRef]
- Hanafy, B.I.; Cave, G.W.; Barnett, Y.; Pierscionek, B. Treatment of Human Lens Epithelium with High Levels of Nanoceria Leads to Reactive Oxygen Species Mediated Apoptosis. molecules 2020, 25, 441. [Google Scholar] [CrossRef]
- Monafo, W.W.; Ayvazian, V.H.; Skinner, A.M. Control of infection in major burn wounds by cerium nitrate/silver sulphadiazine. Burns 1977, 3, 104–107. [Google Scholar] [CrossRef]
- Fox, C.L.; Modak, S.M.; Stanford, J.W. Cerium sulphadiazine as a topical agent for burn wound infections: A comparison with silver sulphadiazine and zinc sulphadiazine. Burns 1978, 4, 233–239. [Google Scholar] [CrossRef]
- Boeckx, W.; Blondeel, P.N.; Vandersteen, K.; De Wolf-Peeters, C.; Schmitz, A. Effect of cerium nitrate-silver sulphadiazine on deep dermal burns: A histological hypothesis. Burns 1992, 18, 456–462. [Google Scholar] [CrossRef]
- Oen, I.M.; van Baar, M.E.; Middelkoop, E.; Nieuwenhuis, M.K. Effectiveness of cerium nitrate–silver sulfadiazine in the treatment of facial burns: A multicenter, randomized, controlled trial. Plast. Reconstr. Surg. 2012, 130, 274e–283e. [Google Scholar] [CrossRef]
- Turin-Moleavin, I.-A.; Fifere, A.; Lungoci, A.-L.; Rosca, I.; Coroaba, A.; Peptanariu, D.; Nastasa, V.; Pasca, S.-A.; Bostanaru, A.-C.; Mares, M. In Vitro and In Vivo Antioxidant Activity of the New Magnetic-Cerium Oxide Nanoconjugates. Nanomaterials 2019, 9, 1565. [Google Scholar] [CrossRef]
- Pinna, A.; Cali, E.; Kerherve, G.; Galleri, G.; Maggini, M.; Innocenzi, P.; Malfatti, L. Fulleropyrrolidine-functionalized ceria nanoparticles as a tethered dual nanosystem with improved antioxidant properties. Nanoscale Adv. 2020, 2, 2387–2396. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, Q.; Hu, Z.; Yang, X.; Yang, B.; Liu, S. Biomimetic Cerium Oxide Loaded Gelatin PCL Nanosystems for Wound Dressing on Cutaneous Care Management of Multidrug-Resistant Bacterial Wound Healing. J. Clust. Sci. 2020, 1–10. [Google Scholar] [CrossRef]
- Kumari, P.; Saifi, M.A.; Khurana, A.; Godugu, C. Cardioprotective effects of nanoceria in a murine model of cardiac remodeling. J. Trace Elem. Med. Biol. 2018, 50, 198–208. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadidi, H.; Hooshmand, S.; Ahmadabadi, A.; Javad Hoseini, S.; Baino, F.; Vatanpour, M.; Kargozar, S. Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering. Molecules 2020, 25, 4559. https://doi.org/10.3390/molecules25194559
Sadidi H, Hooshmand S, Ahmadabadi A, Javad Hoseini S, Baino F, Vatanpour M, Kargozar S. Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering. Molecules. 2020; 25(19):4559. https://doi.org/10.3390/molecules25194559
Chicago/Turabian StyleSadidi, Hossein, Sara Hooshmand, Ali Ahmadabadi, Seyed Javad Hoseini, Francesco Baino, Morvarid Vatanpour, and Saeid Kargozar. 2020. "Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering" Molecules 25, no. 19: 4559. https://doi.org/10.3390/molecules25194559
APA StyleSadidi, H., Hooshmand, S., Ahmadabadi, A., Javad Hoseini, S., Baino, F., Vatanpour, M., & Kargozar, S. (2020). Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering. Molecules, 25(19), 4559. https://doi.org/10.3390/molecules25194559





