Lipid Composition of Latex and Rubber Particles in Hevea brasiliensis and Taraxacum kok-saghyz
Abstract
:1. Introduction
2. Results
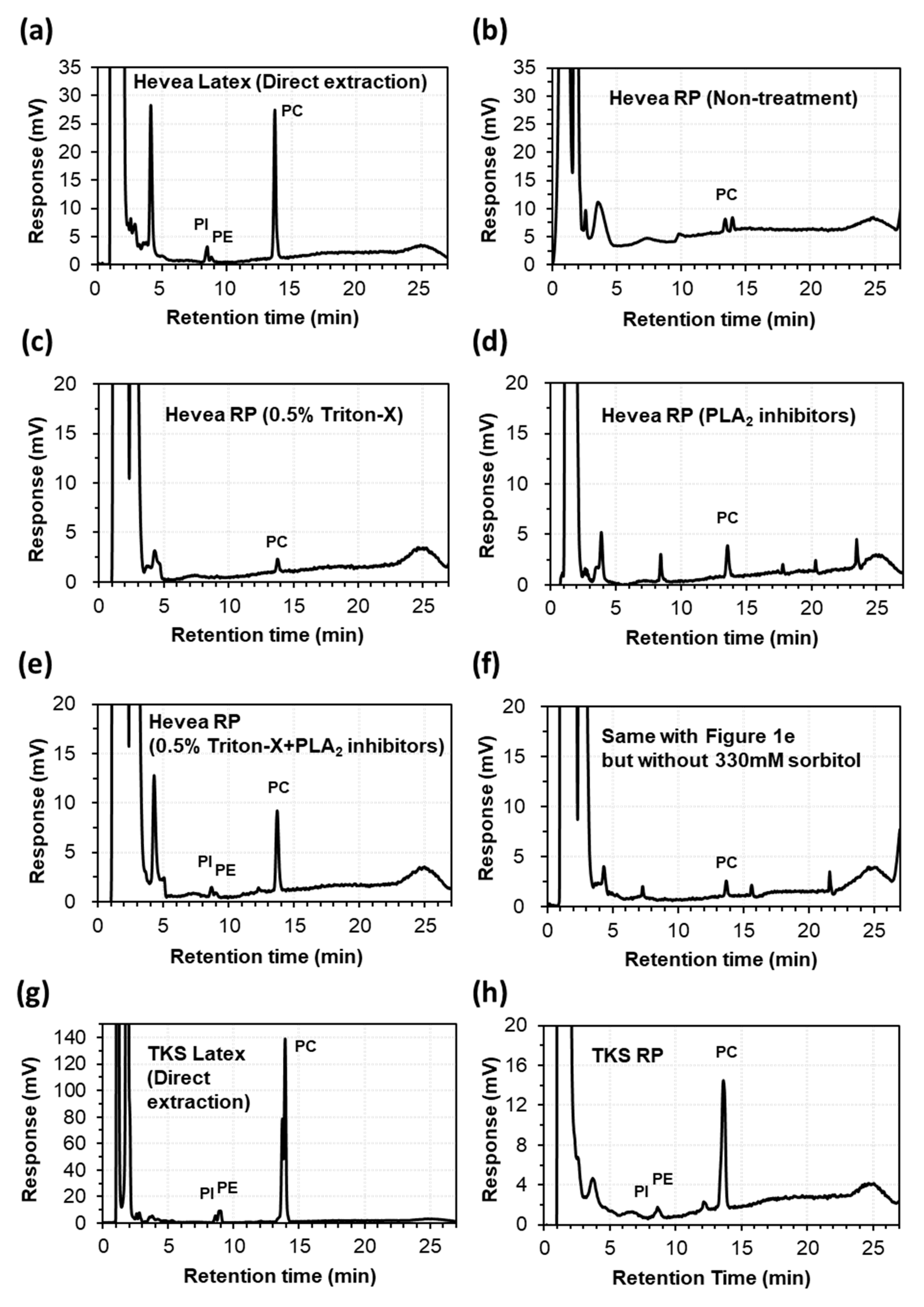
2.1. Extraction of Lipids from the Latex and Rubber Particles of Hevea and TKS
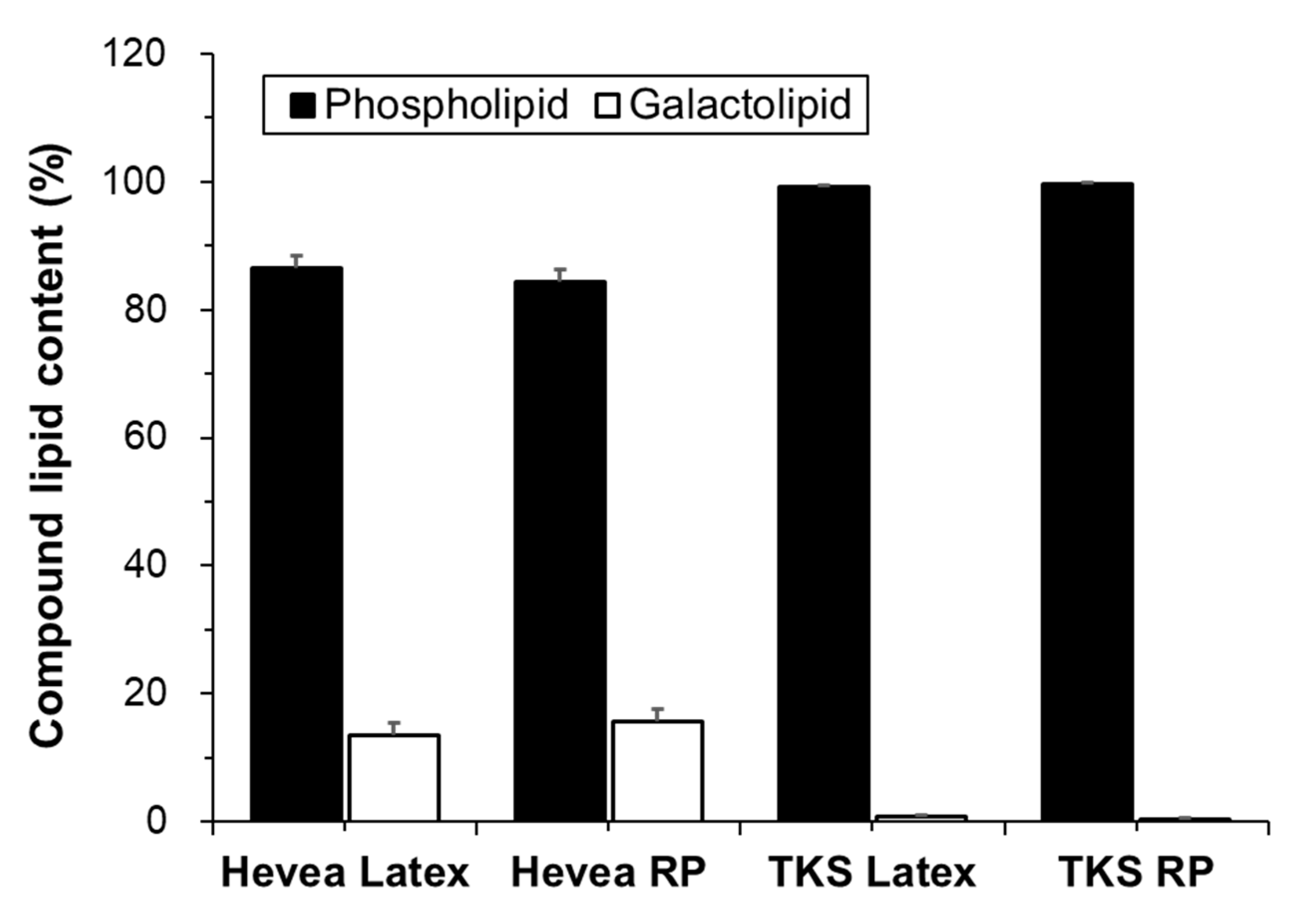
2.2. Lipid Composition
2.2.1. Phospholipids
Group I: Phosphatidylcholines
Group II: Phosphatidylinositols and Phosphatidylethanolamines
Group III: Minor Phospholipids
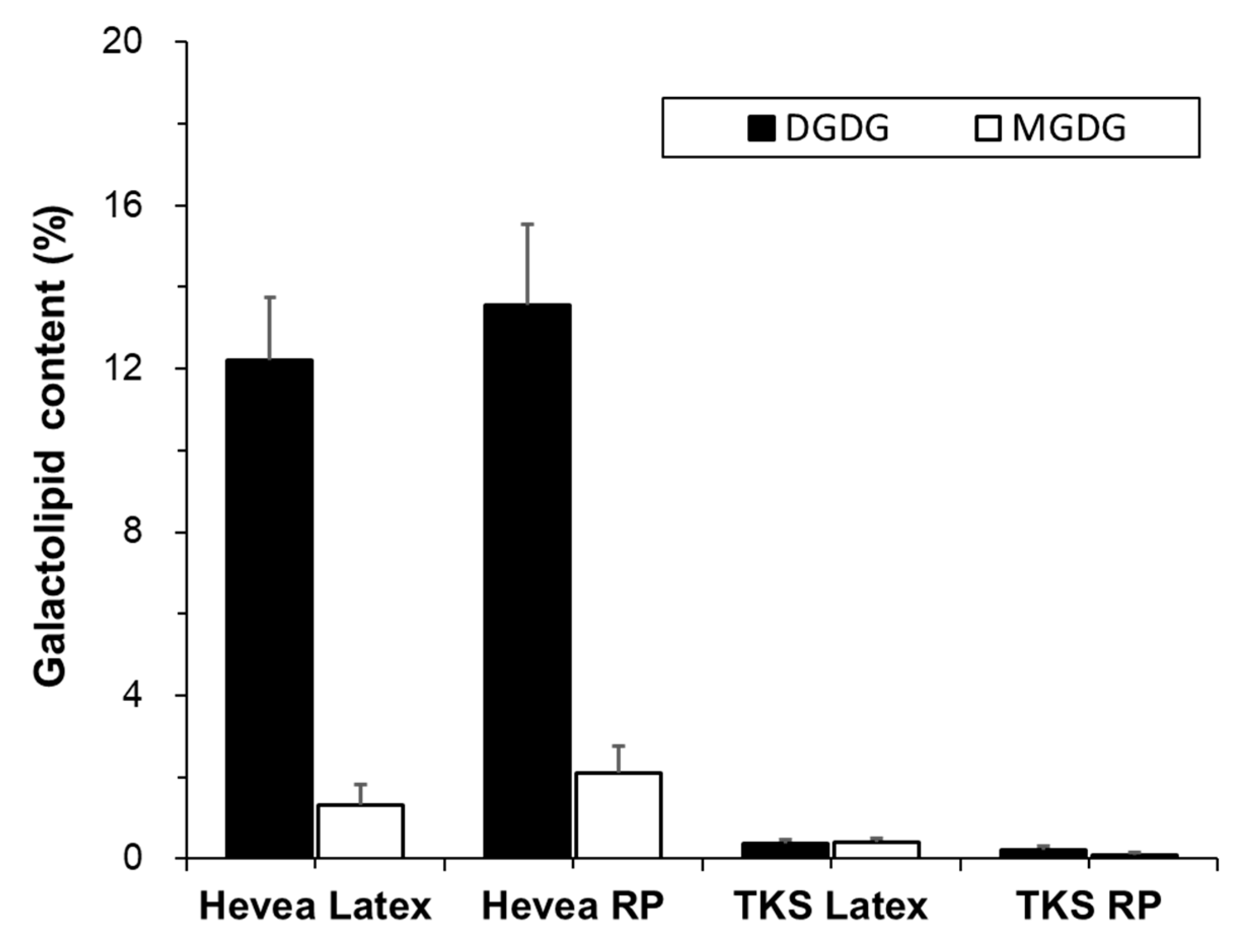
2.2.2. Galactolipids
Digalactosyl Diacylglycerols
Monogalactosyl Diacylglycerols
3. Discussion
4. Materials and Methods
4.1. Collection of Latex and Preparation of Washed Rubber Particles
4.2. Lipid Extraction from Latex and Rubber Particles
4.3. HPLC-ELSD Analysis
4.4. Lipid Analysis Using ESI-MS/MS
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cherian, S.; Ryu, S.B.; Cornish, K. Natural rubber biosynthesis in plants, the rubber transferase complex, and metabolic engineering progress and prospects. Plant Biotechnol. J. 2019, 17, 204–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, W. The Rubber Industry’s Biological Nightmare. Available online: https://money.cnn.com/magazines/fortune/fortune_archive/1997/08/04/229714/index.htm (accessed on 4 August 1997).
- Mooibroek, H.; Cornish, K. Alternative sources of natural rubber. Appl. Microbiol. Biotechnol. 2000, 53, 355–365. [Google Scholar] [CrossRef]
- Van Beilen, J.B.; Poirier, Y. Establishment of new crops for the production of natural rubber. Trends Biotechnol. 2007, 25, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, T.E. Natural Rubber Production Declines, Demand-Supply Gap Rises to 45%. Available online: https://www.business-standard.com/article/economy-policy/natural-rubber-production-declines-demand-supply-gap-rises-to-45-119031300213_1.html (accessed on 13 March 2019).
- Tire Business Staff. Rubber Groups: Demand for Natural Rubber Increases, Supply Decreases. Available online: https://www.tirebusiness.com/news/rubber-groups-demand-natural-rubber-increases-supply-decreases (accessed on 19 July 2019).
- Kirschner, J.; Stepanek, J.; Černý, T.; De Heer, P.; Van Dijk, P.J. Available ex situ germplasm of the potential rubber crop Taraxacum koksaghyz belongs to a poor rubber producer, T. brevicorniculatum (Compositae–Crepidinae). Genet. Resour. Crop. Evol. 2012, 60, 455–471. [Google Scholar] [CrossRef]
- Ramirez-Cadavid, D.A.; Cornish, K.; Michel, F.C. Taraxacum kok-saghyz (TK): Compositional analysis of a feedstock for natural rubber and other bioproducts. Ind. Crop. Prod. 2017, 107, 624–640. [Google Scholar] [CrossRef]
- Ganesh, I.; Choi, S.C.; Bae, S.W.; Park, J.-C.; Ryu, S.B. Heterologous activation of the Hevea PEP16 promoter in the rubber-producing laticiferous tissues of Taraxacum kok-saghyz. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kush, A.; Goyvaerts, E.; Chye, M.-L.; Chua, N.H. Laticifer-specific gene expression in Hevea brasiliensis (rubber tree). Proc. Natl. Acad. Sci. USA 1990, 87, 1787–1790. [Google Scholar] [CrossRef] [Green Version]
- Berthelot, K.; LeComte, S.; Estevez, Y.; Peruch, F. Hevea brasiliensis REF (Hev b 1) and SRPP (Hev b 3): An overview on rubber particle proteins. Biochimie 2014, 106, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cornish, K.; Backhaus, R.A. Rubber transferase activity in rubber particles of guayule. Phytochemistry 1990, 29, 3809–3813. [Google Scholar] [CrossRef]
- Asawatreratanakul, K.; Zhang, Y.-W.; Wititsuwannakul, D.; Wititsuwannakul, R.; Takahashi, S.; Rattanapittayaporn, A.; Koyama, T. Molecular cloning, expression and characterization of cDNA encoding cis-prenyltransferases from Hevea brasiliensis. JBIC J. Biol. Inorg. Chem. 2003, 270, 4671–4680. [Google Scholar] [CrossRef] [Green Version]
- Epping, J.; Van Deenen, N.; Niephaus, E.; Stolze, A.; Fricke, J.; Huber, C.; Eisenreich, W.; Twyman, R.M.; Prüfer, D.; Gronover, C.S. A rubber transferase activator is necessary for natural rubber biosynthesis in dandelion. Nat. Plants 2015, 1, 15048. [Google Scholar] [CrossRef]
- Yamashita, S.; Yamaguchi, H.; Waki, T.; Aoki, Y.; Mizuno, M.; Yanbe, F.; Ishii, T.; Funaki, A.; Tozawa, Y.; Miyagi-Inoue, Y.; et al. Identification and reconstitution of the rubber biosynthetic machinery on rubber particles from Hevea brasiliensis. eLife 2016, 5, e19022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, J.; Moreau, F.; Lance, C.; Jacob, J.-L. Phospholipid composition of the membrane of lutoids from Hevea brasiliensis latex. Phytochemistry 1976, 15, 1215–1217. [Google Scholar] [CrossRef]
- Hasma, H.; Subramaniam, A. Composition of lipids in latex of Hevea brasiliensis clone RRIM 501. J. Nat. Rubb. Res. 1986, 1, 30–40. [Google Scholar]
- Siler, D.J.; Goodrich-Tanrikulu, M.; Cornish, K.; Stafford, A.E.; McKeon, T.A. Composition of rubber particles of Hevea brasiliensis, Parthenium argentatum, Ficus elastica, and Euphorbia lactiflua indicates unconventional surface structure. Plant physiol. Biochem. 1997, 35, 881–889. [Google Scholar]
- Cornish, K.; Wood, D.F.; Windle, J.J. Rubber particles from four different species, examined by transmission electron microscopy and electron-paramagnetic-resonance spin labeling, are found to consist of a homogeneous rubber core enclosed by a contiguous, monolayer biomembrane. Planta 1999, 210, 85–96. [Google Scholar] [CrossRef]
- Liengprayoon, S.; Sriroth, K.; Dubreucq, E.; Vaysse, L. Glycolipid composition of Hevea brasiliensis latex. Phytochemistry 2011, 72, 1902–1913. [Google Scholar] [CrossRef]
- Liengprayoon, S.; Chaiyut, J.; Sriroth, K.; Bonfils, F.; Sainte-Beuve, J.; Dubreucq, E.; Vaysse, L. Lipid compositions of latex and sheet rubber from Hevea brasiliensis depend on clonal origin. Eur. J. Lipid Sci. Technol. 2013, 115, 1021–1031. [Google Scholar] [CrossRef]
- Chan, A.J.; Steenkeste, K.; Eloy, M.; Brosson, D.; Gaboriaud, F.; Fontaine-Aupart, M.-P. Lipid Content in small and large natural rubber particles. Rubber Chem. Technol. 2015, 88, 248–257. [Google Scholar] [CrossRef]
- Laibach, N.; Schmidl, S.; Müller, B.; Bergmann, M.; Prüfer, D.; Gronover, C.S. Small rubber particle proteins from Taraxacum brevicorniculatum promote stress tolerance and influence the size and distribution of lipid droplets and artificial poly(cis -1,4-isoprene) bodies. Plant J. 2018, 93, 1045–1061. [Google Scholar] [CrossRef] [Green Version]
- Bonfils, F.; Ehabe, E.; Aymard, C.; Vaysse, L.; Sainte-Beuve, J. Enhanced solvent extraction of polar lipids associated with rubber particles from hevea brasiliensis. Phytochem. Anal. 2007, 18, 103–108. [Google Scholar] [CrossRef]
- Hasma, H. Lipids associated with rubber particles and their possible role in mechanical stability of latex concentrates. J. Nat. Rubb. Res. 1991, 6, 105–114. [Google Scholar]
- Yang, Y.; Hu, B. Bio-based chemicals from biorefining: Lipid and wax conversion and utilization. In Advances in Biorefineries; Elsevier BV: Amsterdam, Netherlands, 2015; pp. 693–720. [Google Scholar]
- Wadeesirisak, K.; Castano, S.; Berthelot, K.; Vaysse, L.; Bonfils, F.; Peruch, F.; Rattanaporn, K.; Liengprayoon, S.; LeComte, S.; Bottier, C. Rubber particle proteins REF1 and SRPP1 interact differently with native lipids extracted from Hevea brasiliensis latex. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 201–210. [Google Scholar] [CrossRef]
- Dörmann, P.; Benning, C. Galactolipids rule in seed plants. Trends Plant Sci. 2002, 7, 112–118. [Google Scholar] [CrossRef]
- Dowhan, W. Molecular basis for membrane phospholipid diversity: Why are there so many lipids? Annu. Rev. Biochem. 1997, 66, 199–232. [Google Scholar] [CrossRef] [Green Version]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Edward, A.; Gavin, R.G.H.; David, R.S.; Denis, S. Kazakhstan. Available online: https://www.britannica.com/place/Kazakhstan#ref73558 (accessed on 13 March 2020).
- What Is A Continental Climate? Available online: https://www.worldatlas.com/articles/what-is-the-continental-climate.html (accessed on 21 May 2019).
- Jeremy, M.B.S. Tropical Rainforest. Available online: https://www.britannica.com/science/tropical-rainforest (accessed on 13 March 2020).
- Krishan, B. Assessment of drought tolerance in few clones of natural rubber (Hevea brasiliensis) under dry hot climate of Odisha, India. J. Exp. Biol. Agric. Sci. 2017, 5, 106–110. [Google Scholar]
- Brown, D.; Feeney, M.; Ahmadi, M.; Lonoce, C.; Sajari, R.; Di Cola, A.; Frigerio, L. Subcellular localization and interactions among rubber particle proteins from Hevea brasiliensis. J. Exp. Bot. 2017, 68, 5045–5055. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Burke, J.J.; Xin, Z.; Xu, C.; Velten, J. Characterization of the Arabidopsis thermosensitive mutant atts02 reveals an important role for galactolipids in thermotolerance. Plant Cell Environ. 2006, 29, 1437–1448. [Google Scholar] [CrossRef] [Green Version]
- Stubbs, C.D.; Smith, A.D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1984, 779, 89–137. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta (BBA) Biomembr. 2004, 1666, 142–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmreich, E.J. Environmental influences on signal transduction through membranes: A retrospective mini-review. Biophys. Chem. 2002, 100, 519–534. [Google Scholar] [CrossRef]
- Nozawa, Y. Adaptive regulation of membrane lipids and fluidity during thermal acclimation in Tetrahymena. Proc. Jpn. Acad. Ser. B 2011, 87, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Ok, S.H.; Bahn, S.C.; Jang, J.; Oh, S.A.; Park, S.K.; Twell, D.; Ryu, S.B.; Shin, J.S. Endoplasmic Reticulum– and Golgi-Localized Phospholipase A2 Plays Critical Roles in Arabidopsis Pollen Development and Germination. Plant Cell 2011, 23, 94–110. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Devaiah, S.P.; Roth, M.R.; Baughman, E.; Li, M.; Tamura, P.; Jeannotte, R.; Welti, R.; Wang, X. Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a Phospholipase Dα1 knockout mutant. Phytochemistry 2006, 67, 1907–1924. [Google Scholar] [CrossRef] [PubMed]

| Hevea | TKS | |||||
|---|---|---|---|---|---|---|
| Mass | PC | Latex | WRP | Latex | WRP | |
| 756.5 | PC (34:3) | 2.69 ± 0.25 | 3.53 ± 0.70 | 12.63 ± 1.77 | 11.30 ± 0.89 | |
| 758.6 | PC (34:2) | 9.11 ± 0.96 | 10.94 ± 1.66 | 53.43 ± 3.02 | 50.08 ± 1.30 | |
| 760.6 | PC (34:1) | 2.80 ± 0.37 | 3.35 ± 0.57 | 2.59 ± 1.22 | 3.24 ± 2.22 | |
| 780.5 | PC (36:5) | 4.73 ± 0.80 | 5.09 ± 1.84 | 6.95 ± 1.35 | 7.46 ± 1.24 | |
| 782.6 | PC (36:4) | 30.89 ± 7.58 | 27.01 ± 10.12 | 18.51 ± 3.46 | 21.23 ± 3.07 | |
| 784.6 | PC (36:3) | 17.70 ± 2.25 | 17.20 ± 3.48 | 2.13 ± 0.49 | 3.05 ± 1.46 | |
| 786.6 | PC (36:2) | 19.75 ± 1.57 | 18.62 ± 3.07 | 0.69 ± 0.05 | 0.87 ± 0.14 | |
| 788.6 | PC (36:1) | 61.7 ± 1.22 | 5.43 ± 2.68 | 0.02 ± 0.04 | 0.05 ± 0.03 | |
| 810.6 | PC (38:4) | 1.80 ± 2.26 | 3.83 ± 2.70 | 0.10 ± 0.02 | 0.07 ± 0.02 | |
| 812.6 | PC (38:3) | 1.35 ± 1.14 | 1.67 ± 0.88 | 0.12 ± 0.03 | 0.11 ± 0.01 | |
| Hevea | TKS | |||||
|---|---|---|---|---|---|---|
| Mass | PI | Latex | WRP | Latex | WRP | |
| 824.50 | PI (32:2) | 0.33 ± 0.00 | 0.31 ± 0.06 | 1.20 ± 0.32 | 0.98 ± 0.65 | |
| 850.50 | PI (34:3) | 8.62 ± 0.52 | 9.35 ± 2.62 | 8.21 ± 0.55 | 9.12 ± 1.89 | |
| 852.50 | PI (34:2) | 33.69 ± 6.70 | 29.88 ± 2.93 | 82.27 ± 0.70 | 77.41 ± 1.85 | |
| 854.50 | PI (34:1) | 5.45 ± 0.75 | 5.10 ± 0.34 | 1.06 ±0.42 | 2.17 ± 1.74 | |
| 874.50 | PI (36:5) | 2.30 ± 0.31 | 2.29 ± 0.69 | 1.07 ± 0.44 | 1.46 ± 0.62 | |
| 876.50 | PI (36:4) | 9.84 ± 1.28 | 9.33 ± 2.54 | 3.42 ± 1.04 | 4.36 ± 0.97 | |
| 878.50 | PI (36:3) | 10.84 ± 4.89 | 12.80 ± 3.22 | 0.70 ± 0.03 | 1.56 ± 0.59 | |
| 880.60 | PI (36:2) | 24.67 ± 2.55 | 26.21 ± 1.52 | 1.65 ± 0.17 | 2.52 ± 0.85 | |
| 882.60 | PI (36:1) | 3.37 ± 0.81 | 3.87 ± 1.63 | 0.00 ± 0.00 | 0.14 ± 0.15 | |
| Hevea | TKS | |||||
|---|---|---|---|---|---|---|
| Mass | PE | Latex | WRP | Latex | WRP | |
| 688.5 | PE (32:2) | 1.20 ± 0.45 | 0.74 ± 0.76 | 3.97 ± 1.21 | 3.01 ± 1.83 | |
| 690.5 | PE (32:1) | 5.84 ± 4.88 | 12.42 ± 10.02 | 0.09 ± 0.08 | 0.09 ± 0.09 | |
| 692.5 | PE (32:0) | 1.66 ± 0.95 | 10.62 ± 9.92 | 0.03 ± 0.03 | 0.01 ± 0.02 | |
| 714.5 | PE (34:3) | 2.20 ± 0.31 | 1.03 ± 1.64 | 6.53 ± 1.15 | 6.42 ± 2.00 | |
| 716.5 | PE (34:2) | 17.99 ± 4.30 | 8.86 ± 5.59 | 43.63 ± 1.98 | 39.77 ± 2.23 | |
| 718.5 | PE (34:1) | 4.06 ± 1.32 | 5.58 ± 2.39 | 0.43 ± 0.24 | 1.19 ± 0.93 | |
| 736.5 | PE (36:6) | 0.14 ± 0.02 | 0.07 ± 0.07 | 1.03 ± 0.34 | 1.22 ± 0.12 | |
| 738.5 | PE (36:5) | 2.35 ± 0.85 | 0.69 ± 1.19 | 9.29 ± 1.73 | 9.83 ± 1.48 | |
| 740.5 | PE (36:4) | 20.60 ± 5.26 | 16.53 ± 6.56 | 30.17 ± 4.40 | 32.45 ± 2.23 | |
| 742.5 | PE (36:3) | 12.20 ± 2.45 | 13.70 ± 3.05 | 1.59 ± 0.41 | 2.82 ± 1.01 | |
| 744.5 | PE (36:2) | 21.18 ± 3.78 | 20.16 ± 1.83 | 0.77 ± 0.11 | 0.94 ± 0.22 | |
| 746.6 | PE (36:1) | 3.41 ± 0.73 | 2.81 ± 2.43 | 0.00 ±0.00 | 0.00 ± 0.00 | |
| 768.5 | PE (38:4) | 3.53 ± 4.76 | 3.65 ± 2.49 | 0.02 ± 0.02 | 0.02 ± 0.01 | |
| 770.6 | PE (38:3) | 2.29 ± 1.35 | 2.09 ± 1.21 | 0.02 ± 0.03 | 0.05 ± 0.04 | |
| Hevea | TKS | |||||
|---|---|---|---|---|---|---|
| Mass | DGDG | Latex | WRP | Latex | WRP | |
| 932.6 | DGDG (34:3) | 2.60 ± 0.04 | 2.76 ± 1.15 | 11.39 ± 1.97 | 13.47 ± 1.92 | |
| 934.6 | DGDG (34:2) | 5.48 ± 0.80 | 4.89 ± 1.07 | 14.87 ± 3.76 | 23.87 ± 6.50 | |
| 936.6 | DGDG (34:1) | 4.68 ± 0.22 | 4.06 ± 0.61 | 1.56 ± 1.87 | 3.08 ± 2.28 | |
| 954.6 | DGDG (36:6) | 0.72 ± 0.38 | 0.94 ± 0.66 | 20.30 ± 4.29 | 9.87 ± 9.91 | |
| 956.6 | DGDG (36:5) | 5.22 ± 1.83 | 4.36 ± 1.90 | 28.79 ± 7.80 | 20.64 ± 3.08 | |
| 958.6 | DGDG (36:4) | 22.08 ± 6.91 | 19.73 ± 8.16 | 19.16 ± 5.10 | 18.96 ± 2.85 | |
| 960.6 | DGDG (36:3) | 20.90 ± 1.14 | 20.97 ± 1.90 | 2.45 ± 1.66 | 5.61 ± 4.41 | |
| 962.6 | DGDG (36:2) | 21.79 ± 1.77 | 21.56 ± 2.49 | 1.14 ± 1.01 | 3.38 ± 4.91 | |
| 964.7 | DGDG (36:1) | 10.98 ± 1.70 | 10.71 ± 1.79 | 0.15 ± 0.26 | 0.54 ± 0.93 | |
| 986.6 | DGDG (38:4) | 2.15 ± 2.49 | 4.30 ± 3.41 | 0.10 ± 0.17 | 0.39 ± 0.48 | |
| 988.7 | DGDG (38:3) | 2.70 ± 2.77 | 4.76 ± 3.53 | 0.00 ± 0.00 | 0.02 ± 0.03 | |
| Hevea | TKS | |||||
|---|---|---|---|---|---|---|
| Mass | MGDG | Latex | WRP | Latex | WRP | |
| 770.5 | MGDG(34:3) | 1.39 ± 0.96 | 2.15 ± 0.22 | 1.14 ± 1.67 | 1.18 ± 1.02 | |
| 772.6 | MGDG(34:2) | 2.41 ± 0.43 | 2.96 ± 0.36 | 0.32 ± 0.34 | 2.53 ± 3.66 | |
| 774.6 | MGDG(34:1) | 1.56 ± 0.56 | 2.10 ± 0.24 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 792.5 | MGDG(36:6) | 1.87 ± 1.59 | 2.14 ± 2.5 | 22.65 ± 9.01 | 23.25 ± 22.52 | |
| 794.5 | MGDG(36:5) | 9.80 ± 2.50 | 6.43 ± 1.91 | 38.88 ± 9.10 | 39.92 ± 14.45 | |
| 796.6 | MGDG(36:4) | 34.71 ± 11.99 | 25.08 ± 11.54 | 36.96 ± 2.55 | 25.51 ± 5.20 | |
| 798.6 | MGDG(36:3) | 20.08 ± 1.27 | 20.56 ± 2.45 | 0.00 ± 0.00 | 7.53 ± 9.66 | |
| 800.6 | MGDG(36:2) | 13.32 ± 2.55 | 15.49 ± 2.78 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 802.6 | MGDG(36:1) | 6.25 ± 1.32 | 7.51 ± 1.47 | 0.00 ± 0.00 | 0.07 ± 0.13 | |
| 824.6 | MGDG(38:4) | 3.11 ± 2.94 | 6.96 ± 3.92 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 826.6 | MGDG(38:3) | 5.16 ± 5.46 | 8.14 ± 4.88 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| PC | PI | PE | PA | PG | DGDG | MGDG | |
|---|---|---|---|---|---|---|---|
| Hevea Latex | 70.7 ± 2.9 | 11.9 ± 1.6 | 2.1 ± 1.0 | 0.5 ± 0.4 | 0.1 ± 0.1 | 12.2 ± 1.2 | 1.3 ± 0.4 |
| Hevea RP | 69.7 ± 4.0 | 11.4 ± 5.3 | 2.1 ± 2.2 | 0.4 ± 0.3 | 0.1 ± 0.1 | 13.6 ± 1.6 | 2.1 ± 0.5 |
| TKS Latex | 75.4 ± 0.1 | 6.0 ± 0.3 | 14.3 ± 0.5 | 1.4 ± 0.2 | 1.1 ± 0.3 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| TKS RP | 81.1 ± 4.2 | 3.1 ± 0.7 | 10.1 ± 2.7 | 3.2 ± 2.2 | 1.1 ± 0.3 | 0.2 ± 0.1 | 0.1 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.W.; Jung, S.; Choi, S.C.; Kim, M.Y.; Ryu, S.B. Lipid Composition of Latex and Rubber Particles in Hevea brasiliensis and Taraxacum kok-saghyz. Molecules 2020, 25, 5110. https://doi.org/10.3390/molecules25215110
Bae SW, Jung S, Choi SC, Kim MY, Ryu SB. Lipid Composition of Latex and Rubber Particles in Hevea brasiliensis and Taraxacum kok-saghyz. Molecules. 2020; 25(21):5110. https://doi.org/10.3390/molecules25215110
Chicago/Turabian StyleBae, Sung Woo, Sunghee Jung, Sang Chul Choi, Mi Young Kim, and Stephen Beungtae Ryu. 2020. "Lipid Composition of Latex and Rubber Particles in Hevea brasiliensis and Taraxacum kok-saghyz" Molecules 25, no. 21: 5110. https://doi.org/10.3390/molecules25215110
APA StyleBae, S. W., Jung, S., Choi, S. C., Kim, M. Y., & Ryu, S. B. (2020). Lipid Composition of Latex and Rubber Particles in Hevea brasiliensis and Taraxacum kok-saghyz. Molecules, 25(21), 5110. https://doi.org/10.3390/molecules25215110






