Synthesis and Biological Application of Polylactic Acid
Abstract
:1. Introduction
2. PLA Synthesis and Properties
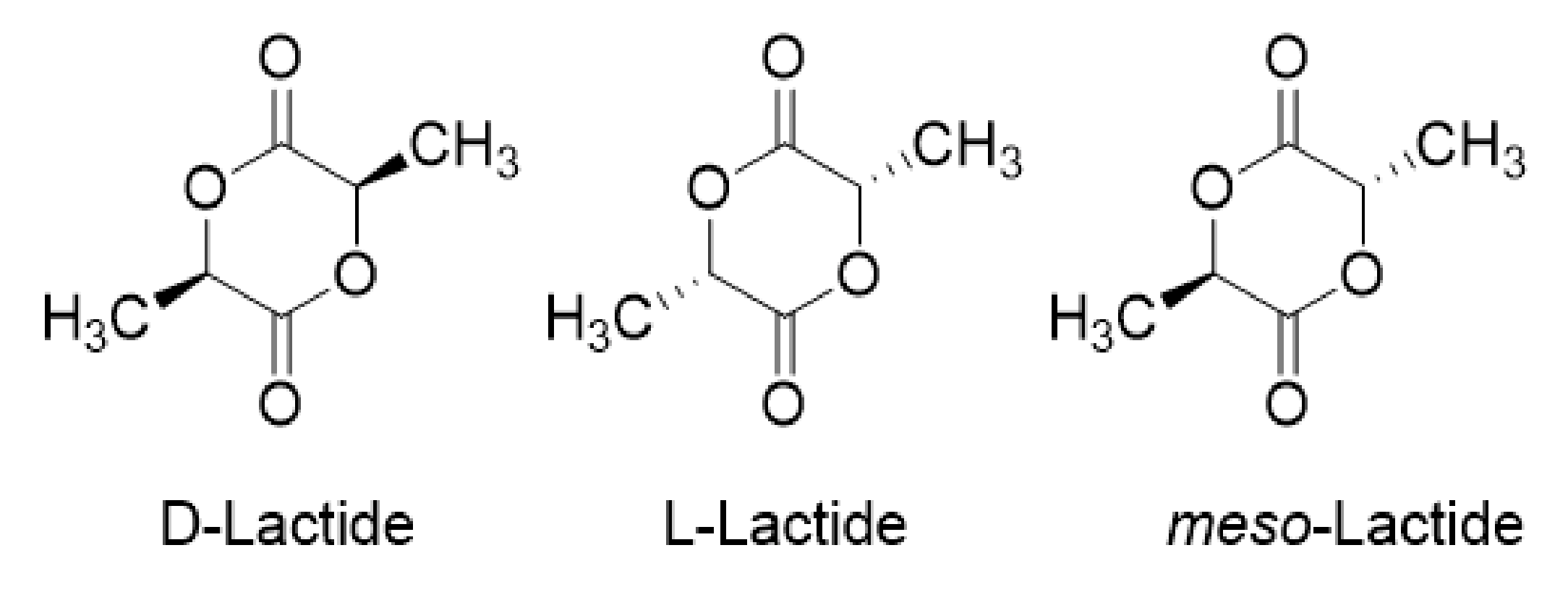
2.1. Lactic Acid
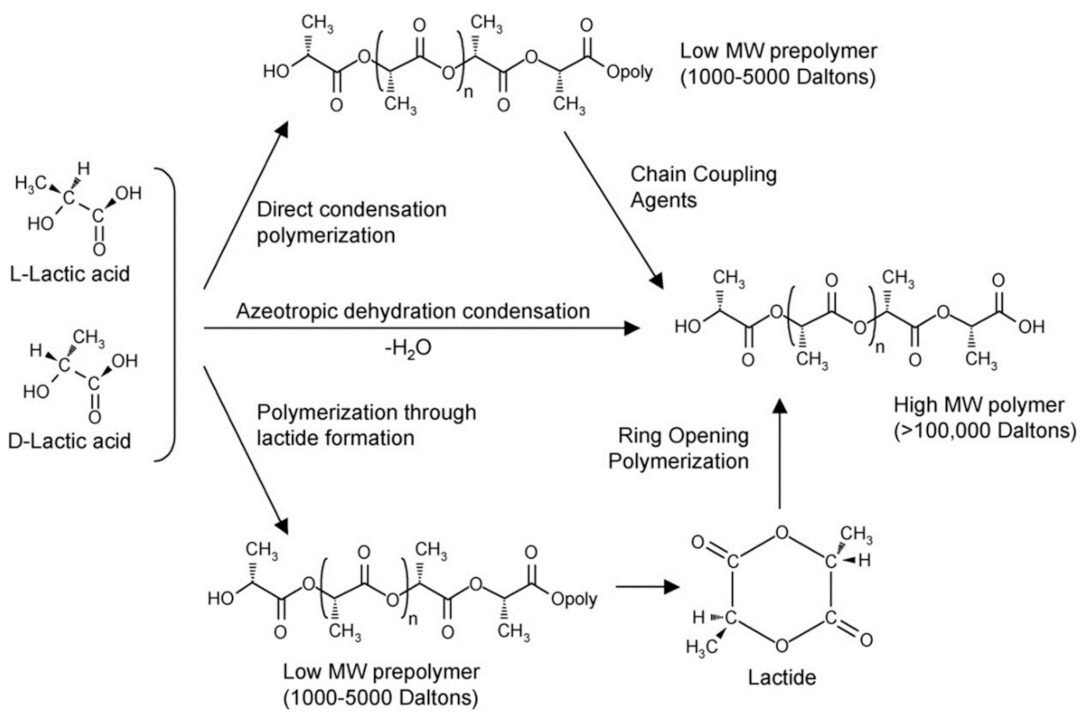
2.2. PLA Polymer
2.3. PLA Properties
2.4. Improvement of PLA Properties
3. Application
3.1. Tissue Engineering
3.2. Drug Delivery
3.3. Implants
4. Conclusions
Funding
Conflicts of Interest
References
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibres, biodegradable polymers and biocomposites: An overview. Macromol. Mater. Eng. 2000, 276, 1–24. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Averous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Adsul, M.G.; Varma, A.J.; Gokhale, D.V. Lactic acid production from waste sugarcane bagasse derived cellulose. Green Chem. 2007, 9, 58–62. [Google Scholar] [CrossRef]
- Singhvi, M.; Joshi, D.; Adsul, M.; Varma, A.; Gokhale, D. d-(−)-Lactic acid production from cellobiose and cellulose by Lactobacillus lactis mutant RM2-24. Green Chem. 2010, 12, 1106–1109. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sonomoto, K. Improved lactic acid productivity by an open repeated batch fermentation system using Enterococcus mundtii QU 25. RSC Adv. 2013, 3, 8437–8445. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Sonomoto, K. Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J. Biotechnol. 2016, 236, 176–192. [Google Scholar] [CrossRef]
- Chen, C.C.; Chueh, J.Y.; Tseng, H.; Huang, H.M.; Lee, S.Y. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar] [CrossRef]
- Martin, O.; Averous, L. Poly (lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Direct lactic acid fermentation: Focus on simultaneous saccharification and lactic acid production. Biotechnol. Adv. 2009, 27, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly (lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Graupner, N.; Herrmann, A.S.; Muessig, J. Natural and man-made cellulose fibre-reinforced poly(lactic acid) (PLA) composites: An overview about mechanical characteristics and application areas. Compos. Part A-Appl. Sci. Manuf. 2009, 40, 810–821. [Google Scholar] [CrossRef]
- Dutkiewicz, S.; Grochowska-Lapienis, D.; Tomaszewski, W. Synthesis of poly(L(+) lactic acid) by polycondensation method in solution. Fibres Text. East. Eur. 2003, 11, 66–70. [Google Scholar]
- Anakhu, P.I.; Bolu, C.C.; Abioye, A.A.; Onyiagha, G.; Boyo, H.; Jolayemi, K.; Azeta, J. Innovative Foundry Technology and Material Using Fused Deposition Modeling and Polylactic Acid Material in Sand Casing. Arch. Foundry Eng. 2018, 18, 65–71. [Google Scholar]
- Deng, K.; Chen, H.; Zhao, Y.; Zhou, Y.; Wang, Y.; Sun, Y. Evaluation of adaptation of the polylactic acid pattern of maxillary complete dentures fabricated by fused deposition modelling technology: A pilot study. PLoS ONE 2018, 13, e0201777. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Wu, B.; Cui, C.; Guo, Y.; Yan, C. A critical review of fused deposition modeling 3D printing technology in manufacturing polylactic acid parts. Int. J. Adv. Manuf. Technol. 2019, 102, 2877–2889. [Google Scholar] [CrossRef]
- Shao, W.; He, J.; Han, Q.; Sang, F.; Wang, Q.; Chen, L.; Cui, S.; Ding, B. A biomimetic multilayer nanofiber fabric fabricated by electrospinning and textile technology from polylactic acid and Tussah silk fibroin as a scaffold for bone tissue engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 67, 599–610. [Google Scholar] [CrossRef]
- Tsukegi, T.; Nagasawa, N.; Horii, T.; Nishida, H. Chemical Recycling Technology of Polylactic Acid/talc-Application into Agricultural Materials. Kobunshi Ronbunshu 2015, 72, 361–368. [Google Scholar] [CrossRef]
- Varadarajan, S.; Miller, D.J. Catalytic upgrading of fermentation-derived organic acids. Biotechnol. Prog. 1999, 15, 845–854. [Google Scholar] [CrossRef]
- Chanfreau, S.; Mena, M.; Porras-Dominguez, J.R.; Ramirez-Gilly, M.; Gimeno, M.; Roquero, P.; Tecante, A.; Barzana, E. Enzymatic synthesis of poly-l-lactide and poly-l-lactide-co-glycolide in an ionic liquid. Bioprocess. Biosyst. Eng. 2010, 33, 629–638. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegradable polymers-Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Yao, K.; Tang, C. Controlled Polymerization of Next-Generation Renewable Monomers and Beyond. Macromolecules 2013, 46, 1689–1712. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. An efficient solid-state polycondensation method for synthesizing stereocomplexed poly (lactic acid)s with high molecular weight. J. Polym. Sci. Part A-Polym. Chem. 2008, 46, 3714–3722. [Google Scholar] [CrossRef]
- Bud, R. A history of lactic-acid making–A chapter in the history of biotechnology-benninga,h. Ann. Sci. 1993, 50, 597. [Google Scholar]
- Lampe, K.J.; Namba, R.M.; Silverman, T.R.; Bjugstad, K.B.; Mahoney, M.J. Impact of Lactic Acid on Cell Proliferation and Free Radical-Induced Cell Death in Monolayer Cultures of Neural Precursor Cells. Biotechnol. Bioeng. 2009, 103, 1214–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Chamberlain, B.M.; Cheng, M.; Moore, D.R.; Ovitt, T.M.; Lobkovsky, E.B.; Coates, G.W. Polymerization of lactide with zinc and magnesium beta-diiminate complexes: Stereocontrol and mechanism. J. Am. Chem. Soc. 2001, 123, 3229–3238. [Google Scholar] [CrossRef]
- Oksman, K.; Skrifvars, M.; Selin, J.F. Natural fibres as reinforcement in polylactic acid (PLA) composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- Tsuji, H. Poly (lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Peres, C.; Matos, A.I.; Conniot, J.; Sainz, V.; Zupancic, E.; Silva, J.M.; Graca, L.; Gaspar, R.S.; Preat, V.; Florindo, H.F. Poly (lactic acid)-based particulate systems are promising tools for immune modulation. Acta Biomater. 2017, 48, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Ahmed, F. Polymersomes. Annu. Rev. Biomed. Eng. 2006, 8, 323–341. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly (lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Oksman, K.; Mathew, A.P.; Bondeson, D.; Kvien, I. Manufacturing process of cellulose whiskers/polylactic acid nanocomposites. Compos. Sci. Technol. 2006, 66, 2776–2784. [Google Scholar] [CrossRef]
- Ray, S.S.; Maiti, P.; Okamoto, M.; Yamada, K.; Ueda, K. New polylactide/layered silicate nanocomposites. 1. Preparation, characterization, and properties. Macromolecules 2002, 35, 3104–3110. [Google Scholar]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Maciel Filho, R. Poly-lactic acid synthesis for application in biomedical devices-A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Biodegradable polylactide and its nanocomposites: Opening a new dimension for plastics and composites. Macromol. Rapid Commun. 2003, 24, 815–840. [Google Scholar] [CrossRef]
- Bax, B.; Muessig, J. Impact and tensile properties of PLA/Cordenka and PLA/flax composites. Compos. Sci. Technol. 2008, 68, 1601–1607. [Google Scholar] [CrossRef] [Green Version]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.-C.; Sodergard, A. From Lactic Acid to Poly(lactic acid) (PLA): Characterization and Analysis of PLA and Its Precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Ochi, S. Mechanical properties of kenaf fibers and kenaf/PLA composites. Mech. Mater. 2008, 40, 446–452. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A-Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Matsumoto, K.I.; Taguchi, S. Enzymatic and whole-cell synthesis of lactate-containing polyesters: Toward the complete biological production of polylactate. Appl. Microbiol. Biotechnol. 2010, 85, 921–932. [Google Scholar] [CrossRef]
- Suryanegara, L.; Nakagaito, A.N.; Yano, H. The effect of crystallization of PLA on the thermal and mechanical properties of microfibrillated cellulose-reinforced PLA composites. Compos. Sci. Technol. 2009, 69, 1187–1192. [Google Scholar] [CrossRef]
- Carrasco, F.; Pages, P.; Gamez-Perez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly (lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J.M. Multifunctional bionanocomposite films of poly (lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Vasir, J.K.; Labhasetwar, V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv. Rev. 2007, 59, 718–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly (lactic acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, J. Research Progress in Toughening Modification of Poly (lactic acid). J. Polym. Sci. Part B-Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Effect of fiber surface-treatments on the properties of laminated biocomposites from poly (lactic acid) (PLA) and kenaf fibers. Compos. Sci. Technol. 2008, 68, 424–432. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Agarwal, M.; Koelling, K.W.; Chalmers, J.J. Characterization of the degradation of polylactic acid polymer in a solid substrate environment. Biotechnol. Prog. 1998, 14, 517–526. [Google Scholar] [CrossRef]
- Gritsch, L.; Conoscenti, G.; La Carrubba, V.; Nooeaid, P.; Boccaccini, A.R. Polylactide-based materials science strategies to improve tissue-material interface without the use of growth factors or other biological molecules. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 94, 1083–1101. [Google Scholar] [CrossRef]
- Codari, F.; Lazzari, S.; Soos, M.; Storti, G.; Morbidelli, M.; Moscatelli, D. Kinetics of the hydrolytic degradation of poly (lactic acid). Polym. Degrad. Stab. 2012, 97, 2460–2466. [Google Scholar] [CrossRef]
- De Jong, S.J.; Arias, E.R.; Rijkers, D.T.S.; van Nostrum, C.F.; Kettenes-van den Bosch, J.J.; Hennink, W.E. New insights into the hydrolytic degradation of poly (lactic acid): Participation of the alcohol terminus. Polymer 2001, 42, 2795–2802. [Google Scholar] [CrossRef]
- Zalusky, A.S.; Olayo-Valles, R.; Wolf, J.H.; Hillmyer, M.A. Ordered nanoporous polymers from polystyrene-polylactide block copolymers. J. Am. Chem. Soc. 2002, 124, 12761–12773. [Google Scholar] [CrossRef]
- Agrawal, C.M.; Athanasiou, K.A. Technique to control pH in vicinity of biodegrading PLA-PGA implants. J. Biomed. Mater. Res. 1997, 38, 105–114. [Google Scholar] [CrossRef]
- Vaz, C.M.; van Tuijl, S.; Bouten, C.V.C.; Baaijens, F.P.T. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. 2005, 1, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable Polymers-A Review on Recent Trends and Emerging Perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Sodergard, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Maiti, P.; Yamada, K.; Okamoto, M.; Ueda, K.; Okamoto, K. New polylactide/layered silicate nanocomposites: Role of organoclays. Chem. Mater. 2002, 14, 4654–4661. [Google Scholar] [CrossRef]
- Paul, M.A.; Alexandre, M.; Degee, P.; Henrist, C.; Rulmont, A.; Dubois, P. New nanocomposite materials based on plasticized poly (l-lactide) and organo-modified montmorillonites: Thermal and morphological study. Polymer 2003, 44, 443–450. [Google Scholar] [CrossRef]
- Paul, M.A.; Delcourt, C.; Alexandre, M.; Degee, P.; Monteverde, F.; Rulmont, A.; Dubois, P. (Plasticized) polylactide/(organo-)clay nanocomposites by in situ intercalative polymerization. Macromol. Chem. Phys. 2005, 206, 484–498. [Google Scholar] [CrossRef]
- Pluta, M.; Paul, M.A.; Alexandre, M.; Dubois, P. Plasticized polylactide/clay nanocomposites. I. The role of filler content and its surface organo-modification on the physico-chemical properties. J. Polym. Sci. Part B-Polym. Phys. 2006, 44, 299–311. [Google Scholar] [CrossRef]
- Tanoue, S.; Hasook, A.; Iemoto, Y.; Unryu, T. Preparation of poly (lactic acid)/poly(ethylene glycol)/organoclay nanocomposites by melt compounding. Polym. Compos. 2006, 27, 256–263. [Google Scholar] [CrossRef]
- Kim, Y.; Dalhaimer, P.; Christian, D.A.; Discher, D.E. Polymeric worm micelles as nano-carriers for drug delivery. Nanotechnology 2005, 16, S484–S491. [Google Scholar] [CrossRef]
- Pluta, M.; Paul, M.A.; Alexandre, M.; Dubois, P. Plasticized polylactide/clay nanocomposites. II. The effect of aging on structure and properties in relation to the filler content and the nature of its organo-modification. J. Polym. Sci. Part B-Polym. Phys. 2006, 44, 312–325. [Google Scholar] [CrossRef]
- Shasteen, C.; Choy, Y.B. Controlling Degradation Rate of Poly (lactic acid) for Its Biomedical Applications. Biomed. Eng. Lett. 2011, 1, 163–167. [Google Scholar] [CrossRef]
- Vieira, A.C.; Guedes, R.M.; Tita, V. Damage-induced hydrolyses modelling of biodegradable polymers for tendons and ligaments repair. J. Biomech. 2015, 48, 3478–3485. [Google Scholar] [CrossRef] [Green Version]
- Grande, R.; Carvalho, A.J.F. Compatible Ternary Blends of Chitosan/poly (vinyl alcohol)/poly(lactic acid) Produced by Oil-in-Water Emulsion Processing. Biomacromolecules 2011, 12, 907–914. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nottelet, B.; Darcos, V.; Coudane, J. Aliphatic polyesters for medical imaging and theranostic applications. Eur. J. Pharm. Biopharm. 2015, 97, 350–370. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.S. Diversity-oriented synthesis: Exploring the intersections between chemistry and biology. Nat. Chem. Biol. 2005, 1, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G.; Goodnough, L.T. Medical progress: Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [Green Version]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar]
- Hebert, P.C.; Wells, G.; Blajchman, M.A.; Marshall, J.; Martin, C.; Pagliarello, G.; Tweeddale, M.; Schweitzer, I.; Yetisir, E.; Canadian Critical Care Trials, G. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N. Engl. J. Med. 1999, 340, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.S. From artificial red blood cells, oxygen carriers, and oxygen therapeutics to artificial cells, nanomedicine, and beyond. Artif. Cells Blood Substit. Biotechnol. 2012, 40, 197–199. [Google Scholar] [CrossRef]
- Chang, T.M.S.; Powanda, D. Analysis of polyethylene-glycol-polylactide nano-dimension artificial red blood cells in maintaining systemic hemoglobin levels and prevention of methemoglobin formation. Artif. Cells Blood Substit. Immobil. Biotechnol. 2003, 31, 231–247. [Google Scholar] [CrossRef]
- Levi, M.; Friederich, P.W.; Middleton, S.; de Groot, P.G.; Wu, Y.P.; Harris, R.; Biemond, B.J.; Heijnen, H.F.G.; Levin, J.; ten Cate, J.W. Fibrinogen-coated albumin microcapsules reduce bleeding in severely thrombocytopenic rabbits. Nat. Med. 1999, 5, 107–111. [Google Scholar] [CrossRef]
- Mansouri, S.; Fatisson, J.; Miao, Z.; Merhi, Y.; Winnik, F.M.; Tabrizian, M. Silencing Red Blood Cell Recognition toward Anti-A Antibody by Means of Polyelectrolyte Layer-by-Layer Assembly in a Two-Dimensional Model System. Langmuir 2009, 25, 14071–14078. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Merhi, Y.; Winnik, F.M.; Tabrizian, M. Investigation of Layer-by-Layer Assembly of Polyelectrolytes on Fully Functional Human Red Blood Cells in Suspension for Attenuated Immune Response. Biomacromolecules 2011, 12, 585–592. [Google Scholar] [CrossRef]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M.; Pairaudeau, G.; Pennie, W.D.; Pickett, S.D.; Wang, J.; et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015, 14, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Sophocleous, A.M.; Schwendeman, S.P. Inhibition of Peptide Acylation in PLGA Microspheres with Water-soluble Divalent Cationic Salts. Pharm. Res. 2009, 26, 1986–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Feng, S.-S. Nanoparticles of poly(d,l-lactide)/methoxy poly(ethylene glycol)-poly(d,l-lactide) blends for controlled release of paclitaxel. J. Biomed. Mater. Res. Part A 2006, 78, 12–19. [Google Scholar] [CrossRef]
- Pan, J.; Feng, S.-S. Targeted delivery of paclitaxel using folate-decorated poly(lactide)-vitamin E TPGS nanoparticles. Biomaterials 2008, 29, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, T.; Byrne, J.D.; Sunaryo, N.; Crowder, S.W.; Kadapakkam, M.; Patel, S.; Casciato, S.; Brannon-Peppas, L. PEGylation strategies for active targeting of PLA/PLGA nanoparticles. J. Biomed. Mater. Res. Part A 2009, 91, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Italia, J.L.; Bhardwaj, V.; Kumar, M.N.V.R. Disease, destination, dose and delivery aspects of ciclosporin: The state of the art. Drug Discov. Today 2006, 11, 846–854. [Google Scholar] [CrossRef]
- Zhao, J.; Mi, Y.; Liu, Y.; Feng, S.-S. Quantitative control of targeting effect of anticancer drugs formulated by ligand-conjugated nanoparticles of biodegradable copolymer blend. Biomaterials 2012, 33, 1948–1958. [Google Scholar] [CrossRef]
- Cheng, C.J.; Tietjen, G.T.; Saucier-Sawyer, J.K.; Saltzman, W.M. A holistic approach to targeting disease with polymeric nanoparticles. Nat. Rev. Drug Discov. 2015, 14, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.-K.; Law, W.-C.; Weinheimer, E.; Sengupta, S.; Prasad, P.N.; Cheng, C. Polylactide-graft-doxorubicin Nanoparticles with Precisely Controlled Drug Loading for pH-Triggered Drug Delivery. Biomacromolecules 2014, 15, 524–532. [Google Scholar] [CrossRef]
- Cunningham, A.; Ko, N.R.; Oh, J.K. Synthesis and reduction-responsive disassembly of PLA-based mono-cleavable micelles. Colloids Surf. B-Biointerfaces 2014, 122, 693–700. [Google Scholar] [CrossRef]
- Zhong, T.; Deng, C.; Gao, Y.; Chen, M.; Zuo, B. Studies of in situ-forming hydrogels by blending PLA-PEG-PLA copolymer with silk fibroin solution. J. Biomed. Mater. Res. Part A 2012, 100A, 1983–1989. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable block copolymers as injectable drug-delivery systems. Nature 1997, 388, 860–862. [Google Scholar] [CrossRef]
- Kim, M.S.; Seo, K.S.; Khang, G.; Cho, S.H.; Lee, H.B. Preparation of methoxy poly(ethylene glycol)/polyester diblock copolymers and examination of the gel-to-sol transition. J. Polym. Sci. Part A-Polym. Chem. 2004, 42, 5784–5793. [Google Scholar] [CrossRef]
- Li, T.; Ci, T.; Chen, L.; Yu, L.; Ding, J. Salt-induced reentrant hydrogel of poly(ethylene glycol)-poly(lactide-co-glycolide) block copolymers. Polym. Chem. 2014, 5, 979–991. [Google Scholar] [CrossRef]
- Mukose, T.; Fujiwara, T.; Nakano, J.; Taniguchi, I.; Miyamoto, M.; Kimura, Y.; Teraoka, I.; Lee, C.W. Hydrogel formation between enantiomeric B-A-B-type block copolymers of polylactides (PLLA or PDLA: A) and polyoxyethylene (PEG: B); PEG-PLLA-PEG and PEG-PDLA-PEG. Macromol. Biosci. 2004, 4, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Nouailhas, H.; El Ghzaoui, A.; Li, S.; Coudane, J. Stereocomplex-Induced Gelation Properties of Polylactide/Poly(ethylene glycol) Diblock and Triblock Copolymers. J. Appl. Polym. Sci. 2011, 122, 1599–1606. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Kim, S.W. Thermoreversible gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions. Macromolecules 1999, 32, 7064–7069. [Google Scholar] [CrossRef]
- Park, M.J.; Char, K. Gelation of PEO-PLGA-PEO triblock copolymers induced by macroscopic phase separation. Langmuir 2004, 20, 2456–2465. [Google Scholar] [CrossRef]
- Lee, D.S.; Shim, M.S.; Kim, S.W.; Lee, H.; Park, I.; Chang, T.Y. Novel thermoreversible gelation of biodegradable PLGA-block-PEO-block-PLGA triblock copolymers in aqueous solution. Macromol. Rapid Commun. 2001, 22, 587–592. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, H.; Ding, J.D. A subtle end-group effect on macroscopic physical gelation of triblock copolymer aqueous solutions. Angew. Chem. Int. Ed. 2006, 45, 2232–2235. [Google Scholar] [CrossRef]
- Lee, H.T.; Lee, D.S. Thermoresponsive phase transitions of PLA-block-PEO-block-PLA triblock stereo-copolymers in aqueous solution. Macromol. Res. 2002, 10, 359–364. [Google Scholar] [CrossRef]
- Tew, G.N.; Sanabria-DeLong, N.; Agrawal, S.K.; Bhatia, S.R. New properties from PLA-PEO-PLA hydrogels. Soft Matter 2005, 1, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Shan, G.; Bao, Y.; Wu, Z.L.; Pan, P. Thermoresponsive physical hydrogels of poly (lactic acid)/poly (ethylene glycol) stereoblock copolymers tuned by stereostructure and hydrophobic block sequence. Soft Matter 2016, 12, 4628–4637. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Mukose, T.; Yamaoka, T.; Yamane, H.; Sakurai, S.; Kimura, Y. Novel thermo-responsive formation of a hydrogel by stereo-complexation between PLLA-PEG-PLLA and PDLA-PEG-PDLA block copolymers. Macromol. Biosci. 2001, 1, 204–208. [Google Scholar] [CrossRef]
- Nouailhas, H.; Li, F.; El Ghzaoui, A.; Li, S.; Coudane, J. Influence of racemization on stereocomplex-induced gelation of water-soluble polylactide-poly (ethylene glycol) block copolymers. Polym. Int. 2010, 59, 1077–1083. [Google Scholar] [CrossRef]
- Jeong, B.; Kibbey, M.R.; Birnbaum, J.C.; Won, Y.Y.; Gutowska, A. Thermogelling biodegradable polymers with hydrophilic backbones: PEG-g-PLGA. Macromolecules 2000, 33, 8317–8322. [Google Scholar] [CrossRef]
- Li, F.; Li, S.M.; Vert, M. Synthesis and rheological properties of polylactide/poly (ethylene glycol) multiblock copolymers. Macromol. Biosci. 2005, 5, 1125–1131. [Google Scholar] [CrossRef]
- Hiemstra, C.; Zhong, Z.Y.; Jiang, X.; Hennink, W.E.; Dijkstra, P.J.; Feijen, J. PEG-PLLA and PEG-PDLA multiblock copolymers: Synthesis and in situ hydrogel formation by stereocomplexation. J. Control. Release 2006, 116, E17–E19. [Google Scholar] [CrossRef]
- Lee, J.; Bae, Y.H.; Sohn, Y.S.; Jeong, B. Thermogelling aqueous solutions of alternating multiblock copolymers of poly(l-lactic acid) and poly(ethylene glycol). Biomacromolecules 2006, 7, 1729–1734. [Google Scholar] [CrossRef]
- Park, S.Y.; Han, D.K.; Kim, S.C. Synthesis and characterization of star-shaped PLLA-PEO block copolymers with temperature-sensitive sol-gel transition behavior. Macromolecules 2001, 34, 8821–8824. [Google Scholar] [CrossRef]
- Park, S.Y.; Han, B.R.; Na, K.M.; Han, D.K.; Kim, S.C. Micellization and gelation of aqueous solutions of star-shaped PLLA-PEO block copolymers. Macromolecules 2003, 36, 4115–4124. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, L.; Wang, Y.; Li, D.; Zhong, Z.; Zhou, S. Redox-responsive star-shaped magnetic micelles with active-targeted and magnetic-guided functions for cancer therapy. Acta Biomater. 2016, 42, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, M.N.; Sudhakara, P.; Subha, M.C.S.; Rao, K.C.; Song, J.I. Novel Thermoresponsive Biodegradable Nanocomposite Hydrogels for Dual Function in Biomedical Applications. Polym. Plast. Technol. Eng. 2015, 54, 1704–1714. [Google Scholar] [CrossRef]
- Zhang, Q.; Ko, N.R.; Oh, J.K. Recent advances in stimuli-responsive degradable block copolymer micelles: Synthesis and controlled drug delivery applications. Chem. Commun. 2012, 48, 7542–7552. [Google Scholar] [CrossRef]
- Hennink, W.E.; De Jong, S.J.; Bos, G.W.; Veldhuis, T.F.J.; van Nostrum, C.F. Biodegradable dextran hydrogels crosslinked by stereocomplex formation for the controlled release of pharmaceutical proteins. Int. J. Pharm. 2004, 277, 99–104. [Google Scholar] [CrossRef]
- Cummings, S.R.; San Martin, J.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, S.T.; Watts, N.B.; Genant, H.K.; McKeever, C.D.; Hangartner, T.; Keller, M.; Chesnut, C.H.; Brown, J.; Eriksen, E.F.; Hoseyni, M.S.; et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis - A randomized controlled trial. Jama-J. Am. Med. Assoc. 1999, 282, 1344–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberman, U.A.; Weiss, S.R.; Broll, J.; Minne, H.W.; Quan, H.; Bell, N.H.; Rodriguez-Portales, J.; Downs, R.W., Jr.; Dequeker, J.; Favus, M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N. Engl. J. Med. 1995, 333, 1437–1443. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C.; Jimenez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [PubMed] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, S.; Bourantas, C.; Serruys, P.W. New concepts in the design of drug-eluting coronary stents. Nat. Rev. Cardiol. 2013, 10, 248–260. [Google Scholar] [CrossRef]
- Gollwitzer, H.; Thomas, P.; Diehl, P.; Steinhauser, E.; Summer, B.; Barnstorf, S.; Gerdesmeyer, L.; Mittelmeier, W.; Stemberger, A. Biomechanical and allergological characteristics of a biodegradable poly(d,l-lactic acid) coating for orthopaedic implants. J. Orthop. Res. 2005, 23, 802–809. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Wang, Y.; Maitz, P.K.; Mirmohseni, F.; Cheng, T.L.; Peacock, L.; Little, D.G.; Schindeler, A.; Dehghani, F. Fabrication of a Biodegradable Implant with Tunable Characteristics for Bone Implant Applications. Biomacromolecules 2017, 18, 1736–1746. [Google Scholar] [CrossRef]
- Mc Conville, C.; Major, I.; Friend, D.R.; Clark, M.R.; Woolfson, A.D.; Malcolm, R.K. Development of polylactide and polyethylene vinyl acetate blends for the manufacture of vaginal rings. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2012, 100, 891–895. [Google Scholar] [CrossRef]
- Wehmoeller, M.; Neuking, K.; Epple, M.; Annen, T.; Eufinger, H. Mechanical characteristics of functionally graded biodegradable implants for skull bone reconstruction. Mater. Und Werkst. 2006, 37, 413–415. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190, 97–110. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Du, Z.; Jiang, W.; Han, X.; Zhao, D.; Han, D.; Li, Q. Three dimensional printed macroporous polylactic acid/hydroxyapatite composite scaffolds for promoting bone formation in a critical-size rat calvarial defect model. Sci. Technol. Adv. Mater. 2016, 17, 136–148. [Google Scholar] [CrossRef] [Green Version]



| Type of Hydrogel | Copolymer | Phase Transition | Ref |
|---|---|---|---|
| diblock | PEG–PLA | gel–sol | [102] |
| PEG–PLLA | gel–sol | [102,103] | |
| PEG–PLGA | gel–sol | [103,104] | |
| PEG–PLLA/PEG–PDLA | gel–sol | [105,106] | |
| triblock | PEG–PLGA–PEG | sol–gel–sol | [107,108] |
| PEG–PLLA–PEG | sol–gel–sol | [102,103] | |
| PLGA–PEG–PLGA | sol–gel | [109,110] | |
| PLA–PEG–PLA | sol–gel | [111] | |
| PLLA–PEG–PLLA | sol–gel | [112,113] | |
| PDLA–PEG–PDLA | sol–gel | [114,115] | |
| graft | PEG–g–PLGA | gel–sol | [116] |
| multiblock | (PEG–PLA)n | gel–sol | [117] |
| (PEG–PLLA)n | gel–sol, sol–gel | [118,119] | |
| star shape | PEG–(PLLA)n | gel–sol | [120] |
| PEG–(PLLA)8/PEG–(PDLA)8 | gel–precipitate | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. https://doi.org/10.3390/molecules25215023
Li G, Zhao M, Xu F, Yang B, Li X, Meng X, Teng L, Sun F, Li Y. Synthesis and Biological Application of Polylactic Acid. Molecules. 2020; 25(21):5023. https://doi.org/10.3390/molecules25215023
Chicago/Turabian StyleLi, Ge, Menghui Zhao, Fei Xu, Bo Yang, Xiangyu Li, Xiangxue Meng, Lesheng Teng, Fengying Sun, and Youxin Li. 2020. "Synthesis and Biological Application of Polylactic Acid" Molecules 25, no. 21: 5023. https://doi.org/10.3390/molecules25215023
APA StyleLi, G., Zhao, M., Xu, F., Yang, B., Li, X., Meng, X., Teng, L., Sun, F., & Li, Y. (2020). Synthesis and Biological Application of Polylactic Acid. Molecules, 25(21), 5023. https://doi.org/10.3390/molecules25215023






