Synthesis of Water-Soluble Copolymers of N-vinylpyrrolidone with N-vinyldithiocarbamate as Multidentate Polymeric Chelation Systems and Their Complexes with Indium and Gallium
Abstract
:1. Introduction
2. Results and Discussion
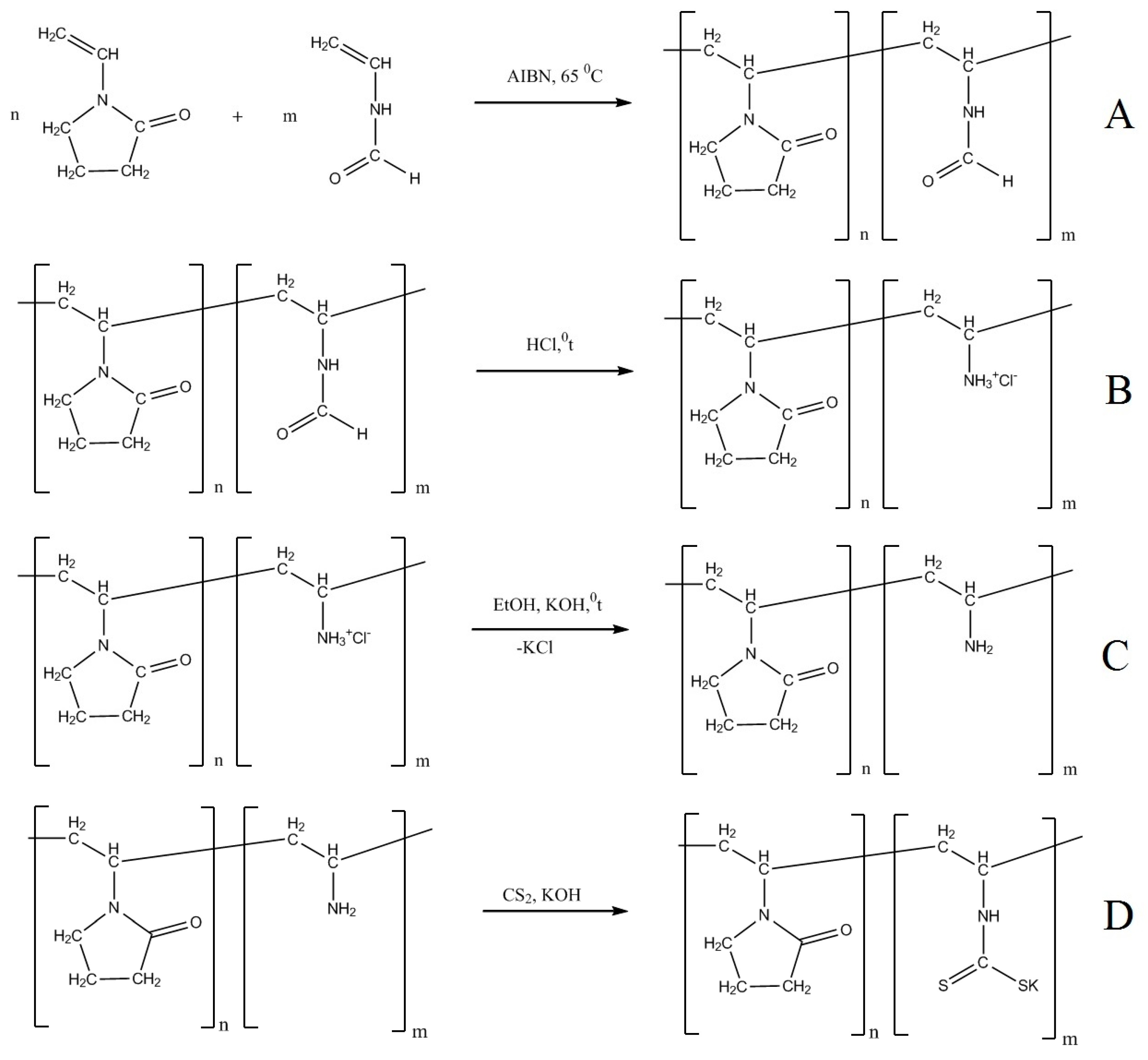
2.1. Synthesis of VP–VDTCCo-Polymers
2.2. UV and IR Spectroscopy of VP–VDTC
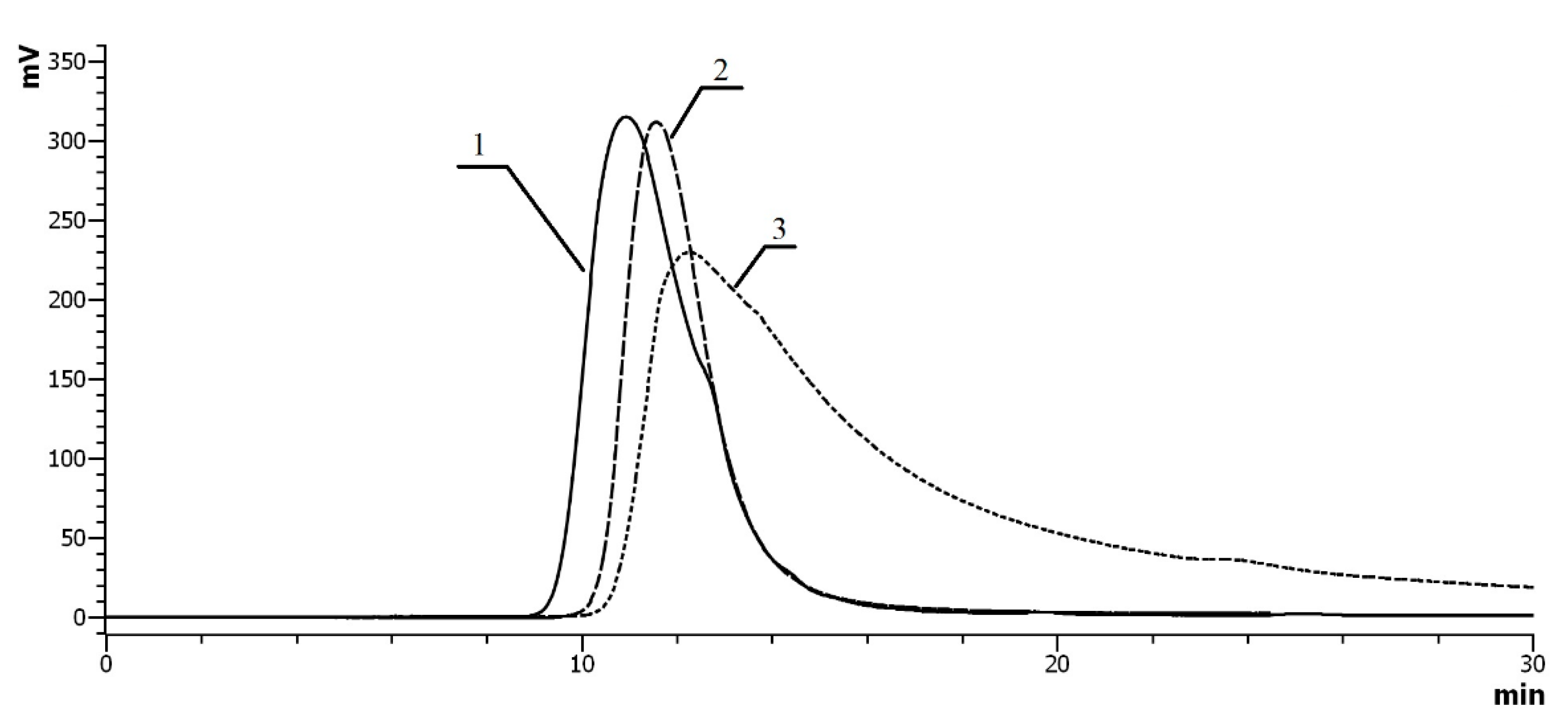
2.3. Size-Exclusion (SEC) Chromatography of Co-Polymers
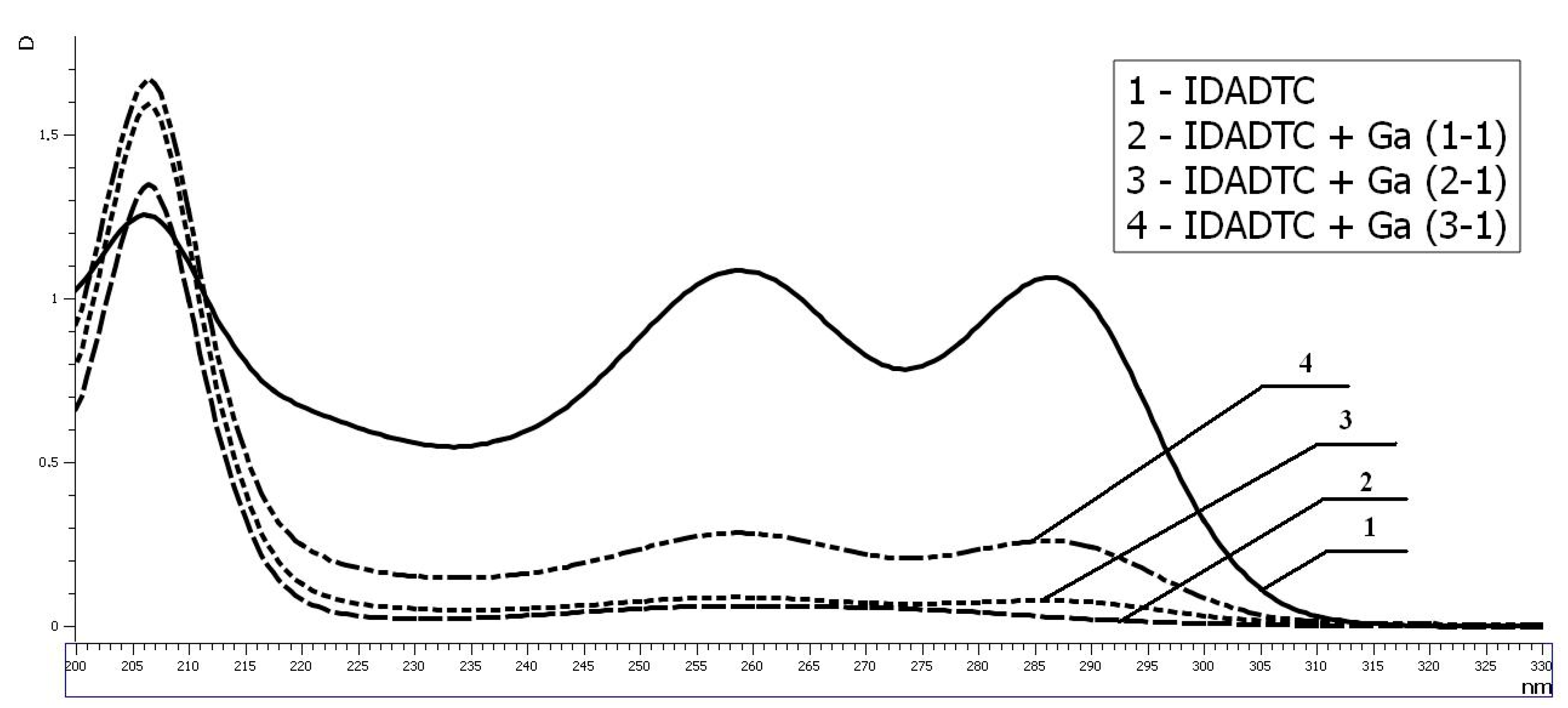
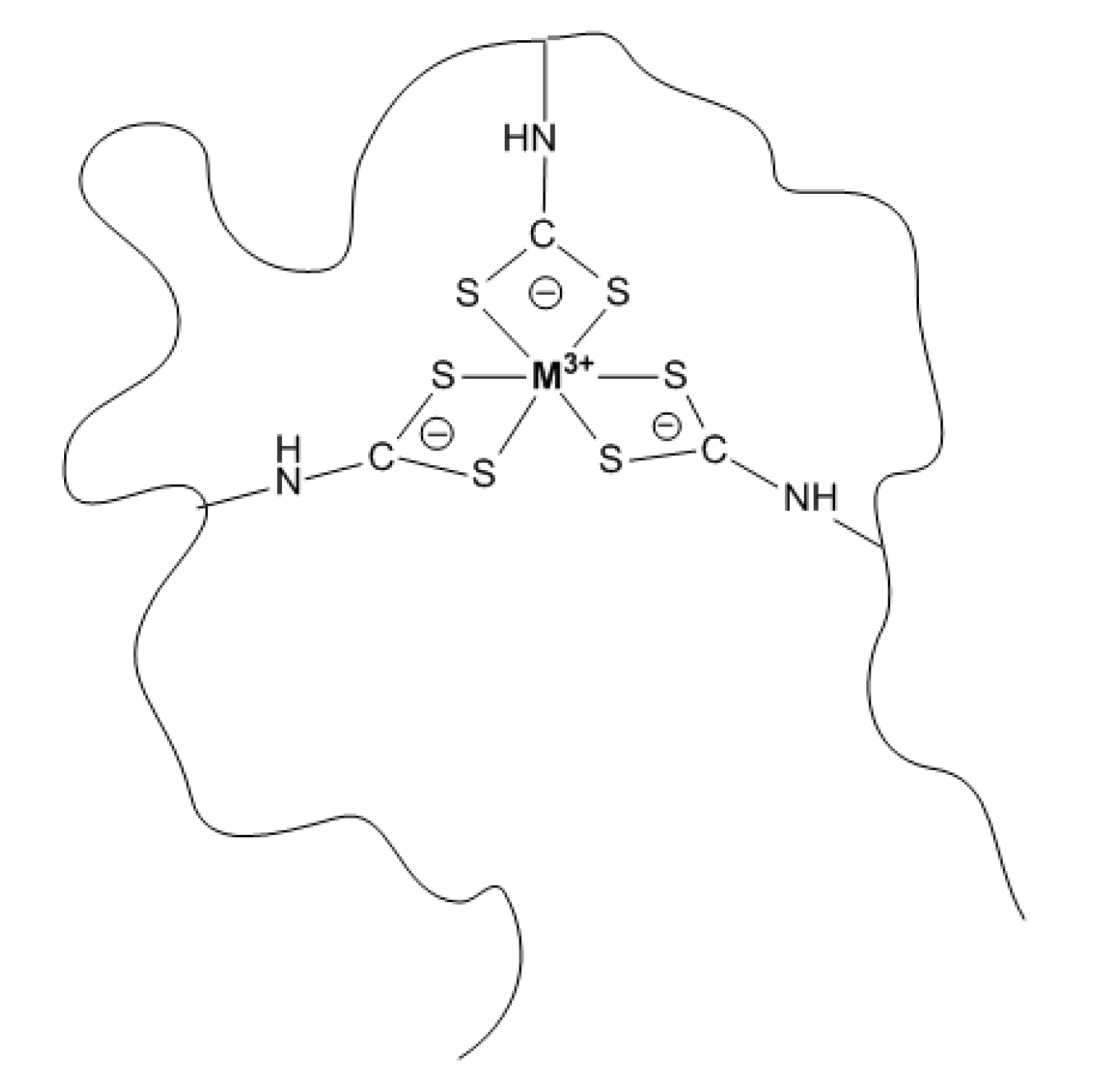
2.4. Synthesis and Investigation of Stoichiometry of M(Ga,In)–IDADTC (Bis (Carboxymethyl) Dithiocarbamate) Model Complex
2.5. Synthesis and Investigation of Ga/In-VP–DTC MPC
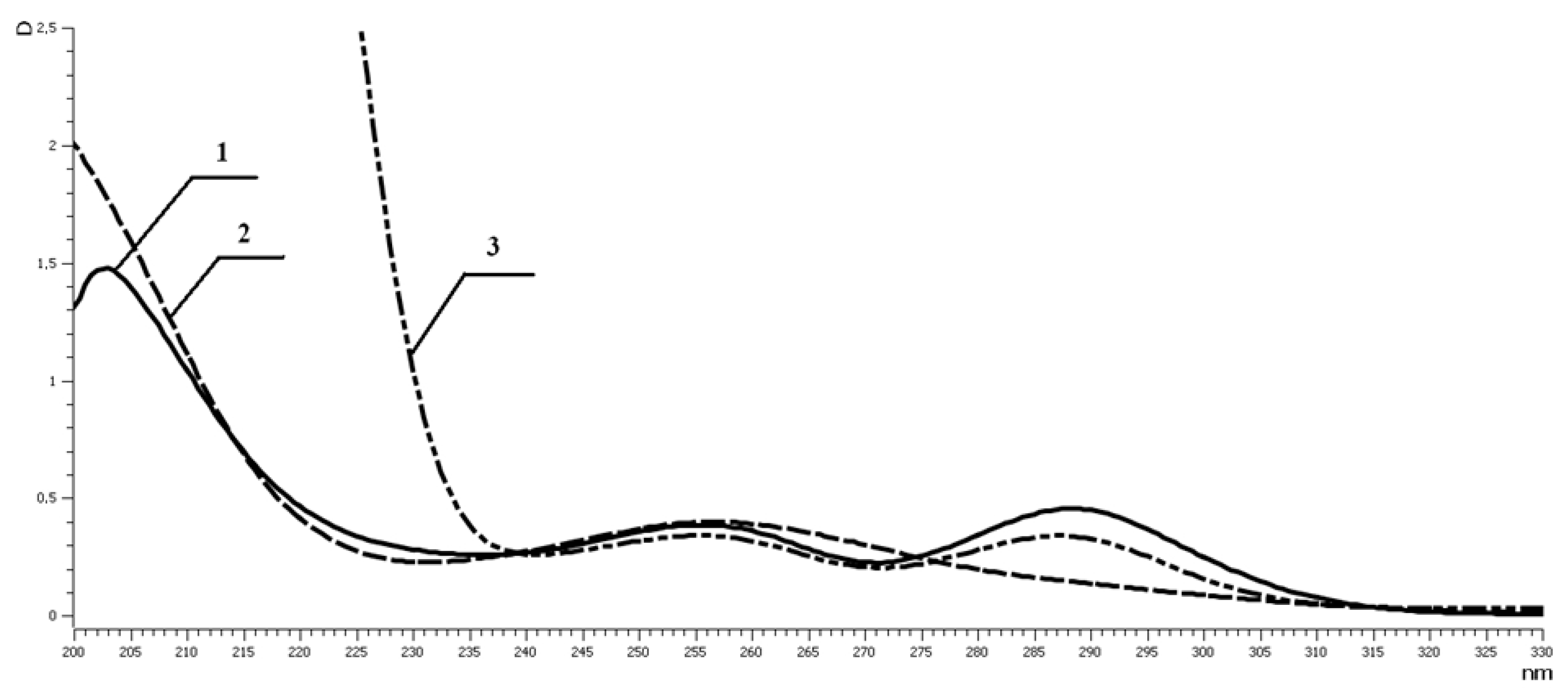
2.5.1. UV Measurements
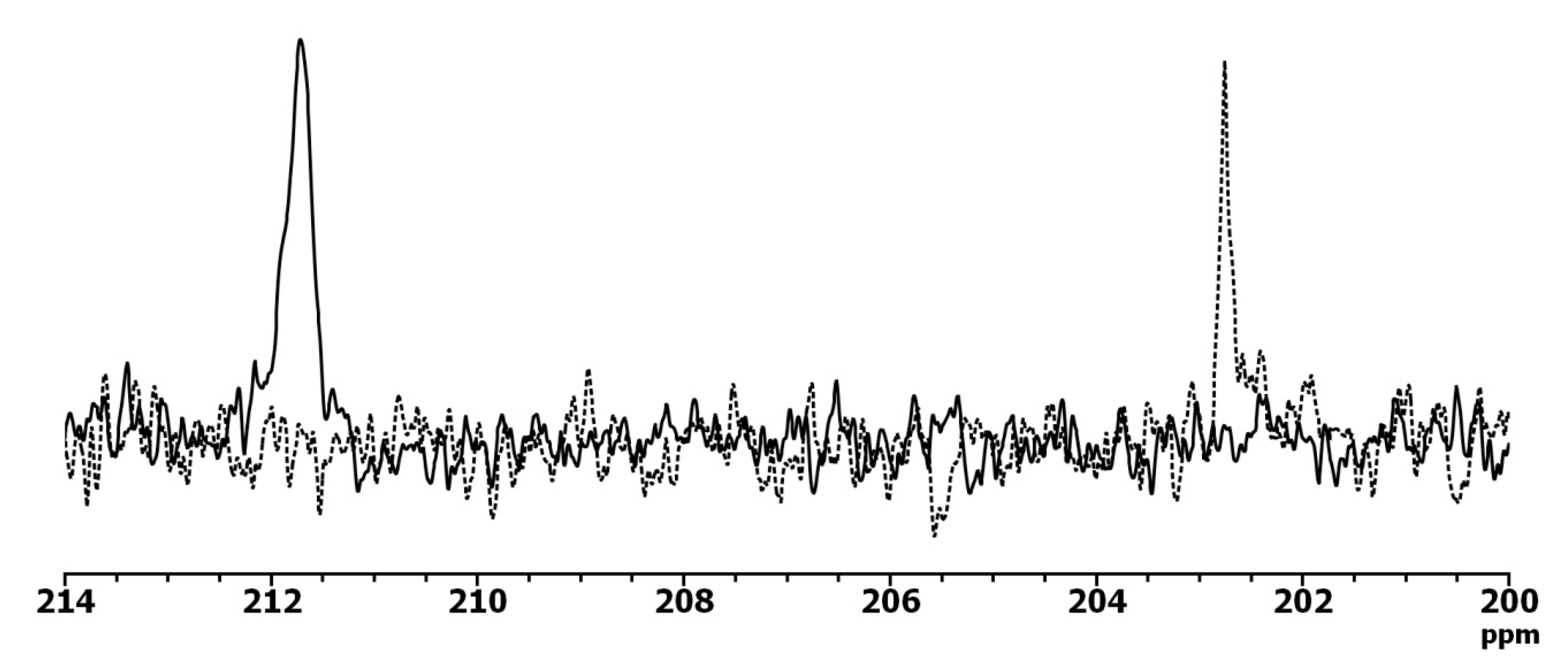
2.5.2. IR Spectroscopy
2.5.3. SEC Measurements
2.6. Molecular Hydrodynamic and Optic Investigation of Copolymers and In-VP–VDTC MPC
2.7. Stability of MPC in Histidine Challenge Reaction (HCR)
3. Materials and Methods
3.1. Chemicals, Reagents and Materials
3.2. Synthesis of N-Vinylpyrrolidone Copolymers
3.2.1. Synthesis of N-Vinylpyrrolidone-N-VFA Copolymers
- 1H NMR (500 MHz, D2O): δ = 1.39–1.83 (CH2CH(C4H6NO), br, 2H), 1.84–2.07 (NCH2CH2CH2CO, br, 2H), 2.08–2.50 (NCH2CH2CH2CO, br, 2H), 2.93–3.38 (NCH2CH2CH2CO, br, 2H), 3.41–3.86 (CH2CH(C4H6NO), br 1H).
- 13C NMR (500 MHz, D2O): δ = 17 (NCH2CH2CH2CO), 31(NCH2CH2CH2CO), 35 (CH2CH(C4H6NO), 44 (NCH2CH2CH2CO), 52 (CH2CH(C4H6NO), 163(-NH-CHO), 177 (C=O).
- IR (KBr) Lactam C=O 1642 cm−1.
- Mn SEC (DMF) = 6.900 Da, Mw/Mn = 1.32.
- Elem. Anal.
- Found: C 18.58%, H 1.21%, N 4.60%.
- Calculated: C 18.56%, H 1.25%, N 4.33%.
3.2.2. Synthesis of Copolymer of N-Vinylpyrrolidone with N-Vinylamine Hydrochloride. Deprotection of PVP–PVFA Copolymers
- 1H NMR (500 MHz, D2O): δ = 1.39–1.83 (CH2CH(C4H6NO), br, 2H), 1.84–2.07 (NCH2CH2CH2CO, br, 2H), 2.08–2.50 (NCH2CH2CH2CO, br, 2H), 2.93–3.38 (NCH2CH2CH2CO, br, 2H), 3.41–3.86 (CH2CH(C4H6NO), br 1H).
- 13C NMR (500 MHz, D2O): δ = 17 (NCH2CH2CH2CO), 31(NCH2CH2CH2CO), 35 (CH2CH(C4H6NO)), 44 (NCH2CH2CH2CO), 52 (CH2CH(C4H6NO)), 177 (C=O).
- IR (KBr) Lactam C=O 1642 cm−1.
- Mn SEC (DMF) = 6.900 Da, Mw/Mn = 1.32.
- Elem. Anal.
- Found: C 51.15%, H 6.75%, N 11.09%.
- Calculated: C 51.01%, H 6.78%, N 11.08%. (
3.2.3. Synthesis of Copolymer of N-Vinylpyrrolidone with Potassium N-Vinyldithiocarbamate
- 1H NMR (500 MHz, D2O): δ = 1.39–1.83 (CH2CH(C4H6NO), br, 2H), 1.84–2.07 (NCH2CH2CH2CO, br, 2H), 2.08–2.50 (NCH2CH2CH2CO, br, 2H), 2.93–3.38 (NCH2CH2CH2CO, br, 2H), 3.41–3.86 (CH2CH(C4H6NO), br 1H).
- 13C NMR (500 MHz, D2O): δ = 17 (NCH2CH2CH2CO), 31(NCH2CH2CH2CO), 35 (CH2CH(C4H6NO)), 44 (NCH2CH2CH2CO), 52 (CH2CH(C4H6NO)), 177 (C=O).
- IR (KBr). Lactam C=O 1642 cm−1
- Mn SEC (DMF) = 6.900 Da, Mw/Mn = 1.32.
- Elem. Anal.
- Found: C 48.66%, H 6.05%, N 10.49%, S 8.08%.
- Calculated: C 48.50%, H 6.00%, N 10.30%, S 8.01%.
3.2.4. Synthesis of Metal–Polymer Complex between Indium and N-Vinylpyrrolidone-Potassium N-vinyldithiocarbamate Copolymer
- 1H NMR (500 MHz, D2O): δ = 1.39–1.83 (CH2CH(C4H6NO), br, 2H), 1.84–2.07 (NCH2CH2CH2CO, br, 2H), 2.08–2.50 (NCH2CH2CH2CO, br, 2H), 2.93–3.38 (NCH2CH2CH2CO, br, 2H), 3.41–3.86 (CH2CH(C4H6NO), br 1H).
- 13C NMR (500 MHz, D2O): δ = 17 (NCH2CH2CH2CO), 31(NCH2CH2CH2CO), 35 (CH2CH(C4H6NO)), 44 (NCH2CH2CH2CO), 52 (CH2CH(C4H6NO)), 177 (C=O).
- IR (KBr). Lactam 1642 cm−1 (νC=O), 976 cm−1 (νC=S), 1126, 1088, 1051(ν N-C=S)
- Mn SEC (DMF) = 6.900 Da, Mw/Mn = 1.32
- Elem. Anal.
- Found: C 48.66%, H 6.05%, N 10.49%, S 8.08%.
- Calculated: C 48.50%, H 6.00%, N 10.30%, S 8.01%3.
3.2.5. Synthesis of PotassiumBis(Carboxymethyl)Dithiocarbamate
- 1H NMR (500 MHz, D2O), δ (ppm): 4.62 (2H, s)
- 13C NMR (500 MHz, D2O), δ (ppm): 211.71 (CSS), 176.54 (−C=O), 171.82(−C=O), 59.34 (CH2), 49.22 (CH2).
- Elem. Anal.
- Found: C 18.58%, H 1.21%, N 4.60%.
- Calculated: C 18.56%, H 1.25%, N 4.33%.
3.3. Instrument and Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oehme, I.; Wolfbeis, O.S. Optical sensors for determination of heavy metal ions. Microchim. Acta 1997, 126, 177–192. [Google Scholar] [CrossRef]
- Rooney, M.T.V.; Seitz, W.R. An optically sensitive membrane for pH based on swellable polymer microspheres in a hydrogel. Anal. Commun. 1999, 36, 267–270. [Google Scholar] [CrossRef]
- Adhikari, B.; Majumdar, S. Polymer in sensor application. Prog. Polym. Sci. 2004, 29, 699. [Google Scholar] [CrossRef]
- Happ, B.; Winter, A.; Hager, M.D.; Schubert, U.S. Photogenerated avenues in macromolecules containing Re(I), Ru(II), Os(II) and Ir(III) metal complexes of pyridine-based ligand. Chem. Soc. Rev. 2012, 41, 2222. [Google Scholar] [CrossRef]
- Cohen, S.M. New approaches for medicinal applications of bioinorganic chemistry. Curr. Opin. Chem. Boil. 2007, 11, 115–120. [Google Scholar] [CrossRef]
- Shen, W.-C.; Beloussow, K.; Meirim, M.G.; Neuse, E.W.; Caldwell, G. Antiproliferative Activity of Polymer-Bound, Monoamine-Coordinated Platinum Complexes Against LNCaP Human Metastatic Prostate Adenocarcinoma Cells. J. Inorg. Organomet. Polym. 2000, 10, 51–60. [Google Scholar] [CrossRef]
- Callari, M.; Aldrich-Wright, J.R.; De Souza, P.L.; Stenzel, M.H. Polymers with platinum drugs and other macromolecular metal complexes for cancer treatment. Prog. Polym. Sci. 2014, 39, 1614–1643. [Google Scholar] [CrossRef]
- Wang, Z.; Niu, G.; Chen, X.S. Polymeric Materials for Theranostic Applications. Pharm. Res. 2013, 31, 1358–1376. [Google Scholar] [CrossRef]
- Pomogailo, A.D.; Kestelman, V.N. Metallopolymer Nanocomposites, LLC; Springer Science and Business Media: Berlin, Germany, 2005. [Google Scholar]
- Gielen, M.; Tiekink, E.R. Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Wang, X.; McHale, R. Metal-Containing Polymers: Building Blocks for Functional (Nano) Materials. Macromol. Rapid Commun. 2009, 31, 331–350. [Google Scholar] [CrossRef]
- Winter, A.; Schubert, U.S. Synthesis and characterization of metallo-supramolecular polymers. Chem. Soc. Rev. 2016, 45, 5311–5357. [Google Scholar] [CrossRef]
- Neumann, L.N.; Urban, D.A.; Lemal, P.; Ramani, S.; Petri-Fink, A.; Balog, S.; Weder, C.; Schrettl, S. Preparation of metallosupramolecular single-chain polymeric nanoparticles and their characterization by Taylor dispersion. Polym. Chem. 2020, 11, 586–592. [Google Scholar] [CrossRef] [Green Version]
- Wöhrle, D.; Pomogailo, A. Metal Complexes and Metals in Macromolecules: Synthesis, Structures and Properites; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- El-Sonbati, A.; Diab, M.; El-Bindary, A. Stoichiometry of Polymer Complexes; IntechOpen: London, UK, 2012. [Google Scholar]
- Hogarth, G. Transition Metal Dithiocarbamates: 1978–2003. In Progress in Inorganic Chemistry Volume 58; Wiley: Hoboken, NJ, USA, 2005; pp. 71–561. [Google Scholar]
- Byr’ko, V.M. Dithiocarbamates; Nauka: Moscow, Russia, 1984. (in Russian) [Google Scholar]
- McClain, A.; Hsieh, Y.-L. Synthesis of polystyrene-supported dithiocarbamates and their complexation with metal ions. J. Appl. Polym. Sci. 2004, 92, 218–225. [Google Scholar] [CrossRef]
- Denizli, A.; Kesenci, K.; Arica, Y.; Pişkin, E.; Arıca, Y. Dithiocarbamate-incorporated monosize polystyrene microspheres for selective removal of mercury ions. React. Funct. Polym. 2000, 44, 235–243. [Google Scholar] [CrossRef]
- Afzali, D.; Favi, A.; Etemadi, F.; Ghazizadeh, A. Application of modified multiwalled carbon nanotubes as solid sorbent for separation and preconcentration of trace amounts of manganese ions. Arab. J. Chem. 2012, 5, 187–191. [Google Scholar] [CrossRef]
- Wang, X.; Jing, S.; Liu, Y.; Qiu, X.; Tan, Y. Preparation of dithiocarbamate polymer brush grafted nanocomposites for rapid and enhanced capture of heavy metal ions. RSC Adv. 2017, 7, 13112–13122. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wu, J.; Li, X.; Ling, Y. Synthesis of poly(dimethyldiallylammonium chloride-co-acrylamide)-graft-triethylenetetramine–dithiocarbamate and its removal performance and mechanism of action towards heavy metal ions. Sep. Purif. Technol. 2013, 103, 92–100. [Google Scholar] [CrossRef]
- Humeres, E.; De Souza, E.P.; Debacher, N.A.; Aliev, A.E. Synthesis and coordinating ability of chitosan dithiocarbamate and analogs towards Cu(II) ions. J. Phys. Org. Chem. 2002, 15, 852–857. [Google Scholar] [CrossRef]
- Liu, B.; Wang, D.; Yu, G.; Meng, X. Removal of F− from aqueous solution using Zr(IV) impregnated dithiocarbamate modified chitosan beads. Chem. Eng. J. 2013, 228, 224–231. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, R.; Ou, S.; Li, Y. Synthesis and adsorption performance of dithiocarbamate-modified glycidyl methacrylate starch. Carbohydr. Polym. 2013, 96, 320–325. [Google Scholar] [CrossRef]
- Abo-Shosha, M.H.; Ibrahim, N.A. Synthesis and Characterization of Starch-Glycidyl Methacrylate-Acrylic Acid Cation Exchange Composites. Starch-Stärke 1992, 44, 466–471. [Google Scholar] [CrossRef]
- Kanchi, S.; Singh, P.; Bisetty, K. Dithiocarbamates as hazardous remediation agent: A critical review on progress in environmental chemistry for inorganic species studies of 20th century. Arab. J. Chem. 2014, 7, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Halls, D.J. The properties of dithiocarbamates A Review. Microchim. Acta 1969, 57, 62–77. [Google Scholar] [CrossRef]
- Oliveira, J.; Rocha, H.A.O.; De Medeiros, W.M.T.Q.; Silva, M.S. Application of Dithiocarbamates as Potential New Antitrypanosomatids-Drugs: Approach Chemistry, Functional and Biological. Molecule 2019, 24, 2806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeyemi, J.O.; Onwudiwe, D.C. Chemistry and Some Biological Potential of Bismuth and Antimony Dithiocarbamate Complexes. Molecules 2020, 25, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.T.; Yum, S.; Heo, J.S.; Kim, W.; Jung, Y.; Kim, Y.M. Dithiocarbamate chitosan as a potential polymeric matrix for controlled drug release. Drug Dev. Ind. Pharm. 2013, 40, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhang, Q.; Zeng, J.; Gu, Z.; Gao, M. Radiolabeling nanomaterials for multimodality imaging: New insights into nuclear medicine and cancer diagnosis. Biomaterials 2019, 228, 119553. [Google Scholar] [CrossRef]
- Arranja, A.G.; Pathak, V.; Lammers, T.; Shi, Y. Tumor-targeted nanomedicines for cancer theranostics. Pharmacol. Res. 2017, 115, 87–95. [Google Scholar] [CrossRef] [Green Version]
- De Kruijff, R.M.; Arranja, A.; Denkova, A. Radiolabeling Methods and Nuclear Imaging Techniques in the Design of New Polymeric Carriers for Cancer Therapy. Curr. Appl. Polym. Sci. 2018, 2, 3–17. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Guo, W.; Jing, H.; Yang, W.; Guo, Z.; Feng, S.; Zhang, X. Radiolabeling of folic acid-modified chitosan with 99mTc as potential agents for folate-receptor-mediated targeting. Bioorganic Med. Chem. Lett. 2011, 21, 6446–6450. [Google Scholar] [CrossRef]
- Talelli, M.; Barz, M.; Rijcken, C.J.; Kiessling, F.; Hennink, W.E.; Lammers, T. Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsh, Y. Water Soluble Poly-N-Vinylamides: Synthesis and Physicochemical Properties; John Wiley& Sons: Chichester, UK, 1998. [Google Scholar]
- Kaneda, Y.; Tsutsumi, Y.; Yoshioka, Y.; Kamada, H.; Yamamoto, Y.; Kodaira, H.; Tsunoda, S.-I.; Okamoto, T.; Mukai, Y.; Shibata, H.; et al. The use of PVP as a polymeric carrier to improve the plasma half-life of drugs. Biomaterials 2004, 25, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Panarin, E.F.; Lavrov, N.A.; Solovskii, M.V.; Shal’nova, L.I. Polymers-carriers of Biologically Active Substances, COP; St.-Petersburg. Professiya: St. Petersburg, Russia, 2014. (in Russian) [Google Scholar]
- Kopeikin, V.; Panarin, Y.; Milevskaya, I.; Redi, N. Tautomeric equilibrium and polymerization activity of methacryloyl acetone. Polym. Sci. U.S.S.R. 1977, 19, 998–1004. [Google Scholar] [CrossRef]
- Urinov, E.; Abdullayev, F.; Mirzayev, U. Molecular and conformational parameters of the copolymer of vinylpyrrolidone with crotonic aldehyde and its complexes with 3d-transition metals in dilute solution. Polym. Sci. U.S.S.R. 1989, 31, 660–666. [Google Scholar] [CrossRef]
- Panarin, E.; Kopeikin, V. On the intermolecular (intra unit) catalysis of hydrolysis of polymeric ketoimine derivatives of amino acids. Bioorg. Chem. 1982, 8, 1207. (in Russian). [Google Scholar]
- Rashidova, S.H.; Urinov, E.U.; Khodjaev, S.G. Role of metal ions in development of specific properties of polymer metal complexes. Makromol. Chemie. Macromol. Symp. 1986, 4, 233–244. [Google Scholar] [CrossRef]
- Tikhonova, L.; Samoilova, O.; Panarin, E.; Yashunskii, V. Iminodiacetic acids based on vinylamine-vinylpyrrolidone copolymers. Polym. Sci. U.S.S.R. 1974, 16, 3081–3087. [Google Scholar] [CrossRef]
- Korshak, V.V.; Shtilman, M.I.; Zalukaeva, T.P.; Kozlov, A.A. Study of some features of co-polymerization of N-vinylpyrrrolidone and some allyl monomers. Polym. Sci. 1980, 22, 591. (in Russian). [Google Scholar]
- Gruz, R.I.; Verkhoglyadova, N.Y.; Panarin, E.F. Synthesis and properties of co-polymers of N-vinylperrolidone and vinylamine. Polym. Sci. U.S.S.R. 1971, 13, 647. [Google Scholar]
- Kirsh, Y.E.; Semina, N.V.; Kalninsh, R.R.; Shatalov, G.V. Radical co-polymerization of N-vinylpyrrolidone and N-vinylformamide. Polym. Sci. 1996, 38, 1905. (in Russian). [Google Scholar]
- Moerlein, S.M.; Welch, M. The chemistry of gallium and indium as related to radiopharmaceutical production. Int. J. Nucl. Med. Boil. 1981, 8, 277–287. [Google Scholar] [CrossRef]
- Gorshkov, N.; Shatik, S.V.; Tokarev, A.V.; Gavrilova, I.I.; Nazarova, O.V.; Murko, A.Y.; Krasikov, V.D.; Panarin, E.F. Synthesis of complexes of N-vinylpyrrolidone–vinylamine or N-vinylpyrrolidone–allylamine containing macrocyclic polyligand 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate (DOTA) with gallium-68 isotope and estimation of their in vivo distribution. Russ. Chem. Bull. 2017, 66, 156–163. [Google Scholar] [CrossRef]
- Gorshkov, N.; Murko, A.Y.; Egorova, O.S.; Malakhova, I.I.; Pokhvoshchev, Y.V.; Krasikov, V.D. Specific features of using ultrashort monolithic columns for analysis of biologically active synthetic polymers labeled with radioactive metal isotopes (99mTc, 161Tb, 68Ga). Prot. Met. Phys. Chem. Surf. 2015, 51, 1094–1099. [Google Scholar] [CrossRef]
- Gorshkov, N.I.; Miroslavov, E.A.; Alekseev, I.E.; Lumpov, A.A.; Murko, A.Y.; Gavrilova, I.I.; Saprykina, N.N.; Bezrukova, M.A.; Kipper, A.I.; Krasikov, V.D.; et al. Study of N-vinilpyrrolidone-N-vinylformamide copolymers labeled with indium-113m. J. Labelld Compd. Radiopharm. 2017, 60, 302. [Google Scholar] [CrossRef]
- Santuryan, Y.G.; Malakhova, I.I.; Gorshkov, N.I.; Krasikov, V.D.; Panarin, E.F. Water-soluble poly(n-vinylamides)as a basis for the synthesis of polymeric carriers of biologically active compounds. Int. J. Polym. Anal. Charact. 2019, 24, 105–113. [Google Scholar] [CrossRef]
- Akintola, O.S.; Saleh, T.A.; Khaled, M.M.; Al-Hamouz, O.C.S. Removal of mercury (II) via a novel series of cross-linked polydithiocarbamates. J. Taiwan Inst. Chem. Eng. 2016, 60, 602–616. [Google Scholar] [CrossRef]
- Al-Hamouz, O.C.S.; Estatie, M.; Saleh, T.A. Removal of cadmium ions from wastewater by dithiocarbamate functionalized pyrrole based terpolymers. Sep. Purif. Technol. 2017, 177, 101–109. [Google Scholar] [CrossRef]
- Gorshkov, N.; Bezrukova, M.A.; Kipper, A.I.; Andreeva, L.N.; Pokhvoschev, Y.V.; Gavrilova, I.I.; Murko, A.Y.; Krasikov, V.D.; Panarin, E.F. Study of complexation between perrhenate ion andN-vinylpyrrolidone/N-vinylamine copolymers. Int. J. Polym. Anal. Charact. 2017, 22, 330–337. [Google Scholar] [CrossRef]
- Jones, M.M.; Burka, L.T.; Hunter, M.E.; Basinger, M.; Campo, G.; Weaver, A.D. Dithiocarbamate chelating agents for toxic heavy metals. J. Inorg. Nucl. Chem. 1980, 42, 775–778. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Wang, S.; Wai, C. Separation of Metal Dithiocarbamate Complexes by High-Performance Liquid Chromatography. J. Chromatogr. Sci. 1994, 32, 506–510. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Andreeva, L.N.; Tsvetkov, N.V. Anisotropy of Segments and Monomer Units of Polymer Molecules. In The Wiley Database of Polymer Properties; Wiley Online Library: Hoboken, NJ, USA, 2002. [Google Scholar]
- Meyers, G.F.; DeKoven, B.M.; Seitz, J.T. Is the molecular surface of polystyrene really glassy? Langmuir 1992, 8, 2330–2335. [Google Scholar] [CrossRef]
- Tsvetkov, V.N. Rigid-Chain Polymers. Die Angew. Makromol. Chem. Appl. Macromol. Chem. Phys. 1989, 109, 1–19. [Google Scholar]
- Pavlov, M.; Panarin, E.F.; Korneeva, E.V.; Kurochkin, K.V.; Baikov, V.E.; Ushakova, V.N. Hydrodynamical properties of molecules of polyvinylpyrrolidone according sedimtntation-diffusion and viscosimetry analysis. Polym. Sci. Ser. B 1990, 32, 1119. [Google Scholar]
Sample Availability: Samples of the original copolymers, synthesized in this work (VP-VFA, VP-VA*HCl, VP-VDTC and corresponding In-VP-VDTC MPC), are available from the authors. |






| Sample | Composition, mol.% | [η], dl/g | D × 107, cm2/s | S, sec | MsD, kDa | Rh, nm | dn/dc, cm3/g | A0 × 1010, erg/K |
|---|---|---|---|---|---|---|---|---|
| VP–VA∙HCl | 94–6 | 0.17 | 7.47 | 0.9 | 27 | 3.3 | 0.154 | 3.32 |
| VP–VDTC | 94–6 | 0.16 | 7.49 | - | - | 4.1 | 0.157 | - |
| VP–VDTC -In | 94–6 | 0.09 | 7.1 | 0.2 | 32 | 1.9 | 0.171 | 3.40 |
| VP–VA∙HCl | 94–6 | 0.10 | 10.94 | 1.2 | 12 | 3.3 | 0.155 | 3.96 |
| VP–VDTC | 94–6 | 0.10 | 10.61 | - | - | 3.8 | 0.158 | - |
| VP–VDTC -In | 94–6 | 0.07 | 10.19 | 2.0 | 14 | 2.4 | 0.178 | 4.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorshkov, N.I.; Murko, A.Y.; Gavrilova, I.I.; Bezrukova, M.A.; Kipper, A.I.; Krasikov, V.D.; Panarin, E.F. Synthesis of Water-Soluble Copolymers of N-vinylpyrrolidone with N-vinyldithiocarbamate as Multidentate Polymeric Chelation Systems and Their Complexes with Indium and Gallium. Molecules 2020, 25, 4681. https://doi.org/10.3390/molecules25204681
Gorshkov NI, Murko AY, Gavrilova II, Bezrukova MA, Kipper AI, Krasikov VD, Panarin EF. Synthesis of Water-Soluble Copolymers of N-vinylpyrrolidone with N-vinyldithiocarbamate as Multidentate Polymeric Chelation Systems and Their Complexes with Indium and Gallium. Molecules. 2020; 25(20):4681. https://doi.org/10.3390/molecules25204681
Chicago/Turabian StyleGorshkov, Nikolay I., Andrey Yu. Murko, Irina I. Gavrilova, Marina A. Bezrukova, Albert I. Kipper, Valerii D. Krasikov, and Evgenii F. Panarin. 2020. "Synthesis of Water-Soluble Copolymers of N-vinylpyrrolidone with N-vinyldithiocarbamate as Multidentate Polymeric Chelation Systems and Their Complexes with Indium and Gallium" Molecules 25, no. 20: 4681. https://doi.org/10.3390/molecules25204681






