Photophysical Properties of Donor-Acceptor Stenhouse Adducts and Their Inclusion Complexes with Cyclodextrins and Cucurbit[7]uril
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizing DASAs as Fluorophores
2.2. Comparative Binding by Cyclodextrins and Cucurbiturils
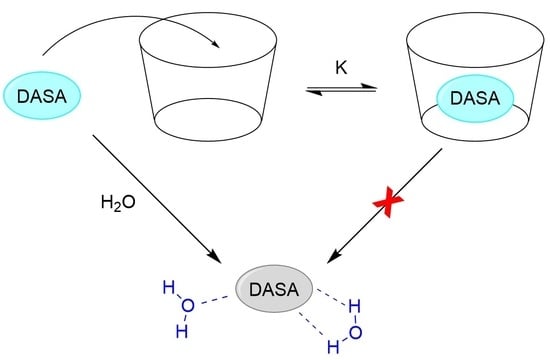
2.3. Dark Switching within Supramolecular Complexes
3. Materials and Methods
3.1. Chemicals and Instrumentation
3.2. Spectroscopic Measurements
3.3. Quantum Yield
3.4. Polarity Sensitivity Factor (PSF)
3.5. Fluorescence Titrations and Binding Constants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lerch, M.M.; Szymański, W.; Feringa, B.L. The (photo)chemistry of Stenhouse photoswitches: Guiding principles and system design. Chem. Soc. Rev. 2018, 47, 1910–1937. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Eisler, S. Photoswitchable double bonds: Synthetic strategies for tunability and versatility. J. Phys. Org. Chem. 2018, 31, e3858. [Google Scholar] [CrossRef]
- Tian, T.; Song, Y.; Wang, J.; Fu, B.; He, Z.; Xu, X.; Li, A.; Zhou, X.; Wang, S.; Zhou, X. Small-molecule-triggered and light-controlled reversible regulation of enzymatic activity. J. Am. Chem. Soc. 2016, 138, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Fong, W.-K.; Graham, B.; Boyd, B.J. Photoswitchable molecules in long-wavelength light-responsive drug delivery: From molecular design to applications. Chem. Mater. 2018, 30, 2873–2887. [Google Scholar] [CrossRef]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–796. [Google Scholar] [CrossRef] [Green Version]
- Minoshima, M.; Kikuchi, K. Photostable and photoswitching fluorescent dyes for super-resolution imaging. J. Biol. Inorg. Chem. 2017, 22, 639–652. [Google Scholar] [CrossRef]
- Beharry, A.A.; Woolley, G.A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011, 40, 4422–4437. [Google Scholar] [CrossRef]
- Kortekaas, L.; Browne, W.R. The evolution of spiropyran: Fundamentals and progress of an extraordinarily versatile photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424. [Google Scholar] [CrossRef] [Green Version]
- Wiedbrauk, S.; Dube, H. Hemithioindigo—An emerging photoswitch. Tetrahedron Lett. 2015, 56, 4266–4274. [Google Scholar] [CrossRef]
- Dempsey, G.T.; Bates, M.; Kowtoniuk, W.E.; Liu, D.R.; Tsien, R.Y.; Zhuang, X. Photoswitching mechanism of cyanine dyes. J. Am. Chem. Soc. 2009, 131, 18192–18193. [Google Scholar] [CrossRef] [Green Version]
- Helmy, S.; Oh, S.; Leibfarth, F.A.; Hawker, C.J.; Read de Alaniz, J. Design and synthesis of donor–acceptor Stenhouse adducts: A visible light photoswitch derived from furfural. J. Org. Chem. 2014, 79, 11316–11329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenhouse, J. Ueber furfuranilin und furfurtoluidin. Justus Liebigs Ann. Chem. 1870, 156, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Honda, K.; Komizu, H.; Kawasaki, M. Reverse photochromism of Stenhouse salts. J. Chem. Soc. Chem. Commun. 1982, 253–254. [Google Scholar] [CrossRef]
- D’Arcy, B.R.; Lewis, K.G.; Mulquiney, C.E. Reactions of Stenhouse salts. III. Transformation products under acidic and basic conditions. Aust. J. Chem. 1985, 38, 953–965. [Google Scholar] [CrossRef]
- Šafár, P.; Provažanec, F.; Prónayová, N.; Baran, P.; Kickelbick, G.; Kožíšek, J.; Breza, M. Dichotomy in the ring opening reaction of 5-[(2-Furyl)methylidene]-2,2-dimethyl-1,3-dioxane-4,6-dione with cyclic secondary amines. Collect. Czech. Chem. Commun. 2000, 65, 1911–1938. [Google Scholar] [CrossRef]
- Helmy, S.; Leibfarth, F.A.; Oh, S.; Poelma, J.E.; Hawker, C.J.; Read de Alaniz, J. Photoswitching using visible light: A new class of organic photochromic molecules. J. Am. Chem. Soc. 2014, 136, 8169–8172. [Google Scholar] [CrossRef] [Green Version]
- Lerch, M.M.; Hansen, M.J.; Velema, W.A.; Szymanski, W.; Feringa, B.L. Orthogonal photoswitching in a multifunctional molecular system. Nat. Commun. 2016, 7, 12054. [Google Scholar] [CrossRef] [Green Version]
- Klikar, M.; Jelínková, V.; Růžičková, Z.; Mikysek, T.; Pytela, O.; Ludwig, M.; Bureš, F. Malonic acid derivatives on duty as electron-withdrawing units in push–pull molecules. Eur. J. Org. Chem. 2017, 2017, 2764–2779. [Google Scholar] [CrossRef]
- Kleinpeter, E.; Schulenburg, A. Quantification of the push–pull effect in substituted alkenes. Tetrahedron Lett. 2005, 46, 5995–5997. [Google Scholar] [CrossRef]
- Lerch, M.M.; Wezenberg, S.J.; Szymanski, W.; Feringa, B.L. Unraveling the photoswitching mechanism in donor–acceptor Stenhouse adducts. J. Am. Chem. Soc. 2016, 138, 6344–6347. [Google Scholar] [CrossRef]
- Di Donato, M.; Lerch, M.M.; Lapini, A.; Laurent, A.D.; Iagatti, A.; Bussotti, L.; Ihrig, S.P.; Medved’, M.; Jacquemin, D.; Szymański, W.; et al. Shedding light on the photoisomerization pathway of donor-acceptor Stenhouse adducts. J. Am. Chem. Soc. 2017, 139, 15596–15599. [Google Scholar] [CrossRef] [PubMed]
- Zulfikri, H.; Koenis, M.A.J.; Lerch, M.M.; Di Donato, M.; Szymański, W.; Filippi, C.; Feringa, B.L.; Buma, W.J. Taming the complexity of donor-acceptor Stenhouse adducts: Infrared motion pictures of the complete switching pathway. J. Am. Chem. Soc. 2019, 141, 7376–7384. [Google Scholar] [CrossRef]
- Laurent, A.D.; Medved’, M.; Jacquemin, D. Using time-dependent functional theory to probe the nature of donor-acceptor stenhoue adduct photochromes. ChemPhysChem 2016, 17, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Ugandi, M.; Roemelt, M. An ab initio computational study of electronic and structural factors in the isomerization of donor-acceptor Stenhouse adducts. J. Phys. Chem. A 2020, 124, 7756–7767. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.M.; Raucci, U.; Ferrerars, K.N.; Martínez, T.J. Putting photochemical switches to work: An ab initio multiple spawning study of donor-acceptor Stenhouse adducts. J. Phys. Chem. Lett. 2020, 11, 7901–7907. [Google Scholar] [CrossRef]
- Poelma, S.O.; Oh, S.S.; Helmy, S.; Knight, A.S.; Burnett, G.L.; Soh, H.T.; Hawker, C.J.; Read de Alaniz, J. Controlled drug release to cancer cells from modular one-photon visible light-responsive micellar system. Chem. Commun. 2016, 52, 10525–10528. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Friedel, K.; Himmerlich, M.; Lei, Y.; Schlingloff, G.; Schober, A. Spatiotemporal photopatterning on polycarbonate surface through visible light responsive polymer bound DASA compounds. ACS Macro Lett. 2015, 4, 1273–1277. [Google Scholar] [CrossRef]
- Diaz, Y.J.; Page, Z.A.; Knight, A.S.; Treat, N.J.; Hemmer, J.R.; Hawker, C.J.; Read de Alaniz, J. A versatile and highly selective colorimetric sensor for the detection of amines. Chem. Eur. J. 2017, 23, 3562–3566. [Google Scholar] [CrossRef] [Green Version]
- Zhong, D.; Cao, Z.; Wu, B.; Zhang, Q.; Wang, G. Polymer dots of DASA-functionalized polyethyleneimine: Synthesis, visible light/pH responsiveness, and their applications as chemosensors. Sens. Actuators B: Chem. 2018, 254, 385–392. [Google Scholar] [CrossRef]
- Yang, S.; Liu, J.; Cao, Z.; Li, M.; Luo, Q.; Qu, D. Fluorescent photochromic donor-acceptor Stenhouse adduct controlled by visible light. Dyes Pigm. 2018, 148, 341–347. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, L.; Zhao, H.; Wu, J.; Wagner, M.; Sun, W.; Liu, X.; Miao, M.; Zheng, Y. Inducing molecular isomerization assisted by water. Commun. Chem. 2019, 2, 118. [Google Scholar] [CrossRef] [Green Version]
- Steed, W.J.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; John Wiley & Son: Chichester, UK, 2009; p. 38. [Google Scholar]
- Johnston, L.J.; Wagner, B.D. Electronic absorption and luminescence. In Comprehensive Supramolecular Chemistry; Physical Methods in Supramolecular Chemistry; Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Vögtle, F., Lehn, J., Ripmeester, J.A., Eds.; Pergamon Press: Oxford, UK, 1996; Volume 8, pp. 537–566. [Google Scholar]
- Saha, R.; Devaraj, A.; Bhattacharyya, S.; Das, S.; Zangrando, E.; Mukherjee, P.S. Unusual behavior of donor-acceptor Stenhouse adducts in confined space of a water-soluble PdII8 molecular vessel. J. Am. Chem. Soc. 2019, 141, 8638–8645. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Montes-Navajas, P.; González-Béjar, M.; Scaiano, J.C.; García, H. Cucurbituril complexes cross the cell membrane. Photochem. Photobiol. Sci. 2009, 8, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Guether, R.; Reddington, M.V. Photostable cyanine dye β-Cyclodextrin conjugates. Tetrahedron Lett. 1997, 38, 6167–6170. [Google Scholar] [CrossRef]
- Gadde, S.; Batchelor, E.K.; Weiss, J.P.; Ling, Y.; Kaifer, A.E. Control of H- and J-aggregate formation via host−guest complexation using cucurbituril hosts. J. Am. Chem. Soc. 2008, 130, 17114–17119. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Gao, C.; Sun, R.; Wang, Q. Enhancing photostability of cyanine dye by cucurbituril encapsulation. Dyes Pigm. 2012, 94, 266–270. [Google Scholar] [CrossRef]
- Wagner, B.D. Fluorescence studies of supramolecular host-guest inclusion complexes. In Handbook of Photochemistry and Photobiology; Supramolecular Photochemistry; Nalwa, H.S., Ed.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2003; Volume 3, pp. 1–57. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer Science + Business Media, LLC: Singapore, 2006; pp. 54–55. [Google Scholar]
- Wagner, B.D.; Arnold, A.E.; Gallant, S.T.; Grinton, C.R.; Locke, J.K.; Mills, N.D.; Snow, C.A.; Uhlig, T.B.; Vessey, C.N. The polarity sensitivity factor of some fluorescent probe molecules used for studying supramolecular systems and other heterogeneous environments. Can. J. Chem. 2018, 96, 629–635. [Google Scholar] [CrossRef]
- Wu, B.; Xue, T.; Wang, W.; Li, S.; Shen, J.; He, Y. Visible light triggered aggregation-induced emission switching with a donor–acceptor Stenhouse adduct. J. Mater. Chem. C 2018, 6, 8538–8545. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Matsubara, Y.; Ochi, T.; Wakamiya, T.; Yoshida, Z. How the π conjugation length affects the fluorescence emission efficiency. J. Am. Chem. Soc. 2008, 130, 13867–13869. [Google Scholar] [CrossRef] [PubMed]
- Bublitz, G.U.; Ortiz, R.; Marder, S.R.; Boxer, S.G. Stark spectroscopy of donor/acceptor substituted polyenes. J. Am. Chem. Soc. 1997, 119, 3365–3376. [Google Scholar] [CrossRef]
- Kulinich, A.V.; Ishchenko, A.A. Merocyanine dyes: Synthesis, structure, properties and applications. Russ. Chem. Rev. 2009, 78, 141–164. [Google Scholar] [CrossRef]
- Kulinich, A.V.; Mikitenko, E.K.; Ishchenko, A.A. Scope of negative solvatochromism and solvatofluorochromism of merocyanines. Phys. Chem. Chem. Phys. 2016, 18, 3444–3453. [Google Scholar] [CrossRef]
- Ishchenko, A.A.; Svidro, V.A.; Derevyanko, N.A. Solvatofluorochromy of cationic cyanine dyes. Dyes Pigm. 1989, 10, 85–96. [Google Scholar] [CrossRef]
- Kim, J.; Jung, I.-S.; Kim, S.-Y.; Lee, E.; Kang, J.-K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New cucurbituril homologues: Syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar] [CrossRef]
- Muňoz de la Peňa, A.; Salinas, F.; Gómez, M.J.; Acedo, M.I.; Sánchez Peňa, M.J. Absorptiometric and spectrofluorimetric study of the inclusion complexes of 2-naphthyloxyacetic acid and 1-naphthylacetic acid with β-cyclodextrin in aqueous solution. Inclus. Phenom. Mol. Recog. Chem. 1993, 15, 131–143. [Google Scholar] [CrossRef]
- Freeman, W.A.; Mock, W.L.; Shih, N.Y. Cucurbituril. J. Am. Chem. Soc. 1981, 103, 7367–7368. [Google Scholar] [CrossRef]
- Assaf, K.I.; Nau, W.M. Cucurbiturils: From synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 2015, 44, 394–418. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Selvapalam, N.; Ko, Y.H.; Park, K.M.; Kim, D.; Kim, J. Functionalized cucurbiturils and their applications. Chem. Soc. Rev. 2007, 36, 267–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Ruspic, C.; Mukhopadhyay, P.; Chakrabarti, S.; Zavalij, P.Y.; Isaacs, L. The Cucurbit[n]uril family: Prime components for self-sorting systems. J. Am. Chem. Soc. 2005, 127, 15959–15967. [Google Scholar] [CrossRef] [PubMed]
- Bigi, F.; Carloni, S.; Ferrari, L.; Maggi, R.; Mazzacani, A.; Sartori, G. Clean synthesis in water. Part 2: Uncatalysed condensation reaction of Meldrum’s acid and aldehydes. Tetrahedron Lett. 2001, 42, 5203–5205. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |








| DASA-M | DASA-B | |||
|---|---|---|---|---|
| Parameter | Water | Ethanol | Water | Ethanol |
| λA,max (nm) | 482 | 521 | 500 | 544 |
| λF,max (nm) | 543 | 557 | 567 | 578 |
| Stokes Shift (nm) | 61 | 36 | 67 | 34 |
| Stokes Shift (cm−1) | 2331 | 1241 | 2344 | 1081 |
| Photophysical Property | DASA-M | DASA-B |
|---|---|---|
| Absorption spectrum red shift * (nm) | 39 | 44 |
| Absorption spectrum red shift * (cm−1) | 1553 | 1618 |
| Emission spectrum red shift * (nm) | 14 | 11 |
| Emission spectrum red shift * (cm−1) | 463 | 335 |
| Quantum yield in water (ΦF) | 2.5 × 10−4 | 12 × 10−4 |
| Polarity sensitivity factor (PSF) | 1.37 | 1.01 |
| K (M−1) | ||
|---|---|---|
| Host | DASA-M | DASA-B |
| HP-γ-CD | 60 | 39 |
| CB[7] | 27000 | 89000 |
| DASA-M | DASA-B | |||
|---|---|---|---|---|
| Condition | k (10−5 s−1) | τ½ (Min.) | k (10−5 s−1) | τ½ (Min.) |
| no host | 14.6 ± 0.2 | 79 ± 1 | 12.1 ± 0.5 | 95 ± 2 |
| 20 mM HP-γ-CD | 7.0 ± 0.1 | 165 ± 2 | 14.5 ± 2.5 | 81 ± 14 |
| 2 mM CB[7] | 4.8 ± 0.4 | 242 ± 19 | 3.5 ± 0.1 | 330 ± 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payne, L.; Josephson, J.D.; Murphy, R.S.; Wagner, B.D. Photophysical Properties of Donor-Acceptor Stenhouse Adducts and Their Inclusion Complexes with Cyclodextrins and Cucurbit[7]uril. Molecules 2020, 25, 4928. https://doi.org/10.3390/molecules25214928
Payne L, Josephson JD, Murphy RS, Wagner BD. Photophysical Properties of Donor-Acceptor Stenhouse Adducts and Their Inclusion Complexes with Cyclodextrins and Cucurbit[7]uril. Molecules. 2020; 25(21):4928. https://doi.org/10.3390/molecules25214928
Chicago/Turabian StylePayne, Liam, Jason D. Josephson, R. Scott Murphy, and Brian D. Wagner. 2020. "Photophysical Properties of Donor-Acceptor Stenhouse Adducts and Their Inclusion Complexes with Cyclodextrins and Cucurbit[7]uril" Molecules 25, no. 21: 4928. https://doi.org/10.3390/molecules25214928