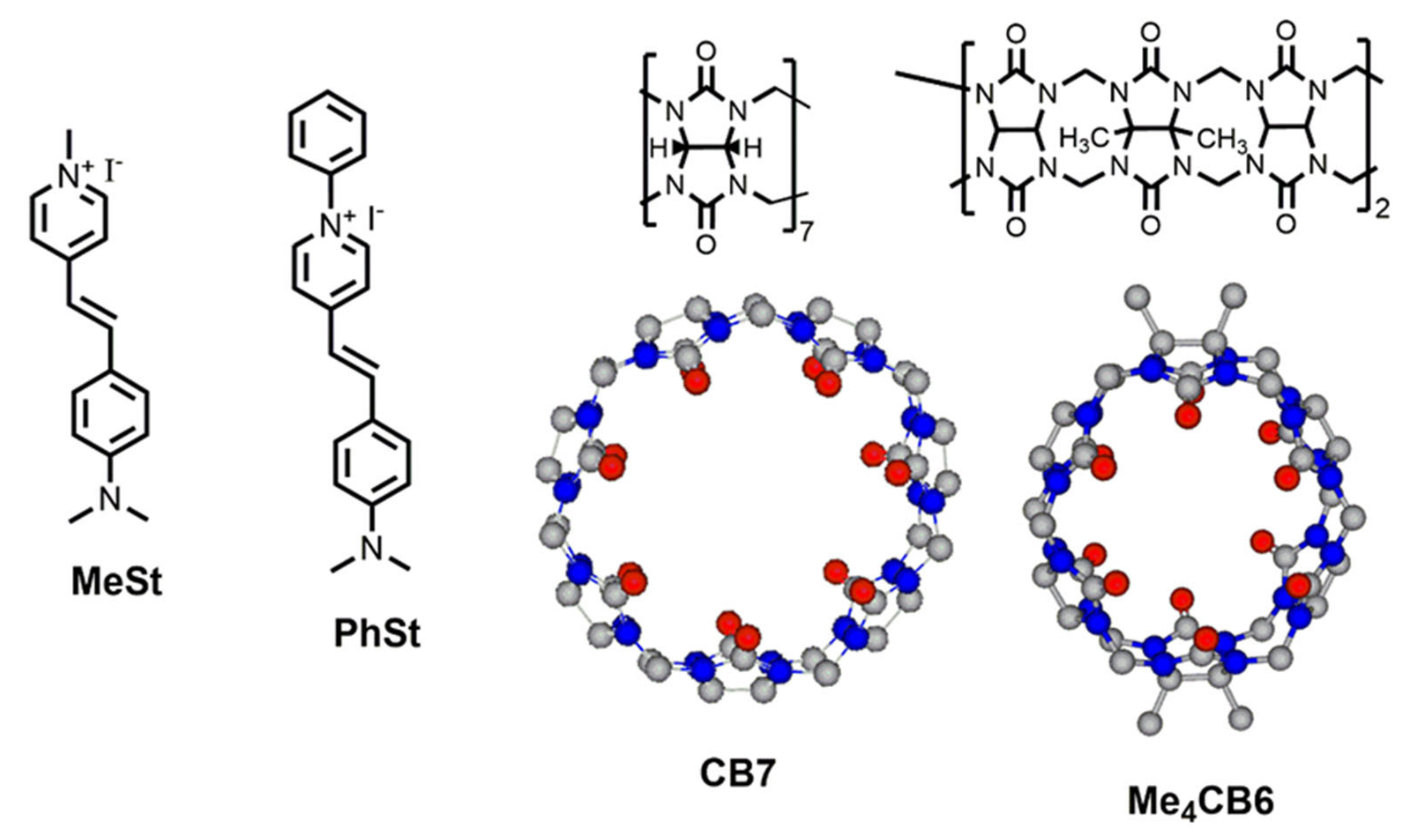
Binding Modes of a Phenylpyridinium Styryl Fluorescent Dye with Cucurbiturils
Abstract
1. Introduction
2. Results and Discussion
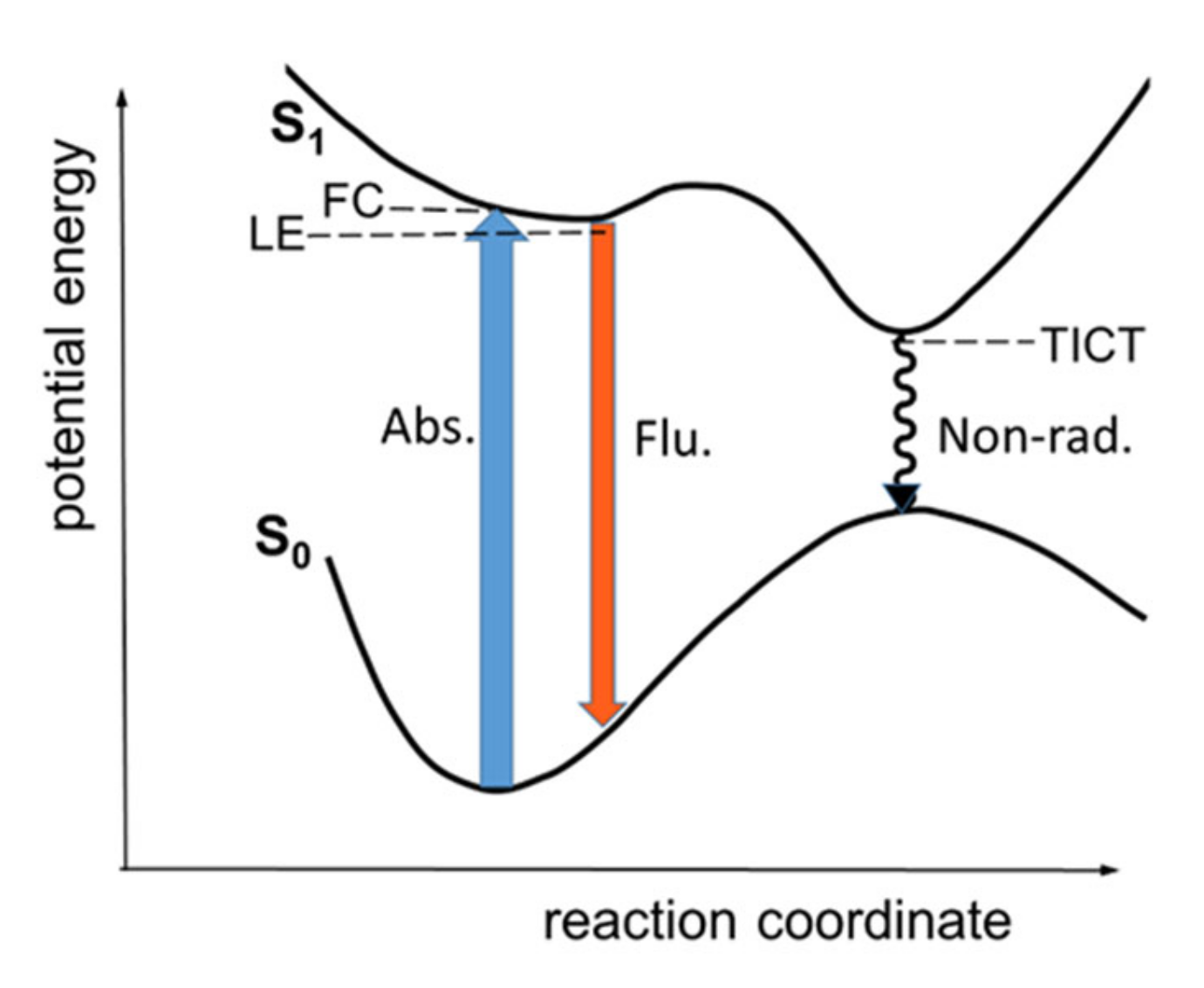
2.1. Scheme of Photoinduced Processes
2.2. Solvatochromism
2.3. Viscosity Dependence of Fluorescence
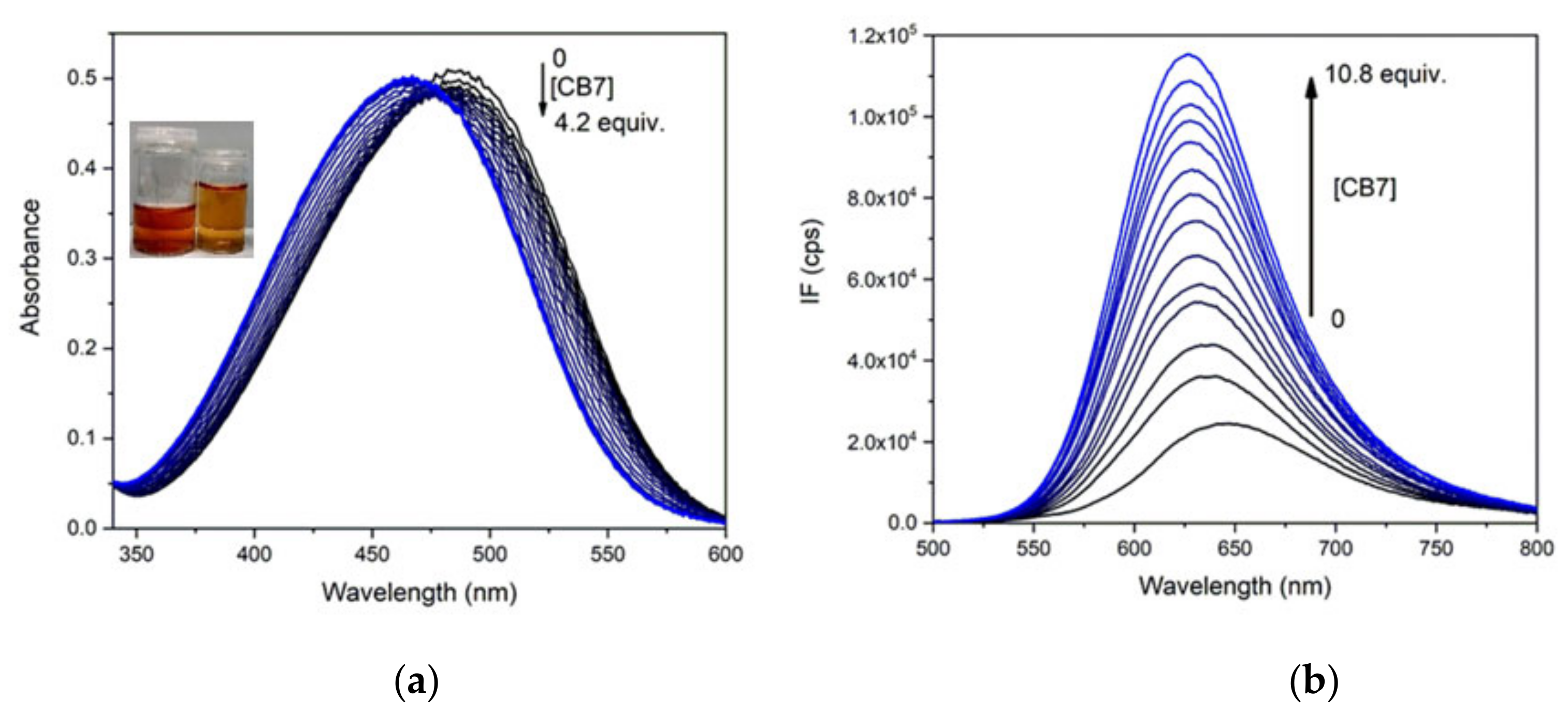
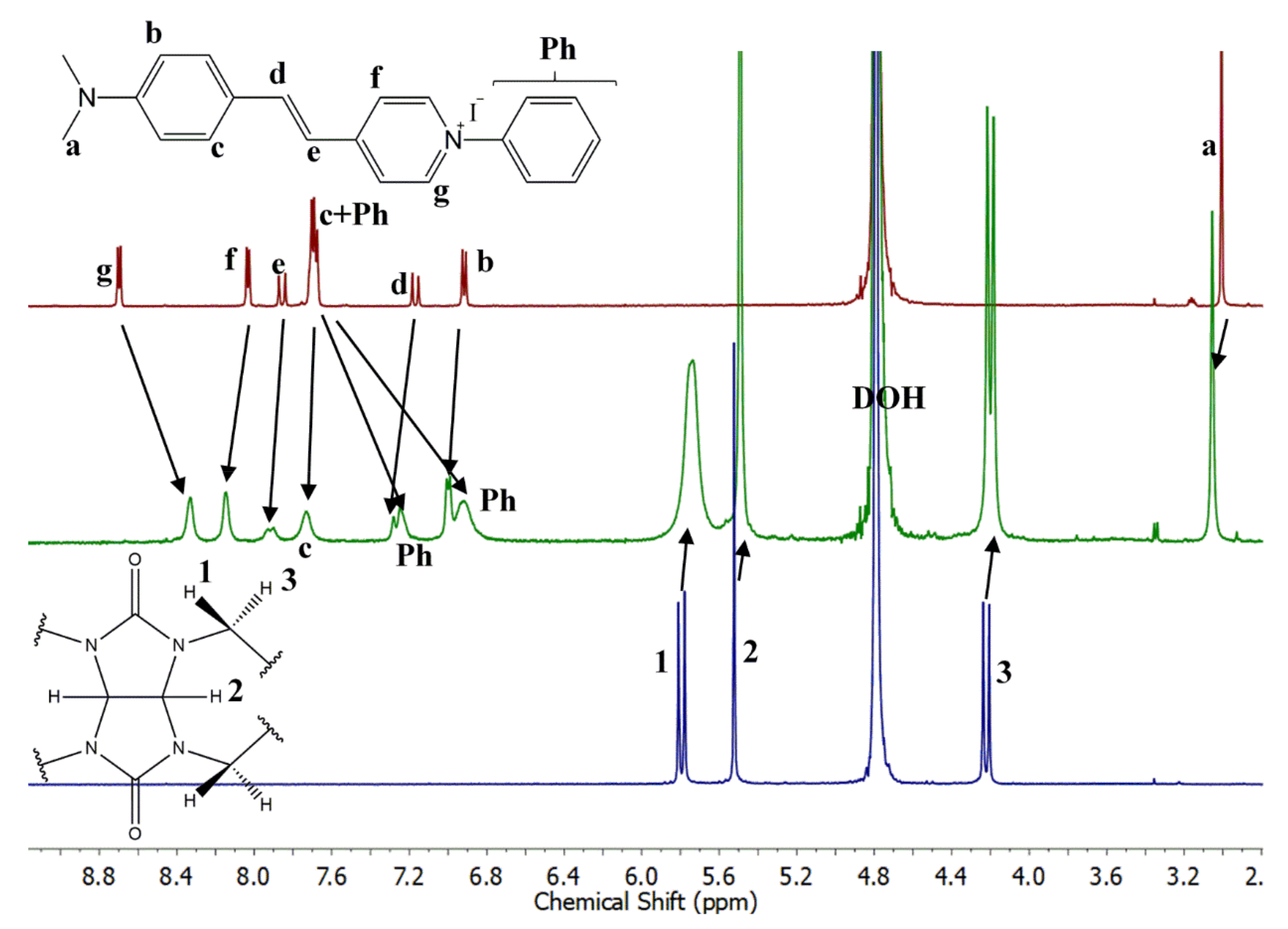
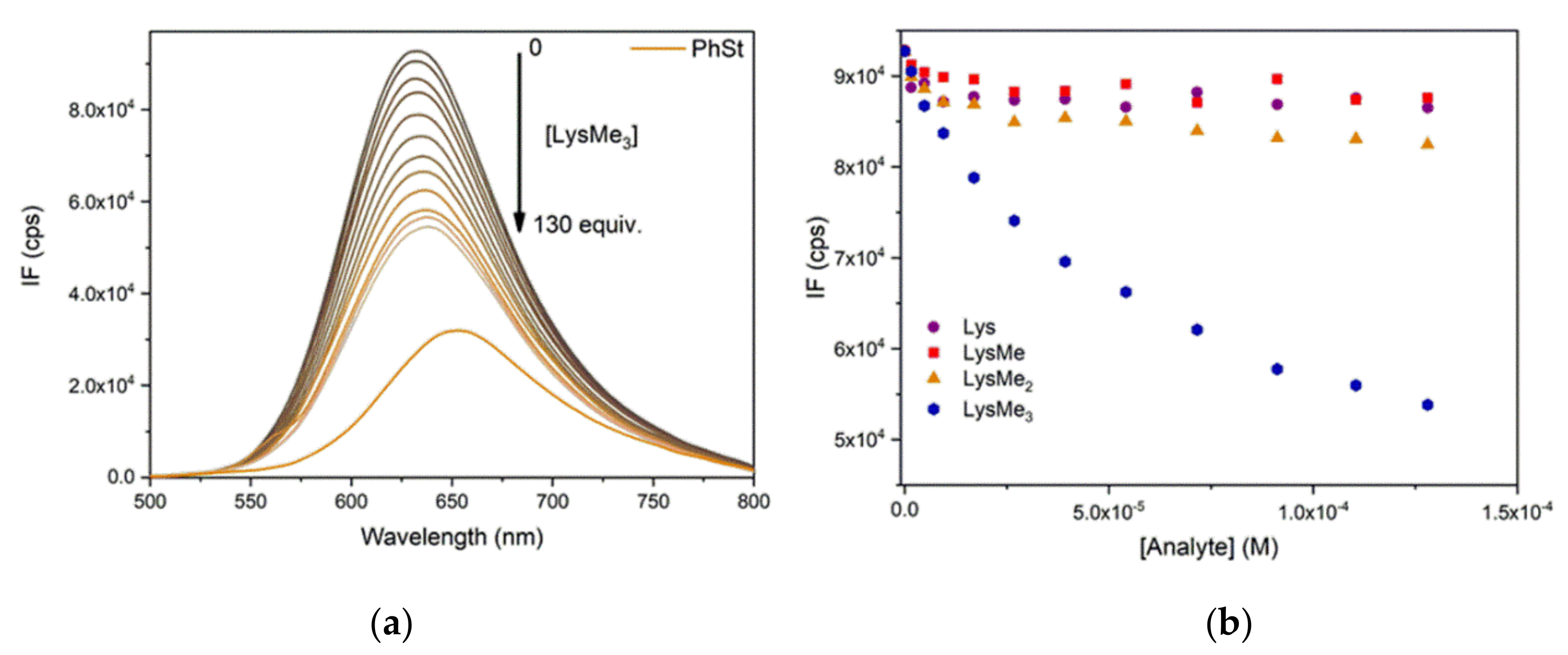
2.4. Complexation of PhSt with CB7
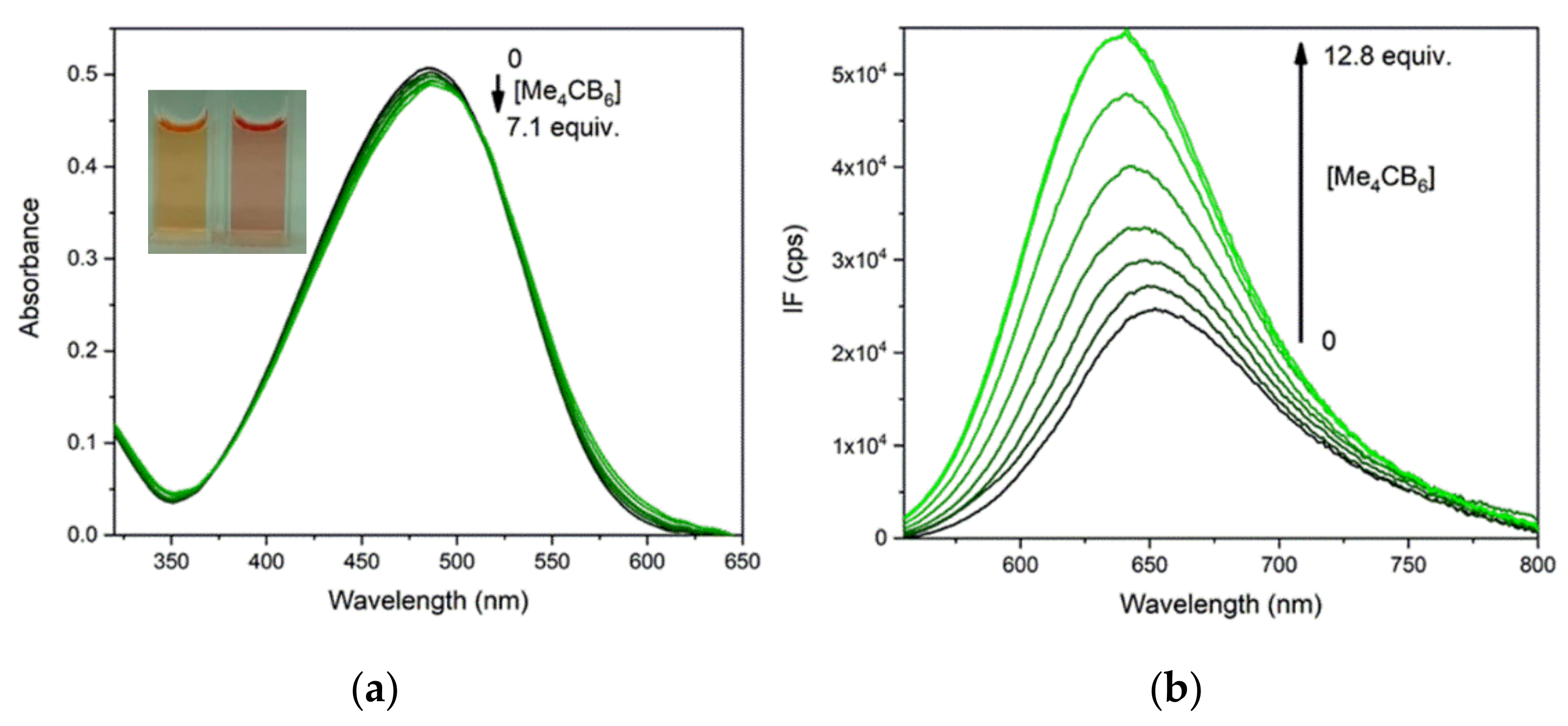
2.5. Complexation of PhSt with Me4CB6
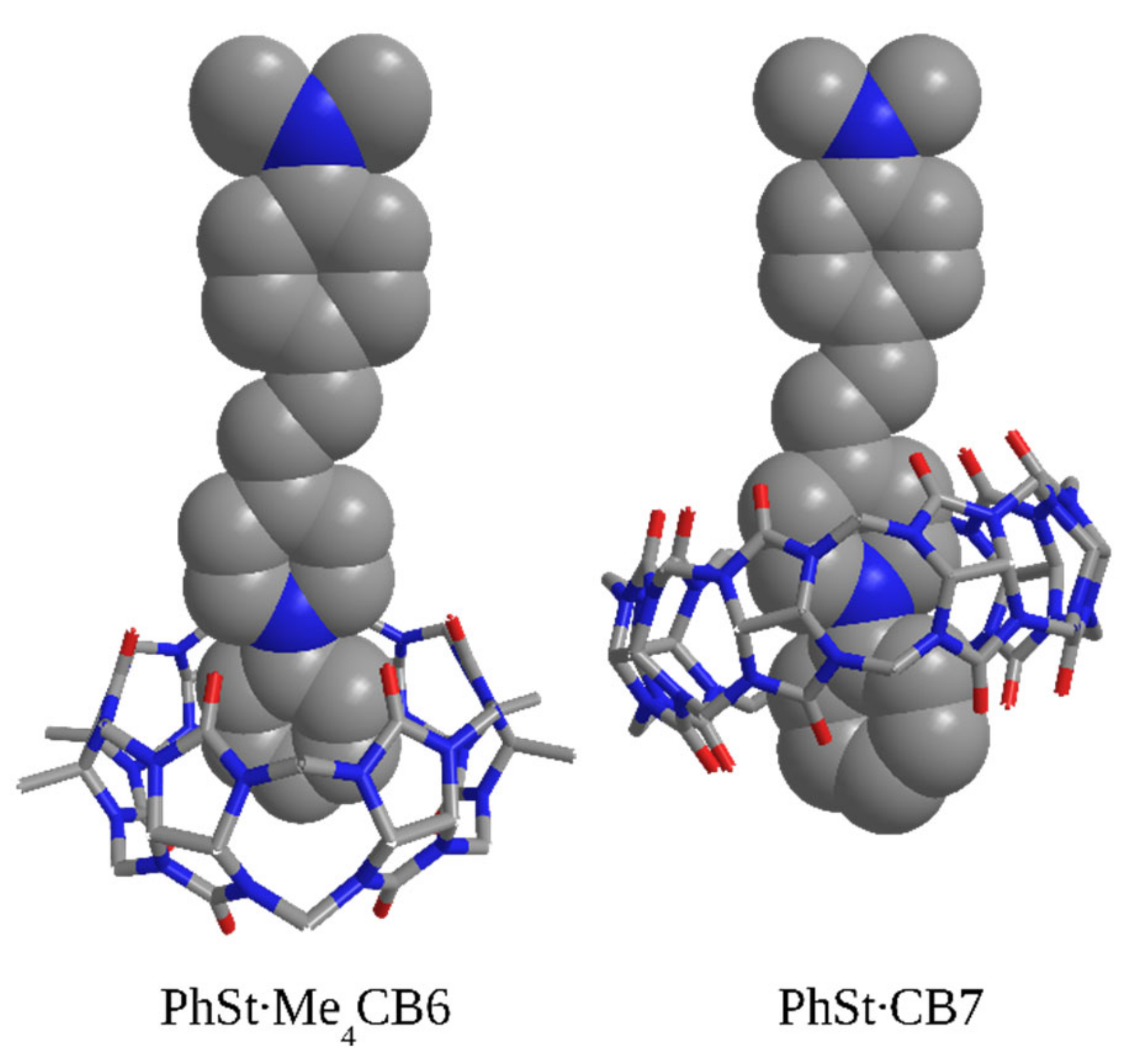
2.6. Calculated Structures of the Complexes
2.7. Size Selectivity of Complexing
2.8. Indicator Displacement
3. Methods
3.1. Synthesis
3.2. Spectroscopic Experiments
3.3. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; Del Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [PubMed]
- Kolman, V.; Babinský, M.; Kulhánek, P.; Marek, R.; Šindelář, V. Redistribution of electron density in pyridinium and pyrazinium guests induced by complexation with cucurbit[6]uril. New J. Chem. 2011, 35, 2854. [Google Scholar] [CrossRef]
- He, S.; Zhou, C.; Zhang, H.; Zhou, X. Binding modes of cucurbit[6]uril and cucurbit[7]uril with a series of bis-pyridinium compounds. J. Incl. Phenom. Macrocycl. Chem. 2012, 76, 333–344. [Google Scholar] [CrossRef]
- Yang, B.; Xiao, X.; Zhang, Y.-Q.; Zhu, Q.-J.; Xue, S.-F.; Tao, Z.; Wei, G. Inclusion of 4-pyrrolidinopyridine derivatives in a symmetrical α,α′,δ,δ′-tetramethyl-cucurbit[6]uril and a Ba2+-driven pseudorotaxane with characteristic UV absorption changes. RSC Adv. 2014, 4, 44359–44366. [Google Scholar] [CrossRef]
- Vedernikov, A.I.; Lobova, N.A.; Kuz’Mina, L.G.; Fomina, M.V.; Strelenko, Y.A.; Howard, J.A.K.; Gromov, S.P. Self-assembly of cucurbiturils and cyclodextrins to supramolecular millstones with naphthalene derivatives capable of translocations in the host cavities. New J. Chem. 2019, 43, 3673–3689. [Google Scholar] [CrossRef]
- Moon, K.; Kaifer, A.E. Modes of Binding Interaction between Viologen Guests and the Cucurbit[7]uril Host. Org. Lett. 2004, 6, 185–188. [Google Scholar] [CrossRef]
- Assaf, K.I.; Alnajjar, M.A.; Nau, W.M. Supramolecular assemblies through host–guest complexation between cucurbiturils and an amphiphilic guest molecule. Chem. Commun. 2018, 54, 1734–1737. [Google Scholar] [CrossRef]
- Huang, Z.; Qin, K.; Deng, G.; Wu, G.; Bai, Y.; Xu, J.-F.; Wang, Z.; Yu, Z.; Scherman, O.A.; Zhang, X. Supramolecular Chemistry of Cucurbiturils: Tuning Cooperativity with Multiple Noncovalent Interactions from Positive to Negative. Langmuir 2016, 32, 12352–12360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, T.-Y.; Zhang, K.; Dai, J.-L.; Zhu, Y.-Y.; Zhao, X. Encapsulation Enhanced Dimerization of a Series of 4-Aryl-N-Methylpyridinium Derivatives in Water: New Building Blocks for Self-Assembly in Aqueous Media. Chem. Asian J. 2014, 9, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhu, X.; Bian, B.; Xiao, X.; Tao, Z.; Redshaw, C. A Study of the Interaction between Cucurbit[7]uril and Alkyl Substituted 4-Pyrrolidinopyridinium Salts. Chemistry 2020, 2, 17. [Google Scholar] [CrossRef]
- Xu, W.; Kan, J.; Yang, B.; Prior, T.J.; Bian, B.; Xiao, X.; Tao, Z.; Redshaw, C. A Study of the Interaction Between Cucurbit[8]uril and Alkyl-Substituted 4-Pyrrolidinopyridinium Salts. Chem. Asian J. 2018, 14, 235–242. [Google Scholar] [CrossRef]
- Tcyrulnikov, N.A.; Varadharajan, R.; Tikhomirova, A.A.; Pattabiraman, M.; Ramamurthy, V.; Wilson, R.M. Modulation of Reduction Potentials of Bis(pyridinium)alkane Dications through Encapsulation within Cucurbit[7]uril. J. Org. Chem. 2019, 84, 8759–8765. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, S.; Liu, F.; Pang, Y.; Fan, J.; Song, F.; Peng, X. Large fluorescence enhancement of a hemicyanine by supramolecular interaction with cucurbit[6]uril and its application as resettable logic gates. Dyes Pigment. 2012, 93, 1401–1407. [Google Scholar] [CrossRef]
- Parvari, G.; Reany, O.; Keinan, E. Applicable Properties of Cucurbiturils. Isr. J. Chem. 2011, 51, 646–663. [Google Scholar] [CrossRef]
- Hennig, A.; Nau, W. Cucurbituril-based Sensors and Assays. In Monographs in Supramolecular Chemistry: Cucurbiturils and Related Macrocycles; Kim, K., Ed.; Royal Society of Chemistry: London, UK, 2020; pp. 154–196. [Google Scholar]
- Deng, X.Y.; Chen, K.; Chen, M.-D.; Bin Lü, L.; Tao, Z. Recognition of Different Metal Cations by a trans -4-[4-(Dimethylamino)styryl]-1-methylpyridinium Iodide@Tetramethylcucurbit[6]uril Probe. Eur. J. Inorg. Chem. 2019, 2019, 1212–1219. [Google Scholar] [CrossRef]
- Sun, S.; Yuan, Y.; Li, Z.; Zhang, S.; Zhang, H.; Peng, X. Interaction of a hemicyanine dye and its derivative with DNA and cucurbit[7]uril. New J. Chem. 2014, 38, 3600–3605. [Google Scholar] [CrossRef]
- Park, J.W.; Park, K.H. Inclusion of (aminostyryl)-1-methylpyridinium dyes by?-cyclodextrin and its use for fluorescent-probe studies on association of cationic and neutral molecules with?-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 1994, 17, 277–290. [Google Scholar] [CrossRef]
- Korbakov, N.; Timmerman, P.; Lidich, N.; Urbach, B.; Sa’Ar, A.; Yitzchaik, S. Acetylcholine Detection at Micromolar Concentrations with the Use of an Artificial Receptor-Based Fluorescence Switch. Langmuir 2008, 24, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Bojtár, M.; Hessz, D.; Szakács, Z.; Kubinyi, M.; Bitter, I. Optical spectroscopic studies on the complexation of stilbazolium dyes with a water soluble pillar[5]arene. RSC Adv. 2015, 5, 26504–26508. [Google Scholar] [CrossRef]
- Paudics, A.; Kubinyi, M.; Bitter, I.; Bojtár, M. Carboxylato-pillar[6]arene-based fluorescent indicator displacement assays for the recognition of monoamine neurotransmitters. RSC Adv. 2019, 9, 16856–16862. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, J.; Ko, Y.H.; Ahn, Y.; Murray, J.; Li, M.; Shrinidhi, A.; Kim, K. Dye-Cucurbit[n]uril Complexes as Sensor Elements for Reliable Pattern Recognition of Biogenic Polyamines. Bull. Chem. Soc. Jpn. 2018, 91, 95–99. [Google Scholar] [CrossRef]
- Nilam, M.; Gribbon, P.; Reinshagen, J.; Cordts, K.; Schwedhelm, E.; Nau, W.M.; Hennig, A. A Label-Free Continuous Fluorescence-Based Assay for Monitoring Ornithine Decarboxylase Activity with a Synthetic Putrescine Receptor. SLAS Discov. Adv. Life Sci. 2017, 22, 906–914. [Google Scholar] [CrossRef]
- Coe, B.J.; Harris, J.A.; Asselberghs, I.; Clays, K.; Olbrechts, G.; Persoons, A.; Hupp, J.T.; Johnson, R.C.; Coles, S.J.; Hursthouse, M.B.; et al. Quadratic Nonlinear Optical Properties ofN-Aryl Stilbazolium Dyes. Adv. Funct. Mater. 2002, 12, 110–116. [Google Scholar] [CrossRef]
- Figi, H.; Mutter, L.; Hunziker, C.; Coe, B.J.; Jazbinsek, M.; Günter, P. Extremely large nonresonant second-order nonlinear optical response in crystals of the stilbazolium salt DAPSH. J. Opt. Soc. Am. B 2008, 25, 1786. [Google Scholar] [CrossRef]
- Coe, B.J.; Beljonne, D.; Vogel, H.; Garín, J.; Orduna, J. Theoretical Analyses of the Effects on the Linear and Quadratic Nonlinear Optical Properties ofN-Arylation of Pyridinium Groups in Stilbazolium Dyes. J. Phys. Chem. A 2005, 109, 10052–10057. [Google Scholar] [CrossRef]
- Bojtár, M.; Szakács, Z.; Hessz, D.; Bazsó, F.L.; Kállay, M.; Kubinyi, M.; Bitter, I. Supramolecular FRET modulation by pseudorotaxane formation of a ditopic stilbazolium dye and carboxylato-pillar[5]arene. Dye. Pigment. 2016, 133, 415–423. [Google Scholar] [CrossRef]
- Zhao, Y. Synthesis of a symmetrical tetrasubstituted cucurbit[6]uril and its host-guest inclusion complex with 2,2?-bipyridine. Chin. Sci. Bull. 2004, 49, 1111. [Google Scholar] [CrossRef]
- Malaspina, T.; Fileti, E.E.; Chaban, V.V. Peculiar Aqueous Solubility Trend in Cucurbiturils Unraveled by Atomistic Simulations. J. Phys. Chem. B 2016, 120, 7511–7516. [Google Scholar] [CrossRef]
- Sinn, S.; Biedermann, F. Chemical Sensors Based on Cucurbit[n ]uril Macrocycles. Isr. J. Chem. 2018, 58, 357–412. [Google Scholar] [CrossRef]
- Jee, A.-Y.; Bae, E.; Lee, M. Internal motion of an electronically excited molecule in viscoelastic media. J. Chem. Phys. 2010, 133, 14507. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tolbert, R.W.; McHale, J.L.; Edwards, W.D. Theoretical Study of Solvent Effects on the Intramolecular Charge Transfer of a Hemicyanine Dye. J. Phys. Chem. A 1998, 102, 2739–2748. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, T.; Li, F.; Luo, C.; Huang, C.-H.; Cai, Z.; Zeng, X.; Zhou, J. Photophysical Studies on the Mono- and Dichromophoric Hemicyanine Dyes II. Solvent Effects and Dynamic Fluorescence Spectra Study in Chloroform and in LB Films. J. Phys. Chem. B 2002, 106, 10031–10040. [Google Scholar] [CrossRef]
- Strehmel, B.; Seifert, H.; Rettig, W. Photophysical Properties of Fluorescence Probes. 2. A Model of Multiple Fluorescence for Stilbazolium Dyes Studied by Global Analysis and Quantum Chemical Calculations†. J. Phys. Chem. B 1997, 101, 2232–2243. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, M. Volume Increment Effect on the Photoisomerization of Hemicyanine Dyes in Oligo(ethylene glycol)s. J. Phys. Chem. A 2013, 117, 12878–12883. [Google Scholar] [CrossRef]
- Shim, T.; Lee, M.; Kim, S.; Sung, J.; Rhee, B.K.; Kim, D.; Kim, H.; Yoon, K.B. Photoluminescence decay lifetime measurements of hemicyanine derivatives of different alkyl chain lengths. Mater. Sci. Eng. C 2004, 24, 83–85. [Google Scholar] [CrossRef]
- Panigrahi, M.; Patel, S.; Mishra, B. Solvatochromism of some hemicyanines. J. Mol. Liq. 2013, 177, 335–342. [Google Scholar] [CrossRef]
- Tessore, F.; Cariati, E.; Cariati, F.; Roberto, D.; Ugo, R.; Mussini, P.R.; Zuccacciaa, C.; Macchionia, A. The Role of Ion Pairs in the Second-Order NLO Response of 4-X-1-Methylpiridinium Salts. Chem. Phys. Chem. 2010, 11, 495–507. [Google Scholar] [CrossRef]
- Carlotti, B.; Consiglio, G.; Elisei, F.; Fortuna, C.G.; Mazzucato, U.; Spalletti, A. Intramolecular Charge Transfer of Push–Pull Pyridinium Salts in the Singlet Manifold. J. Phys. Chem. A 2014, 118, 3580–3592. [Google Scholar] [CrossRef]
- Aschi, M.; Barone, V.; Carlotti, B.; Daidone, I.; Elisei, F.; Amadei, A. Photoexcitation and relaxation kinetics of molecular systems in solution: towards a complete in silico model. Phys. Chem. Chem. Phys. 2016, 18, 28919–28931. [Google Scholar] [CrossRef]
- Laage, D.; Thompson, W.H.; Blanchard-Desce, M.; Hynes, J.T. Charged Push−Pull Polyenes in Solution: Anomalous Solvatochromism and Nonlinear Optical Properties. J. Phys. Chem. A 2003, 107, 6032–6046. [Google Scholar] [CrossRef]
- Loutfy, R.O.; Arnold, B.A. Effect of viscosity and temperature on torsional relaxation of molecular rotors. J. Phys. Chem. 1982, 86, 4205–4211. [Google Scholar] [CrossRef]
- Carlotti, B.; Consiglio, G.; Elisei, F.; Fortuna, C.G.; Mazzucato, U.; Spalletti, A. Intramolecular Charge Transfer of Push–Pull Pyridinium Salts in the Triplet Manifold. J. Phys. Chem. A 2014, 118, 7782–7787. [Google Scholar] [CrossRef]
- Zhang, S.; Grimm, L.; Miskolczy, Z.; Biczók, L.; Biedermann, F.; Nau, W.M. Binding affinities of cucurbit[n]urils with cations. Chem. Commun. 2019, 55, 14131–14134. [Google Scholar] [CrossRef]
- Šindelář, V.; Moon, K.; Kaifer, A.E. Binding Selectivity of Cucurbit[7]uril: Bis(pyridinium)-1,4-xylylene versus 4,4‘-Bipyridinium Guest Sites. Org. Lett. 2004, 6, 2665–2668. [Google Scholar] [CrossRef]
- Yamada, S.; Misono, T.; Tsuzuki, S. Cation−π Interactions of a Thiocarbonyl Group and a Carbonyl Group with a Pyridinium Nucleus. J. Am. Chem. Soc. 2004, 126, 9862–9872. [Google Scholar] [CrossRef]
- Gruber, T. Synthetic Receptors for the Recognition and Discrimination of Post-Translationally Methylated Lysines. Chem. Bio. Chem. 2018, 19, 2324–2340. [Google Scholar] [CrossRef]
- Huque, M.E.; Vogel, H.J. Carbon-13 NMR studies of the lysine side chains of calmodulin and its proteolytic fragments. Protein J. 1993, 12, 695–707. [Google Scholar] [CrossRef]
- Henchoz, Y.; Schappler, J.; Geiser, L.; Prat, J.; Carrupt, P.-A.; Veuthey, J.-L. Rapid determination of pK a values of 20 amino acids by CZE with UV and capacitively coupled contactless conductivity detections. Anal. Bioanal. Chem. 2007, 389, 1869–1878. [Google Scholar] [CrossRef]
- Gamal-Eldin, M.A.; Macartney, D.H. Selective molecular recognition of methylated lysines and arginines by cucurbit[6]uril and cucurbit[7]uril in aqueous solution. Org. Biomol. Chem. 2013, 11, 488–495. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision B.01; Computational Chemistry Software; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Nagy, P.R.; Samu, G.; Kállay, M. Optimization of the Linear-Scaling Local Natural Orbital CCSD(T) Method: Improved Algorithm and Benchmark Applications. J. Chem. Theory Comput. 2018, 14, 4193–4215. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- MRCC, a quantum chemical program suite written by Kállay, M.; Nagy, P. R.; Mester, D.; Rolik, Z.; Samu, G.; Csontos, J.; Csóka, J.; Szabó, P. B.; Gyevi-Nagy, L.; Hégely, B.; Ladjánszki, I.; Szegedy, L.; Ladóczki, B.; Petrov, K.; Farkas, M.; Mezei, P. D.; Ganyecz, A. Available online: www.mrcc.hu (accessed on 1 September 2020).
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound PhSt. are available from the authors. |
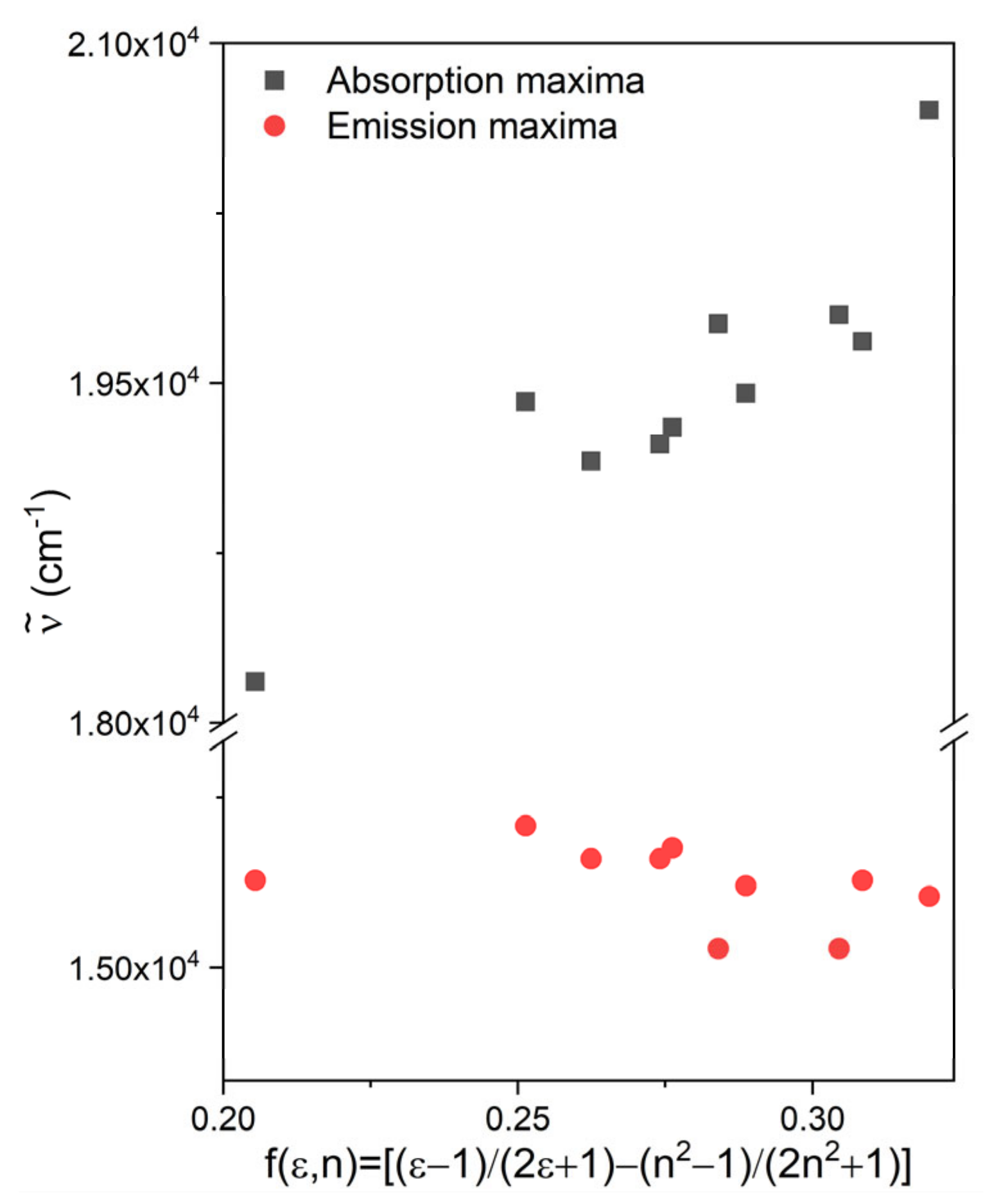



| Solvent | f(ε,n) | λabs / nm (ε/M−1cm−1) | λF / nm (IF/100 cps) |
|---|---|---|---|
| water | 0.3198 | 483 (30300) | 653 (75) |
| methanol | 0.3085 | 508 (58300) | 650 (388) |
| ethanol | 0.2887 | 514 (44200) | 651 (758) |
| 1-propanol | 0.2741 | 520 (43800) | 646 (1530) |
| 2-propanol | 0.2762 | 518 (40800) | 644 (1530) |
| 1-butanol | 0.2624 | 522 (46800) | 646 (2480) |
| t-butanol | 0.2513 | 515 (38500) | 640 (2880) |
| acetonitrile | 0.3045 | 505 (43700) | 663 (151) |
| acetone | 0.2840 | 506 (43100) | 663 (228) |
| dichloromethane | 0.2054 | 550 (61900) | 650 (4980) |
| Solvent | η/ cP | ΦF | τF / ps | kr / 108 s−1 | knr / 109 s−1 |
|---|---|---|---|---|---|
| PEG-200 | 64.0 | 0.0695 | 127 | 1.58 | 2.11 |
| PEG-300 | 92.6 | 0.0919 | 441 | 1.64 | 1.62 |
| PEG-400 | 123.3 | 0.101 | 561 | 1.59 | 1.41 |
| PEG-600 | 178.3 | 0.133 | 637 | 1.79 | 1.17 |
| 90% PEG-600 10% PEG-1000 | 192.3 | 0.155 | 744 | 2.00 | 1.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paudics, A.; Hessz, D.; Bojtár, M.; Gyarmati, B.; Szilágyi, A.; Kállay, M.; Bitter, I.; Kubinyi, M. Binding Modes of a Phenylpyridinium Styryl Fluorescent Dye with Cucurbiturils. Molecules 2020, 25, 5111. https://doi.org/10.3390/molecules25215111
Paudics A, Hessz D, Bojtár M, Gyarmati B, Szilágyi A, Kállay M, Bitter I, Kubinyi M. Binding Modes of a Phenylpyridinium Styryl Fluorescent Dye with Cucurbiturils. Molecules. 2020; 25(21):5111. https://doi.org/10.3390/molecules25215111
Chicago/Turabian StylePaudics, Adrien, Dóra Hessz, Márton Bojtár, Benjámin Gyarmati, András Szilágyi, Mihály Kállay, István Bitter, and Miklós Kubinyi. 2020. "Binding Modes of a Phenylpyridinium Styryl Fluorescent Dye with Cucurbiturils" Molecules 25, no. 21: 5111. https://doi.org/10.3390/molecules25215111
APA StylePaudics, A., Hessz, D., Bojtár, M., Gyarmati, B., Szilágyi, A., Kállay, M., Bitter, I., & Kubinyi, M. (2020). Binding Modes of a Phenylpyridinium Styryl Fluorescent Dye with Cucurbiturils. Molecules, 25(21), 5111. https://doi.org/10.3390/molecules25215111






