Design of Fluorescent Coumarin-Hydroxamic Acid Derivatives as Inhibitors of HDACs: Synthesis, Anti-Proliferative Evaluation and Docking Studies
Abstract
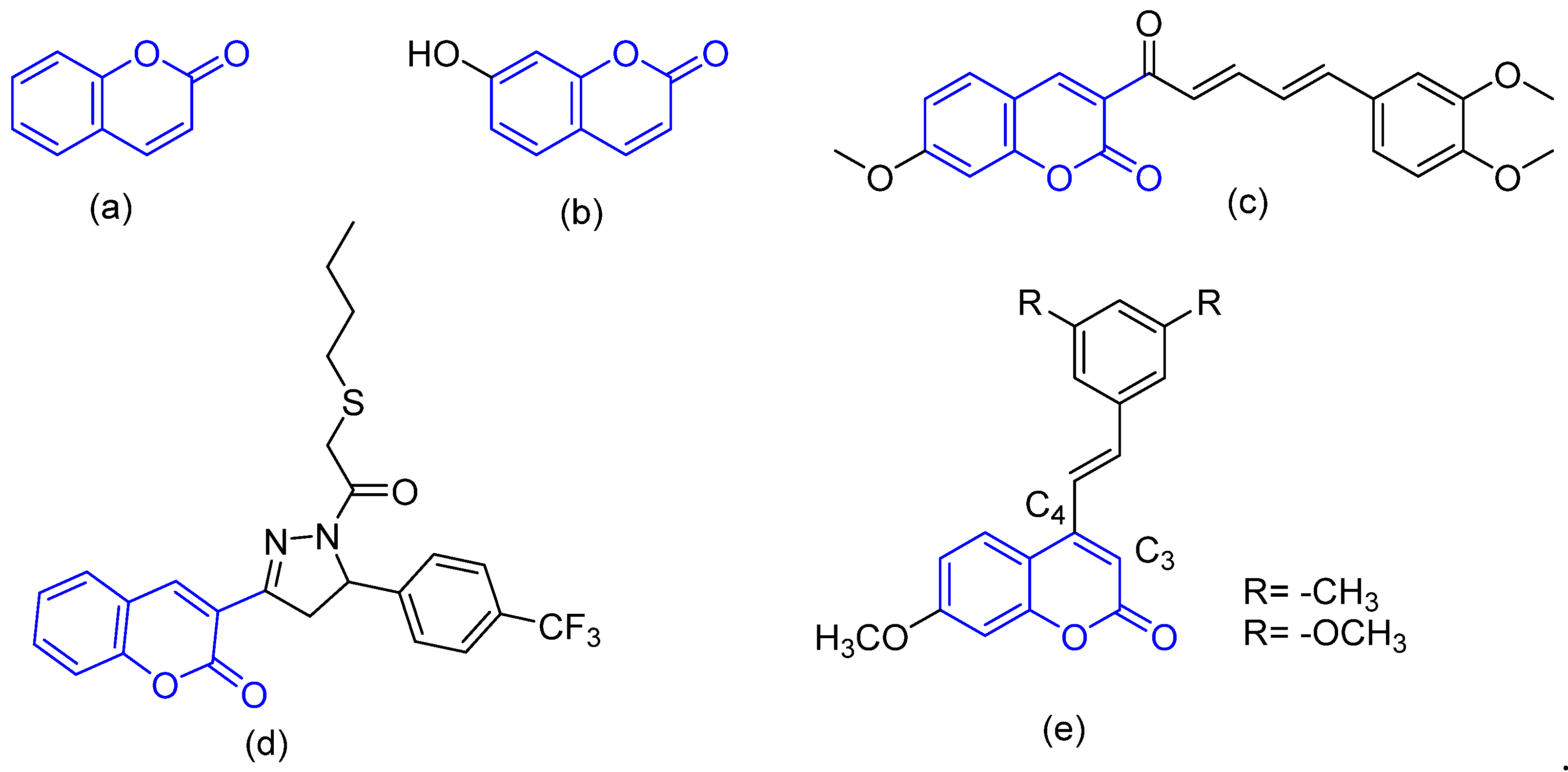
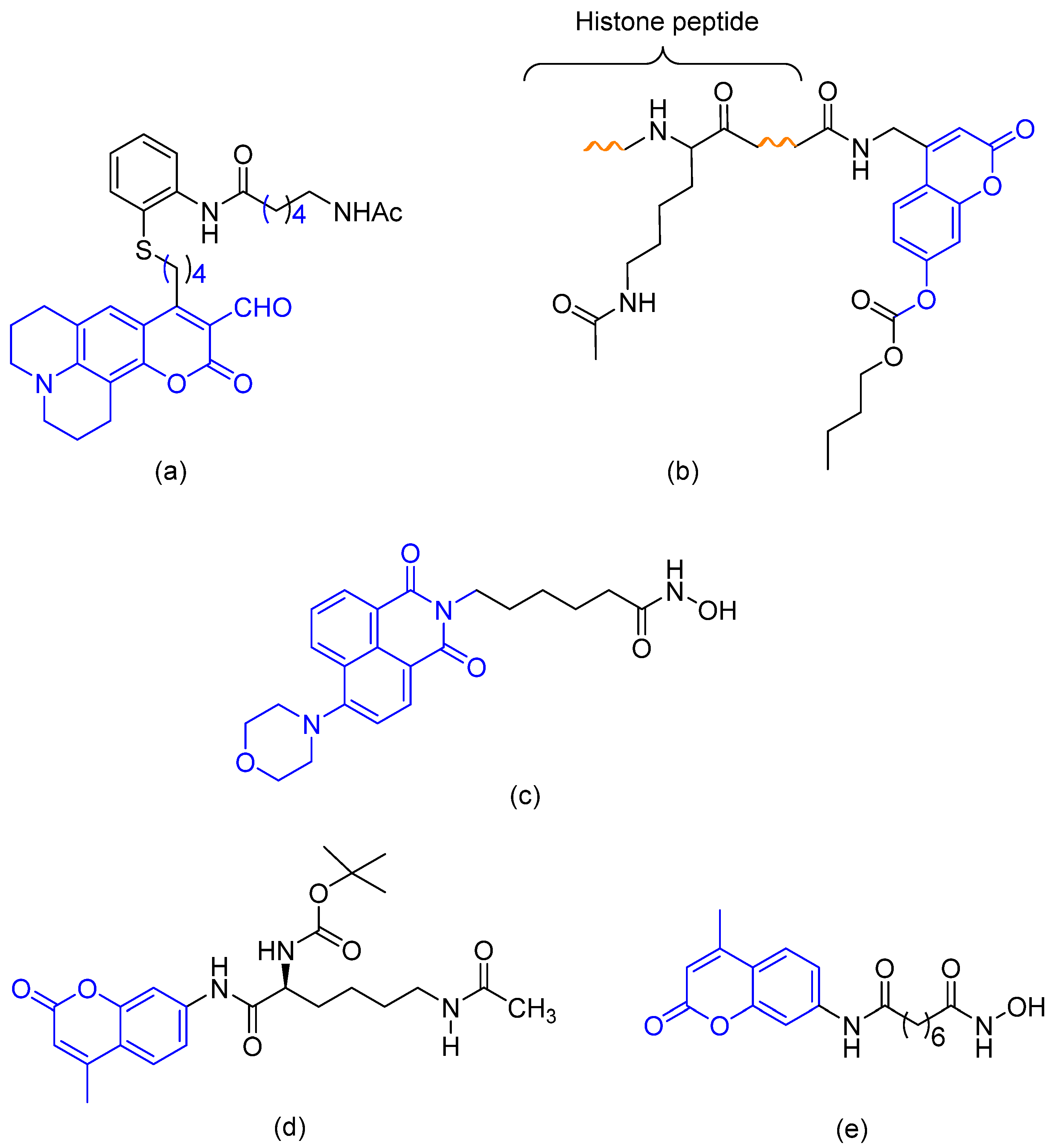
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Effects of SAHA Analogues on the Expression of Cell Cycle Regulatory Genes
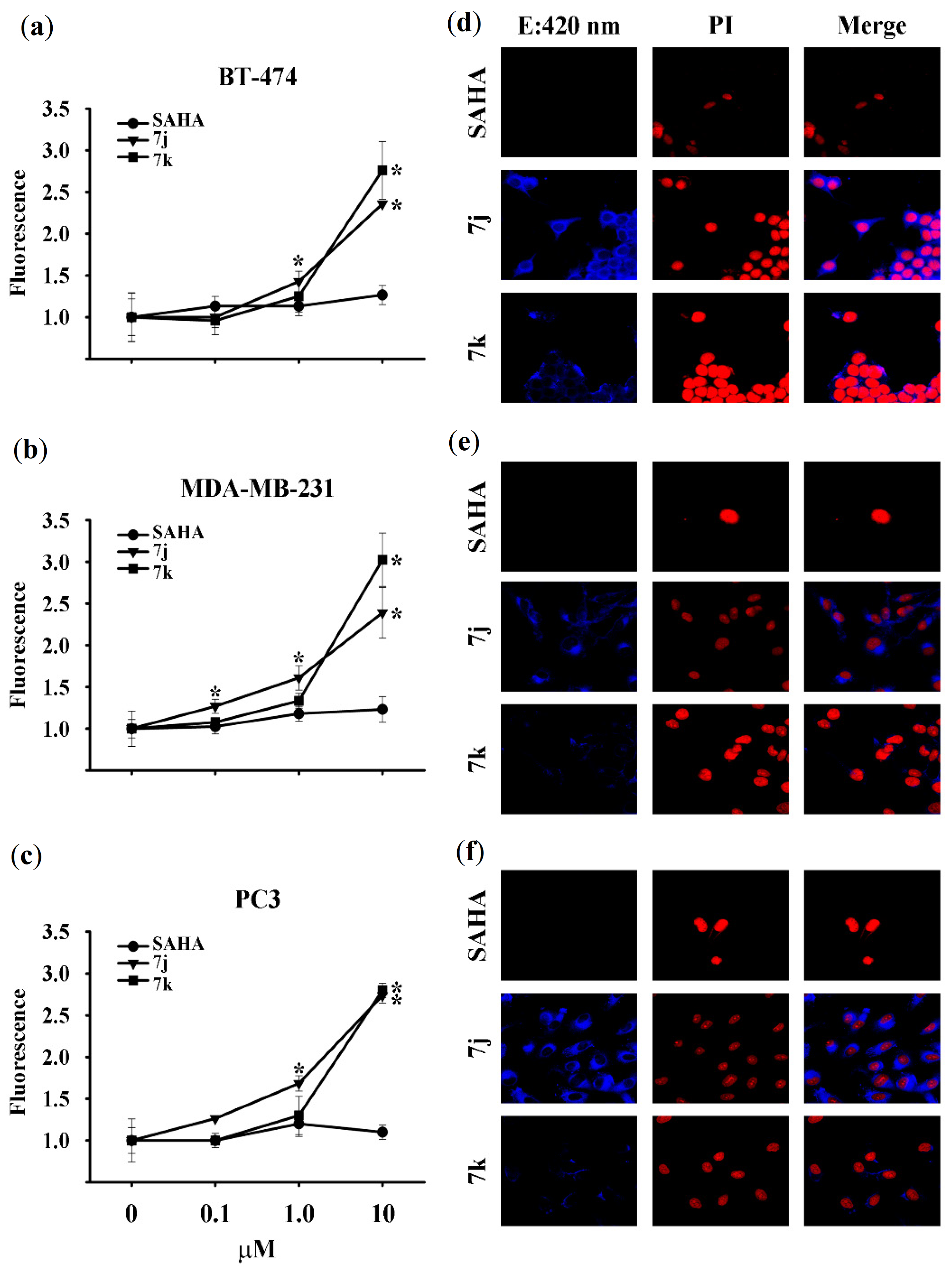
2.2.2. Novel Fluorescent SAHA Analogues
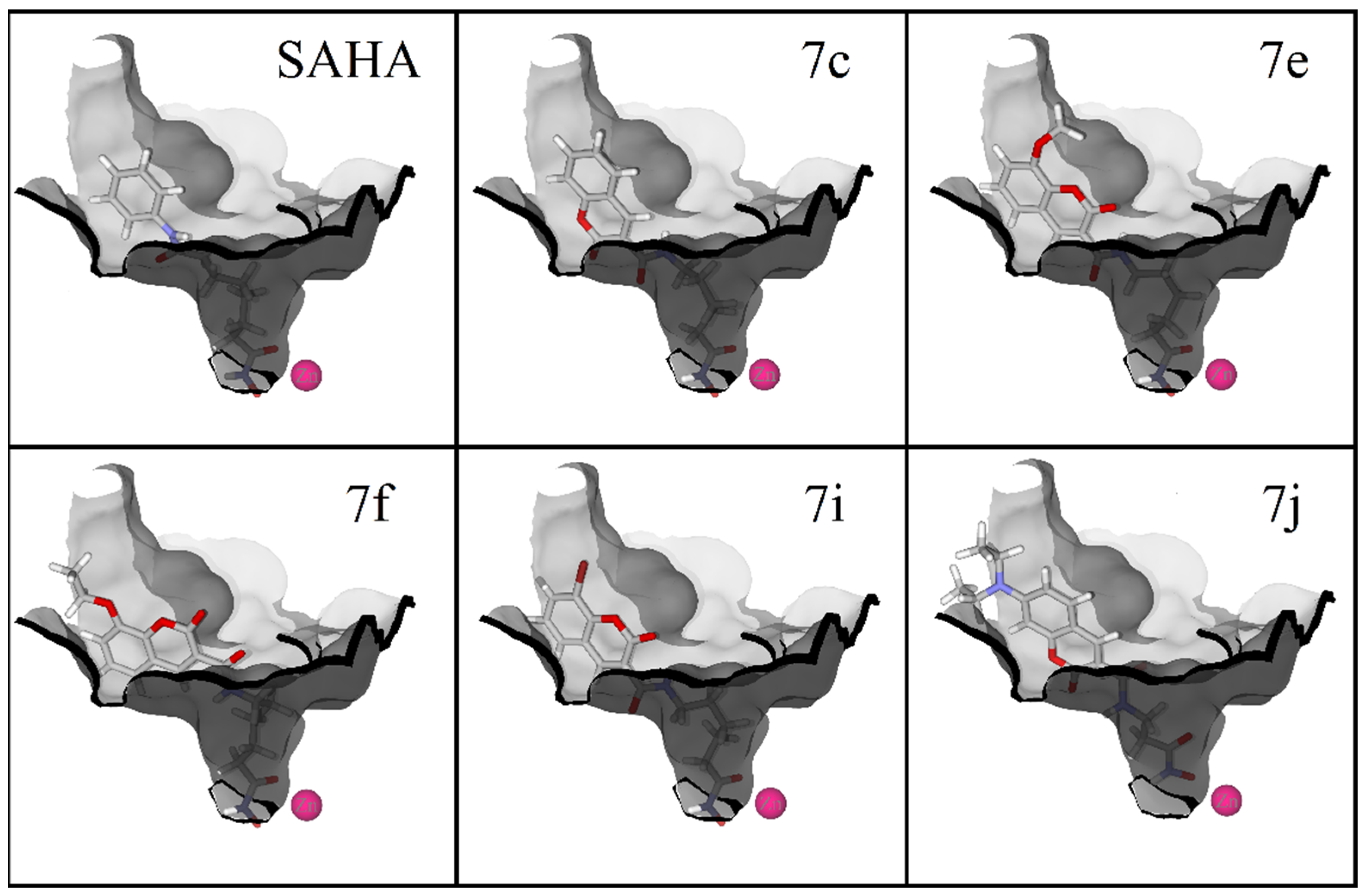
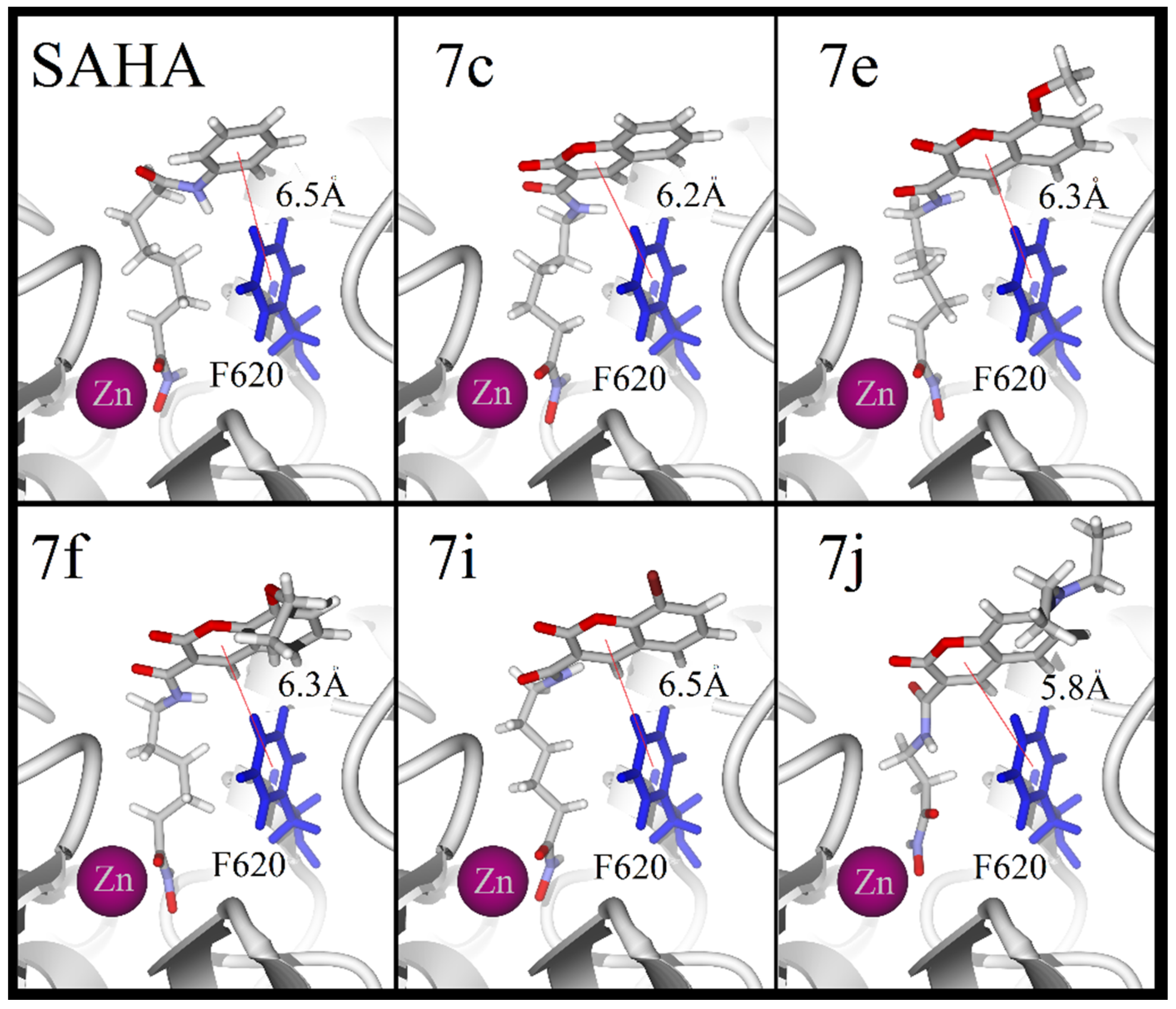
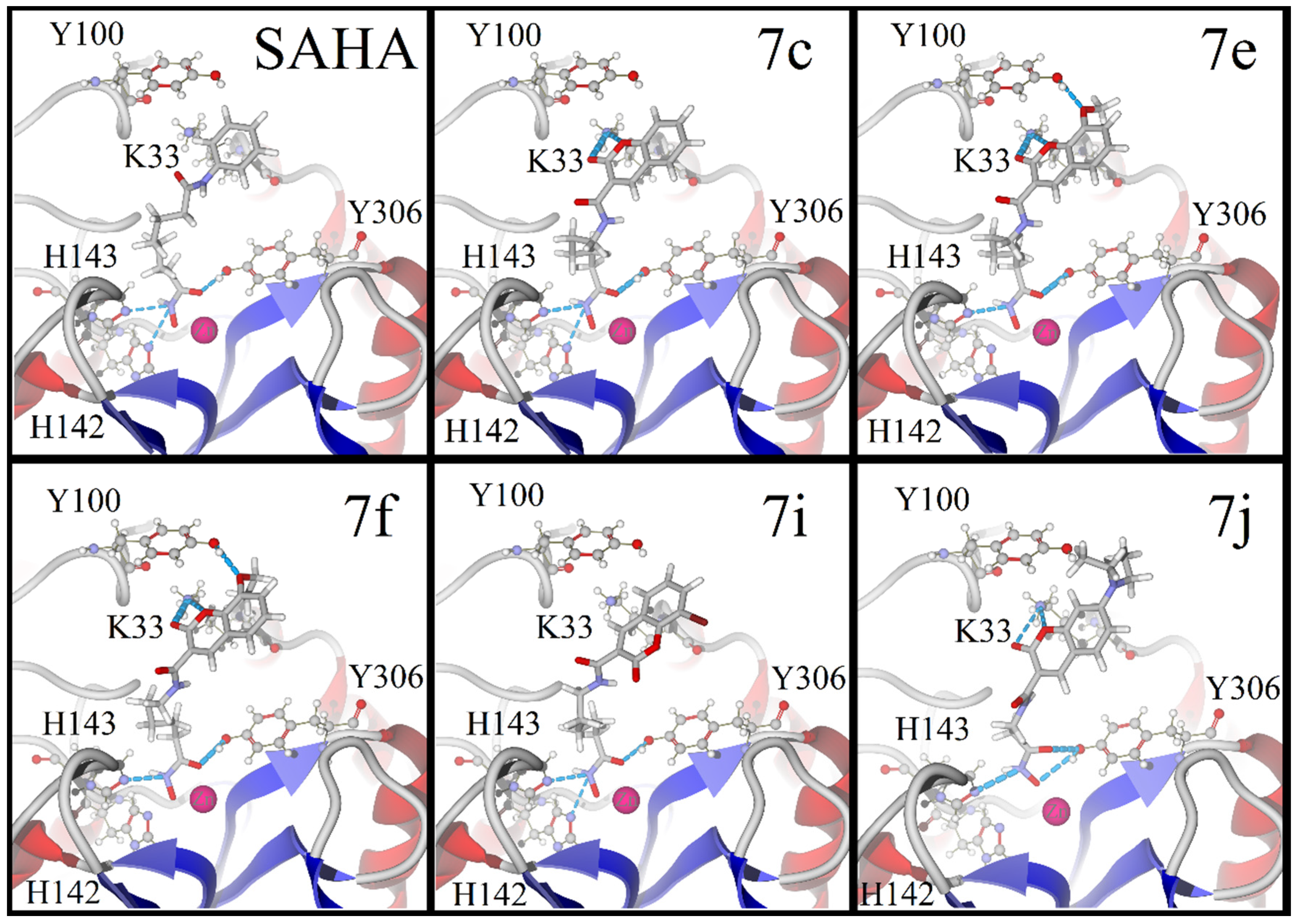
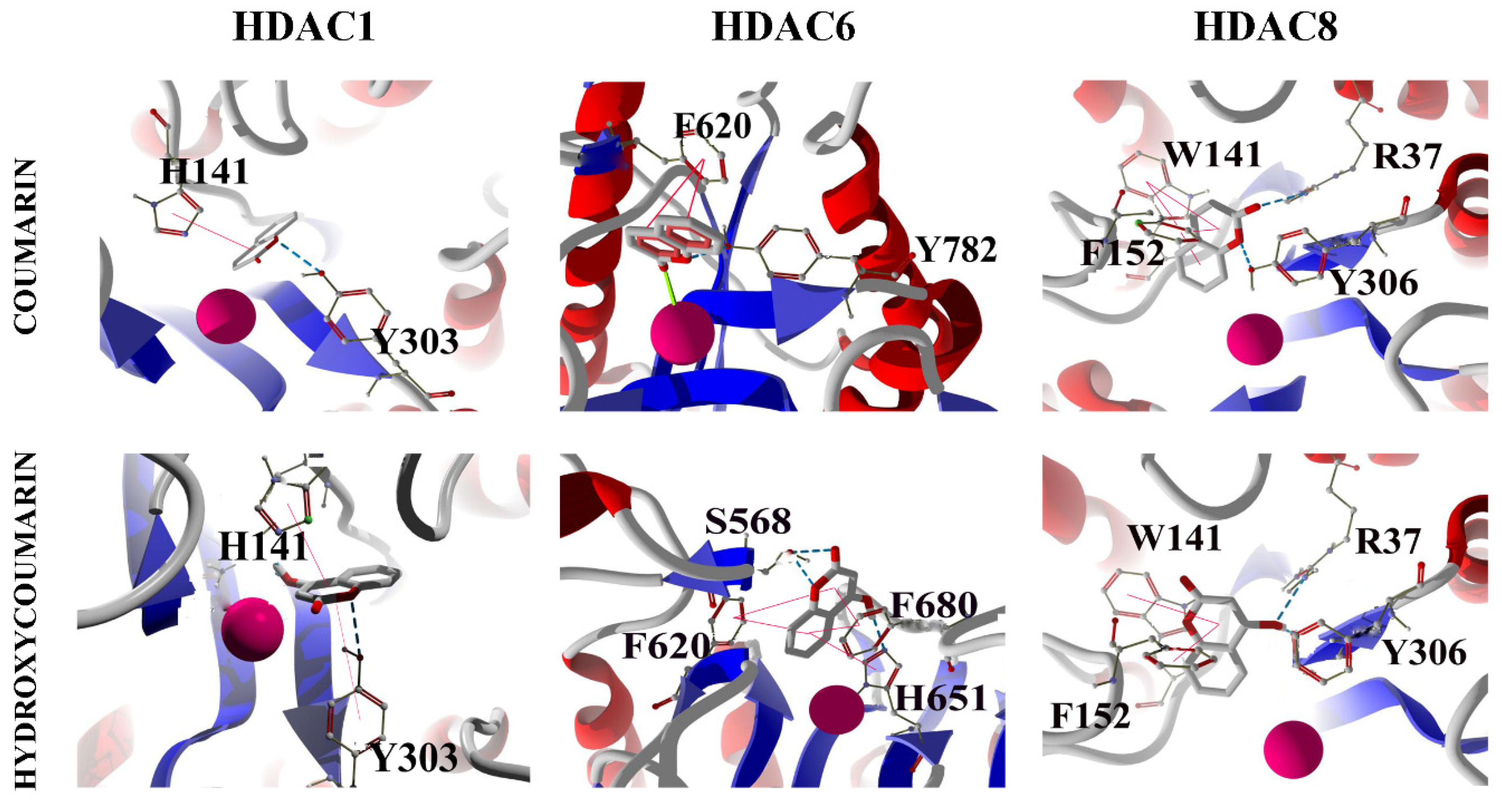
2.3. Molecular Docking
2.4. ADMET Properties for SAHA Analogues
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Preparation of Coumarin-3-Carboxylic Acids (3a–f)
3.1.2. General Procedure for the Preparation of Coumarin-3-Carboxamides (5a–k)
3.1.3. General Procedure for the Preparation of N-(2-Oxo-2H-Chromene-3-Carboxamide) Acids (6a–k)
3.1.4. General Procedure for the Preparation of the Title Compounds (7a–k)
3.2. Computational Methodology: Molecular Docking
3.3. Biological Assay
3.3.1. Cell Culture
3.3.2. Sulforhodamine B (SRB) Assay
3.3.3. Fluorescence Microscopy and Spectrofluorometric Quantification
3.3.4. Real Time RT-PCR
3.3.5. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and Synthetic Coumarin Derivatives with Anti-Inflammatory / Antioxidant Activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef]
- Saeedi, M.; Goli, F.; Mahdavi, M.; Dehghan, G.; Faramarzi, M.A.; Foroumadi, A.; Shafiee, A. Synthesis and Biological Investigation of Some Novel Sulfonamide and Amide Derivatives Containing Coumarin Moieties. Iran. J. Pharm. Res. 2014, 13, 881–892. [Google Scholar]
- Atmaca, M.; Bilgin, H.M.; Obay, B.D.; Diken, H.; Kelle, M.; Kale, E. The hepatoprotective effect of coumarin and coumarin derivates on carbon tetrachloride-induced hepatic injury by antioxidative activities in rats. J. Physiol. Biochem. 2011, 67, 569–576. [Google Scholar] [CrossRef]
- Peng, X.-M.; Damu, G.L.V.; Zhou, C.-H. Current Developments of Coumarin Compounds in Medicinal Chemistry. Curr. Pharm. Des. 2013, 19, 3884–3930. [Google Scholar] [CrossRef]
- Završnik, D.; Muratović, S.; Makuc, D.; Plavec, J.; Cetina, M.; Nagl, A.; De Clercq, E.; Balzarini, J.; Mintas, M. Benzylidene-bis-(4-Hydroxycoumarin) and Benzopyrano-Coumarin Derivatives: Synthesis, 1H/13C-NMR Conformational and X-ray Crystal Structure Studies and In Vitro Antiviral Activity Evaluations. Molecules 2011, 16, 6023–6040. [Google Scholar] [CrossRef]
- Wang, S.-F.; Yin, Y.; Wu, X.; Qiao, F.; Sha, S.; Lv, P.-C.; Zhao, J.; Zhu, H.-L. Synthesis, molecular docking and biological evaluation of coumarin derivatives containing piperazine skeleton as potential antibacterial agents. Bioorg. Med. Chem. 2014, 22, 5727–5737. [Google Scholar] [CrossRef]
- Ostrov, D.A.; Hernández Prada, J.A.; Corsino, P.E.; Finton, K.A.; Le, N.; Rowe, T.C. Discovery of novel DNA gyrase inhibitors by high-throughput virtual screening. Antimicrob. Agents Chemother. 2007, 51, 3688–3698. [Google Scholar] [CrossRef]
- Shamsa, F.; Foroumadi, A.; Shamsa, H.; Samadi, N.; Faramarzi, M.A.; Shafiee, A. Synthesis and In-vitro Antibacterial Activities of Acetylanthracene and Acetylphenanthrene Derivatives of Some Fluoroquinolones. Iran. J. Pharm. Res. 2011, 10, 225–231. [Google Scholar]
- Patel, R.V.; Patel, P.K.; Kumari, P.; Rajani, D.P.; Chikhalia, K.H. Synthesis of benzimidazolyl-1,3,4-oxadiazol-2ylthio-N-phenyl (benzothiazolyl) acetamides as antibacterial, antifungal and antituberculosis agents. Eur. J. Med. Chem. 2012, 53, 41–51. [Google Scholar] [CrossRef]
- Keri, R.S.; Sasidhar, B.S.; Nagaraja, B.M.; Santos, M.A. Recent progress in the drug development of coumarin derivatives as potent antituberculosis agents. Eur. J. Med. Chem. 2015, 100, 257–269. [Google Scholar] [CrossRef]
- Nair, R.V.; Fisher, E.P.; Safe, S.H.; Cortez, C.; Harvey, R.G.; DiGiovanni, J. Novel coumarins as potential anticarcinogenic agents. Carcinogenesis 1991, 12, 65–69. [Google Scholar] [CrossRef]
- Gu, X.; Zhou, Y.; Wu, X.; Wang, F.; Zhang, C.-Y.; Du, C.; Shen, L.; Chen, X.; Shi, J.; Liu, C.; et al. Antidepressant-like effects of auraptenol in mice. Sci. Rep. 2014, 4, 4433. [Google Scholar] [CrossRef]
- Capra, J.C.; Cunha, M.P.; Machado, D.G.; Zomkowski, A.D.E.; Mendes, B.G.; Santos, A.R.S.; Pizzolatti, M.G.; Rodrigues, A.L.S. Antidepressant-like effect of scopoletin, a coumarin isolated from Polygala sabulosa (Polygalaceae) in mice: Evidence for the involvement of monoaminergic systems. Eur. J. Pharmacol. 2010, 643, 232–238. [Google Scholar] [CrossRef]
- Yuce, B.; Danis, O.; Ogan, A.; Sener, G.; Bulut, M.; Yarat, A. Antioxidative and lipid lowering effects of 7,8-dihydroxy-3-(4-methylphenyl) coumarin in hyperlipidemic rats. Arzneimittelforschung 2009, 59, 129–134. [Google Scholar] [CrossRef]
- Orhan, I.E.; Gulcan, H.O. Coumarins: Auspicious Cholinesterase and Monoamine Oxidase Inhibitors. Curr. Top. Med. Chem. 2015, 15, 1673–1682. [Google Scholar] [CrossRef]
- Brühlmann, C.; Ooms, F.; Carrupt, P.-A.; Testa, B.; Catto, M.; Leonetti, F.; Altomare, C.; Carotti, A. Coumarins Derivatives as Dual Inhibitors of Acetylcholinesterase and Monoamine Oxidase. J. Med. Chem. 2001, 44, 3195–3198. [Google Scholar] [CrossRef] [PubMed]
- Katsori, A.-M.; Hadjipavlou-Litina, D. Coumarin derivatives: An updated patent review (2012–2014). Expert Opin. Ther. Pat. 2014, 24, 1323–1347. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef]
- Egan, D.; James, P.; Cooke, D.; O’Kennedy, R. Studies on the cytostatic and cytotoxic effects and mode of action of 8-nitro-7-hydroxycoumarin. Cancer Lett. 1997, 118, 201–211. [Google Scholar] [CrossRef]
- Seidel, C.; Schnekenburger, M.; Zwergel, C.; Gaascht, F.; Mai, A.; Dicato, M.; Kirsch, G.; Valente, S.; Diederich, M. Novel inhibitors of human histone deacetylases: Design, synthesis and bioactivity of 3-alkenoylcoumarines. Bioorg. Med. Chem. Lett. 2014, 24, 3797–3801. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Huang, C.; Jia, Y.-M.; Song, B.-A.; Li, J.; Liu, X.-H. Novel coumarin-dihydropyrazole thio-ethanone derivatives: Design, synthesis and anticancer activity. Eur. J. Med. Chem. 2014, 74, 717–725. [Google Scholar] [CrossRef]
- Belluti, F.; Fontana, G.; Bo, L.D.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorg. Med. Chem. 2010, 18, 3543–3550. [Google Scholar] [CrossRef]
- Ding, J.; Liu, J.; Zhang, Z.; Guo, J.; Cheng, M.; Wan, Y.; Wang, R.; Fang, Y.; Guan, Z.; Jin, Y. Design, synthesis and biological evaluation of coumarin-based N-hydroxycinnamamide derivatives as novel histone deacetylase inhibitors with anticancer activities. Bioorg. Chem. 2020, 101, 104023. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, N.; Song, J.; Zhu, K.; Jiang, C.-S.; Shan, P.; Zhang, H. Design, Synthesis and Biological Evaluation of Novel Coumarin-Based Hydroxamate Derivatives as Histone Deacetylase (Hdac) Inhibitors with Antitumor Activities. Molecules 2019, 24, 2569. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, F.; Han, L.; Qu, Y.; Ge, D.; Zhang, H. Development of Coumarin-Based Hydroxamates as Histone Deacetylase Inhibitors with Antitumor Activities. Molecules 2020, 25, 717. [Google Scholar] [CrossRef]
- Manal, M.; Chandrasekar, M.J.N.; Gomathi Priya, J.; Nanjan, M.J. Inhibitors of histone deacetylase as antitumor agents: A critical review. Bioorg. Chem. 2016, 67, 18–42. [Google Scholar] [CrossRef]
- Qiu, X.; Xiao, X.; Li, N.; Li, Y. Histone deacetylases inhibitors (HDACis) as novel therapeutic application in various clinical diseases. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 72, 60–72. [Google Scholar] [CrossRef]
- Tasior, M.; Kim, D.; Singha, S.; Krzeszewski, M.; Ahn, K.H.; Gryko, D.T. π-Expanded coumarins: Synthesis, Optical Properties and Applications. J. Mater. Chem. C 2015, 3, 1421–1446. [Google Scholar] [CrossRef]
- Yan, F.; Sun, X.; Zu, F.; Bai, Z.; Jiang, Y.; Fan, K.; Wang, J. Fluorescent probes for detecting cysteine. Methods Appl. Fluoresc. 2018, 6, 1–48. [Google Scholar] [CrossRef]
- Jung, Y.; Jung, J.; Huh, Y.; Kim, D. Benzo[g]coumarin-Based Fluorescent Probes for Bioimaging Applications. J. Anal. Methods Chem. 2018, 2018, 5249765. [Google Scholar] [CrossRef]
- Rooker, D.R.; Buccella, D. Real-time detection of histone deacetylase activity with a small molecule fluorescent and spectrophotometric probe. Chem. Sci. 2015, 6, 6456–6461. [Google Scholar] [CrossRef]
- Baba, R.; Hori, Y.; Mizukami, S.; Kikuchi, K. Development of a Fluorogenic Probe with a Transesterification Switch for Detection of Histone Deacetylase Activity. J. Am. Chem. Soc. 2012, 134, 14310–14313. [Google Scholar] [CrossRef]
- Hoffmann, K.; Brosch, G.; Loidl, P.; Jung, M. A non-isotopic assay for histone deacetylase activity. Nucleic Acids Res. 1999, 27, 2057–2058. [Google Scholar] [CrossRef] [PubMed]
- Heltweg, B.; Dequiedt, F.; Marshall, B.L.; Brauch, C.; Yoshida, M.; Nishino, N.; Verdin, E.; Jung, M. Subtype Selective Substrates for Histone Deacetylases. J. Med. Chem. 2004, 47, 5235–5243. [Google Scholar] [CrossRef]
- Singh, R.K.; Mandal, T.; Balasubramanian, N.; Cook, G.; Srivastava, D.K. Coumarin-suberoylanilide hydroxamic acid as a fluorescent probe for determining binding affinities and off-rates of histone deacetylase inhibitors. Anal. Biochem. 2011, 408, 309–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Yacoub, N.; Fecker, L.F.; Möbs, M.; Plötz, M.; Braun, F.K.; Sterry, W.; Eberle, J. Apoptosis Induction by SAHA in Cutaneous T-Cell Lymphoma Cells Is Related to Downregulation of c-FLIP and Enhanced TRAIL Signaling. J. Investig. Dermatol. 2012, 132, 2263–2274. [Google Scholar] [CrossRef]
- Wittine, K.; Ratkaj, I.; Benci, K.; Suhina, T.; Mandić, L.; Ilić, N.; Pavelić, S.K.; Pavelić, K.; Mintas, M. The novel coumarin[3,2-c]thiophene and its hydroxamic acid and ureido derivatives: Synthesis and cytostatic activity evaluations. Med. Chem. Res. 2016, 25, 728–737. [Google Scholar] [CrossRef]
- Abdizadeh, R.; Hadizadeh, F.; Abdizadeh, T. QSAR analysis of coumarin-based benzamides as histone deacetylase inhibitors using CoMFA, CoMSIA and HQSAR methods. J. Mol. Struct. 2020, 1199, 126961. [Google Scholar] [CrossRef]
- Garcia, S.; Vazquez, J.L.; Renteria, M.; Aguilar-Garduño, I.G.; Delgado, F.; Trejo-Duran, M.; Garcia-Revilla, M.A.; Alvarado-Mendez, E.; Vazquez, M.A. Synthesis and experimental-computational characterization of nonlinear optical properties of triazacyclopentafluorene-coumarin derivatives. Opt. Mater. (Amst.) 2016, 62, 231–239. [Google Scholar] [CrossRef]
- Bahena, L.; Cervantes, C.; Soto-Arredondo, K.J.; Martínez-Alfaro, M.; Zarco, N.; García-Revilla, M.A.; Alcaraz-Contreras, Y.; Tirado, L.P.; Vázquez, M.A.; Robles, J. In Silico, Synthesis and Biological Investigations of Pyrrolo[3,4-C]Pyrrole Hydroxamic Acid Derivatives as Potential Anticancer Agents. J. Mex. Chem. Soc. 2017, 61, 297–308. [Google Scholar] [CrossRef]
- Song, A.; Wang, X.; Lam, K.S. A convenient synthesis of coumarin-3-carboxylic acids via Knoevenagel condensation of Meldrum’s acid with ortho-hydroxyaryl aldehydes or ketones. Tetrahedron Lett. 2003, 44, 1755–1758. [Google Scholar] [CrossRef]
- Maggi, R.; Bigi, F.; Carloni, S.; Mazzacani, A.; Sartori, G. Uncatalysed reactions in water: Part 2. Preparation of 3-carboxycoumarins. Green Chem. 2001, 3, 173–174. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, S.-N.; Kim, Y.K. Involvement of HDAC1 in E-cadherin expression in prostate cancer cells; its implication for cell motility and invasion. Biochem. Biophys. Res. Commun. 2011, 404, 915–921. [Google Scholar] [CrossRef]
- Huang, L.; Pardee, A.B. Suberoylanilide hydroxamic acid as a potential therapeutic agent for human breast cancer treatment. Mol. Med. 2000, 6, 849–866. [Google Scholar] [CrossRef]
- Butler, L.M.; Agus, D.B.; Scher, H.I.; Higgins, B.; Rose, A.; Cordon-Cardo, C.; Thaler, H.T.; Rifkind, R.A.; Marks, P.A.; Richon, V.M. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000, 60, 5165–5170. [Google Scholar]
- Patra, N.; De, U.; Kim, T.H.; Lee, Y.J.; Ahn, M.Y.; Kim, N.D.; Yoon, J.H.; Choi, W.S.; Moon, H.R.; Lee, B.M.; et al. A novel histone deacetylase (HDAC) inhibitor MHY219 induces apoptosis via up-regulation of androgen receptor expression in human prostate cancer cells. Biomed. Pharmacother. 2013, 67, 407–415. [Google Scholar] [CrossRef]
- Knutson, A.K.; Welsh, J.; Taylor, T.; Roy, S.; Wang, W.-L.W.; Tenniswood, M. Comparative effects of histone deacetylase inhibitors on p53 target gene expression, cell cycle and apoptosis in MCF-7 breast cancer cells. Oncol. Rep. 2012, 27, 849–853. [Google Scholar] [CrossRef]
- Kong, Y.; Jung, M.; Wang, K.; Grindrod, S.; Velena, A.; Lee, S.A.; Dakshanamurthy, S.; Yang, Y.; Miessau, M.; Zheng, C.; et al. Histone Deacetylase Cytoplasmic Trapping by a Novel Fluorescent HDAC Inhibitor. Mol. Cancer Ther. 2011, 10, 1591–1599. [Google Scholar] [CrossRef]
- Longworth, M.S.; Laimins, L.A. Histone deacetylase 3 localizes to the plasma membrane and is a substrate of Src. Oncogene 2006, 25, 4495–4500. [Google Scholar] [CrossRef]
- Gui, C.-Y.; Ngo, L.; Xu, W.S.; Richon, V.M.; Marks, P.A. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc. Natl. Acad. Sci. USA 2004, 101, 1241–1246. [Google Scholar] [CrossRef]
- Greer, C.B.; Tanaka, Y.; Kim, Y.J.; Xie, P.; Zhang, M.Q.; Park, I.-H.; Kim, T.H. Histone Deacetylases Positively Regulate Transcription through the Elongation Machinery. Cell Rep. 2015, 13, 1444–1455. [Google Scholar] [CrossRef]
- De, U.; Kundu, S.; Patra, N.; Ahn, M.Y.; Ahn, J.H.; Son, J.Y.; Yoon, J.H.; Moon, H.R.; Lee, B.M.; Kim, H.S. A New Histone Deacetylase Inhibitor, MHY219, Inhibits the Migration of Human Prostate Cancer Cells via HDAC1. Biomol. Ther. (Seoul) 2015, 23, 434–441. [Google Scholar] [CrossRef]
- Park, S.Y.; Jun, J.A.; Jeong, K.J.; Heo, H.J.; Sohn, J.S.; Lee, H.Y.; Park, C.G.; Kang, J. Histone deacetylases 1, 6 and 8 are critical for invasion in breast cancer. Oncol. Rep. 2011, 25, 1677–1681. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Herrera-Martínez, M.; Orozco-Samperio, E.; Montaño, S.; Ariza-Ortega, J.A.; Flores-García, Y.; López-Contreras, L. Vorinostat as potential antiparasitic drug. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7412–7419. [Google Scholar] [CrossRef]
- Iwamoto, M.; Friedman, E.J.; Sandhu, P.; Agrawal, N.G.B.; Rubin, E.H.; Wagner, J.A. Clinical pharmacology profile of vorinostat, a histone deacetylase inhibitor. Cancer Chemother. Pharmacol. 2013, 72, 493–508. [Google Scholar] [CrossRef]
- Mravljak, J.; Ojsteršek, T.; Pajk, S.; Sollner Dolenc, M. Coumarin-based dual fluorescent spin-probes. Tetrahedron Lett. 2013, 54, 5236–5238. [Google Scholar] [CrossRef]
- Shao, Y.; Molnar, L.F.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.T.; Gilbert, A.T.B.; Slipchenko, L.V.; Levchenko, S.V.; O’Neill, D.P.; et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar] [CrossRef]
- Wavefunction, Inc. Available online: https://www.wavefun.com (accessed on 30 October 2020).
- Rocha, G.B.; Freire, R.O.; Simas, A.M.; Stewart, J.J.P. RM1: A reparameterization of AM1 for H, C, N, O, P, S, F, Cl, Br, and I. J. Comput. Chem. 2006, 27, 1101–1111. [Google Scholar] [CrossRef]
- Watson, P.J.; Millard, C.J.; Riley, A.M.; Robertson, N.S.; Wright, L.C.; Godage, H.Y.; Cowley, S.M.; Jamieson, A.G.; Potter, B.V.L.; Schwabe, J.W.R. Insights into the activation mechanism of class I HDAC complexes by inositol phosphates. Nat. Commun. 2016, 7, 11262. [Google Scholar] [CrossRef]
- Hai, Y.; Christianson, D.W. Histone deacetylase 6 structure and molecular basis of catalysis and inhibition. Nat. Chem. Biol. 2016, 12, 741–747. [Google Scholar] [CrossRef]
- Somoza, J.R.; Skene, R.J.; Katz, B.A.; Mol, C.; Ho, J.D.; Jennings, A.J.; Luong, C.; Arvai, A.; Buggy, J.J.; Chi, E.; et al. Structural Snapshots of Human HDAC8 Provide Insights into the Class I Histone Deacetylases. Structure 2016, 12, 1325–1334. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: A New Technique for High-Accuracy Molecular Docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Keepers, Y.P.; Pizao, P.E.; Peters, G.J.; van Ark-Otte, J.; Winograd, B.; Pinedo, H.M. Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur. J. Cancer Clin. Oncol. 1991, 27, 897–900. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
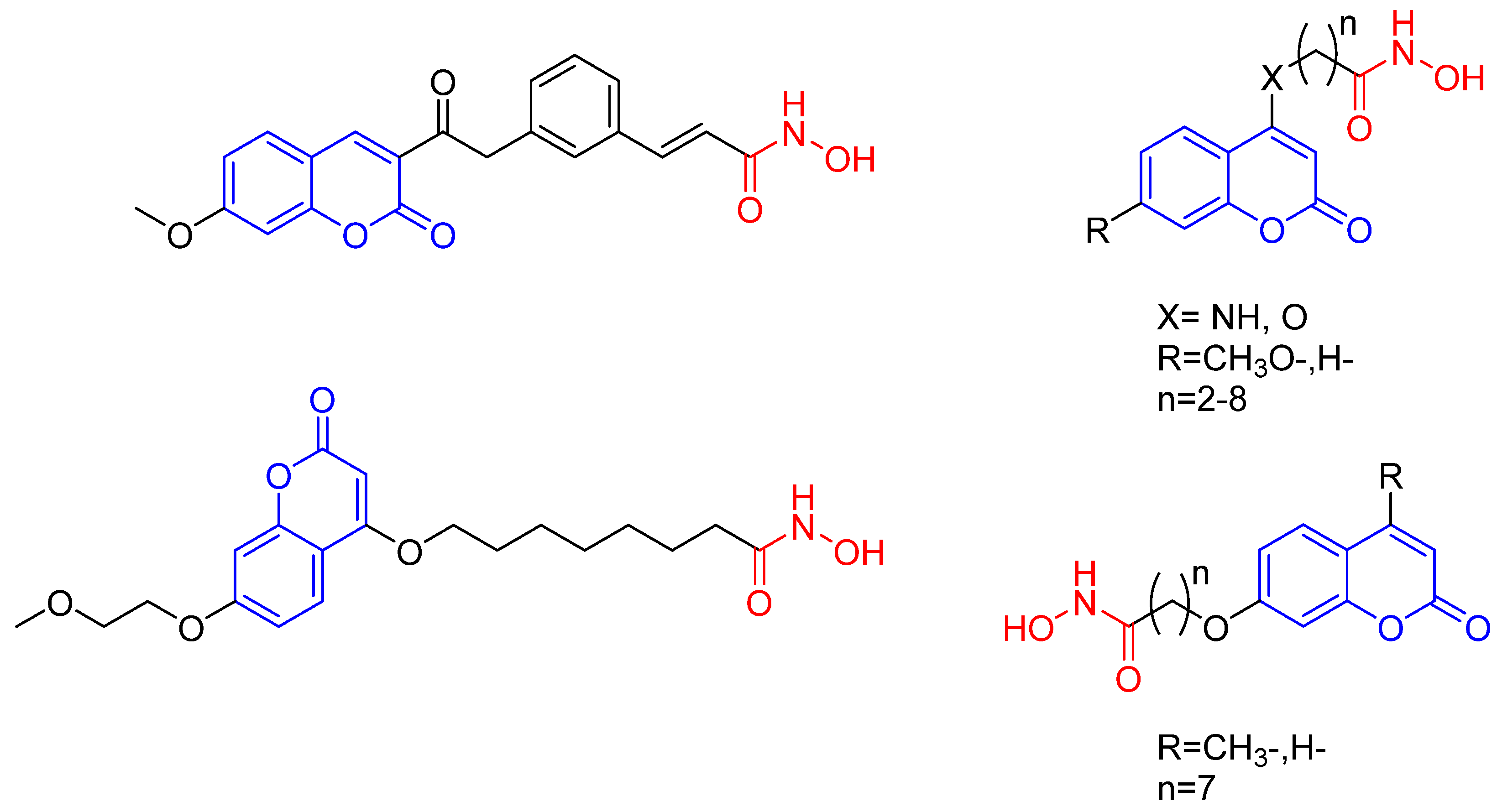
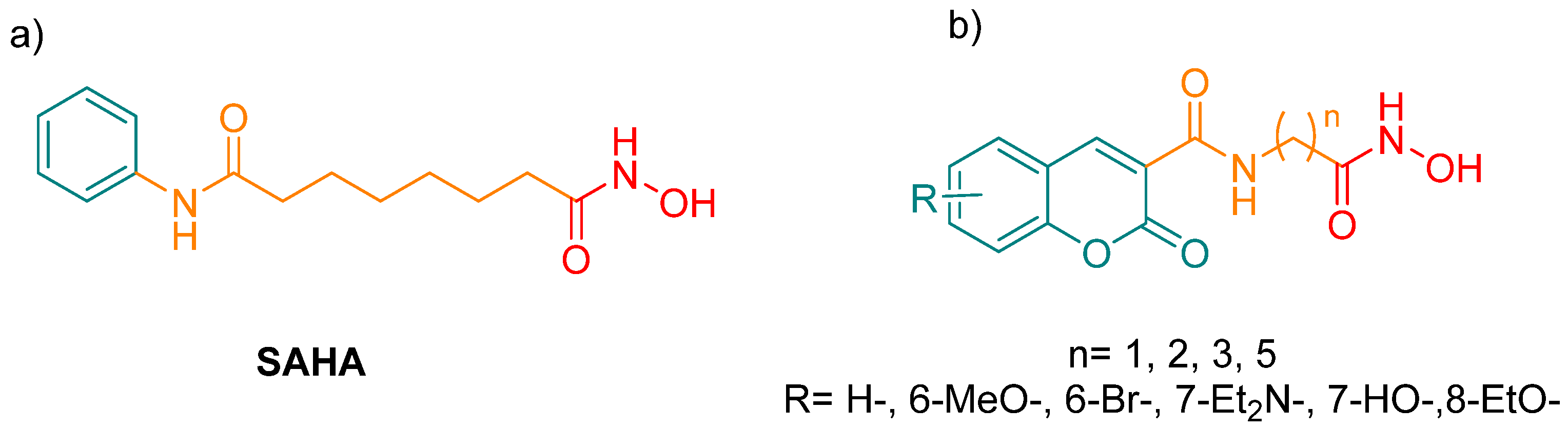
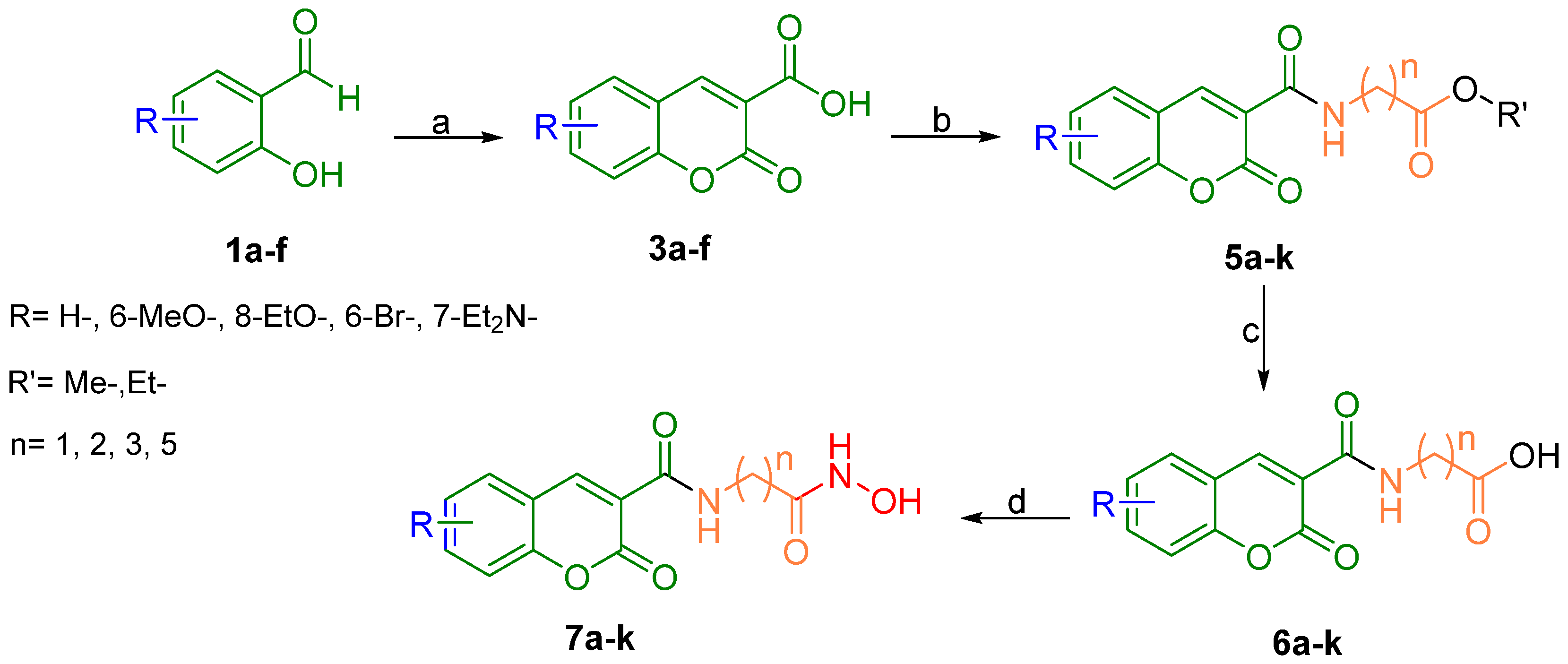
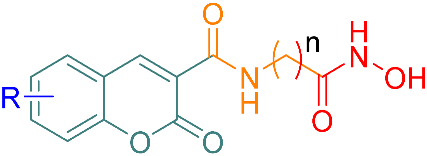
| Product | R | n | Yield (%) | λab (nm) | λem (nm) |
|---|---|---|---|---|---|
| 7a | H- | 1 | 50 | 297 | 406 |
| 7b | H- | 2 | 45 | 303 | 405 |
| 7c | H- | 5 | 50 | 329 | 405 |
| 7d | 6-MeO- | 3 | 30 | 363 | 458 |
| 7e | 6-MeO- | 5 | 87 | 365 | 458 |
| 7f | 8-EtO- | 5 | 48 | 311 | 486 |
| 7g | 6-Br- | 1 | 46 | 294 | 424 |
| 7h | 6-Br- | 2 | 45 | 295 | 415 |
| 7i | 6-Br- | 5 | 65 | 283 | 424 |
| 7j | 7-Et2N- | 2 | 82 | 419 | 470 |
| 7k | 7-Et2N- | 5 | 82 | 419 | 470 |
| Compound | BT-474 | MDA-MB-231 | PC3 |
|---|---|---|---|
| SAHA | 98.22 ± 1.82 * | 97.54 ± 1.59 * | 87.86 ± 7.85 * |
| 7a | 9.90 ± 7.79 | 17.39 ± 15.15 | 11.31 ± 7.65 |
| 7b | 40.06 ± 18.94 * | 47.81 ± 11.06 * | 22.89 ± 19.30 |
| 7c | 95.98 ± 2.61 * | 91.61 ± 2.49 * | 68.82 ± 22.14 * |
| 7d | 33.24 ± 15.25 | 16.57 ± 8.78 | 8.97 ± 16.21 |
| 7e | 82.08 ± 25.72 * | 92.28 ± 5.43 * | 71.02 ± 11.55 * |
| 7f | 91.88 ± 12.54 * | 100.08 ± 0.29 * | 88.62 ± 14.78 * |
| 7g | 6.73 ± 7.40 | 14.00 ± 7.13 | 5.10 ± 8.93 |
| 7h | 61.83 ± 11.82 * | 64.30 ± 17.82 * | 32.64 ± 14.31 |
| 7i | 93.47 ± 4.29 * | 93.64 ± 3.27 * | 75.85 ± 16.43 * |
| 7j | 70.84 ± 18.62 * | 78.50 ± 5.79 * | 53.02 ± 13.35 * |
| 7k | 7.05 ± 13.26 | 0.00 ± 3.9 | 7.69 ± 21.95 |
| BT-474 | |||
| Compound | p21 | p53 | CD1 |
| SAHA | 17.39 ± 3.70 * | 0.12 ± 0.08 * | 0.02 ± 0.00 * |
| 7c | 3.81 ± 1.08 * | 0.57 ± 0.27 * | 0.51 ± 0.09 * |
| 7d | 1.47 ± 0.41 | 0.47 ± 0.08 * | 0.92 ± 0.06 |
| 7e | 5.47 ± 2.01 * | 0.08 ± 0.00 * | 0.57 ± 0.07 * |
| 7f | 23.65 ± 6.69 * | 0.51 ± 0.22 * | 0.40 ± 0.36 |
| 7i | 0.44 ± 0.02 * | 0.74 ± 0.21 | 0.91 ± 0.05 |
| 7j | 26.56 ± 0.44 * | 0.57 ± 0.24 * | 0.32 ± 0.09 * |
| PC3 | |||
| Compound | p21 | p53 | CD1 |
| SAHA | 7.44 ± 2.24 * | 0.12 ± 0.14 * | 0.04 ± 0.01 * |
| 7c | 3.48 ± 0.55 * | 0.25 ± 0.17 * | 0.61 ± 0.13 |
| 7d | 1.41 ± 0.27 | 0.49 ± 0.23 * | 0.64 ± 0.19 |
| 7e | 3.90 ± 0.43 * | 0.18 ± 0.11 * | 0.30 ± 0.02 * |
| 7f | 3.60 ± 0.78 * | 0.16 ± 0.20 * | 0.32 ± 0.22 * |
| 7i | 2.07 ± 0.66 | 0.42 ± 0.39 | 0.90 ± 0.30 |
| 7j | 3.80 ± 2.33 | 0.16 ± 0.06 * | 0.43 ± 0.27 * |
| Ligand | HDAC1 (kcal/mol) | HDAC6 (kcal/mol) | HDAC8 (kcal/mol) |
|---|---|---|---|
| SAHA | −86.16 | −55.33 | −20.42 |
| 7c | −89.78 | −73.66 | −25.20 |
| 7e | −118.77 | −68.80 | −31.56 |
| 7f | −111.23 | −79.28 | −30.17 |
| 7i | −108.99 | −68.82 | −29.20 |
| 7j | −113.59 | −80.73 | −48.89 |
| Compound | PSA (Å2) | Log P | Solubility (mg/mL) | Solubility Class |
|---|---|---|---|---|
| SAHA | 108.64 | 1.92 | 0.509 | Soluble |
| 7c | 91.57 | 3.41 | 0.0665 | Soluble |
| 7e | 117.87 | 1.75 | 0.636 | Soluble |
| 7f | 117.87 | 1.75 | 0.636 | Soluble |
| 7i | 107.89 | 1.77 | 0.38 | Soluble |
| 7j | 98.74 | 1.52 | 1.04 | Soluble |
| Compound | HIA | BBB | Pgp Substrate | CYP1A2 Inhibitor |
|---|---|---|---|---|
| SAHA | High | No | Yes | No |
| 7c | High | No | No | Yes |
| 7e | High | No | Yes | No |
| 7f | High | No | Yes | No |
| 7i | High | No | Yes | No |
| 7j | High | No | Yes | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, S.; Mercado-Sánchez, I.; Bahena, L.; Alcaraz, Y.; García-Revilla, M.A.; Robles, J.; Santos-Martínez, N.; Ordaz-Rosado, D.; García-Becerra, R.; Vazquez, M.A. Design of Fluorescent Coumarin-Hydroxamic Acid Derivatives as Inhibitors of HDACs: Synthesis, Anti-Proliferative Evaluation and Docking Studies. Molecules 2020, 25, 5134. https://doi.org/10.3390/molecules25215134
García S, Mercado-Sánchez I, Bahena L, Alcaraz Y, García-Revilla MA, Robles J, Santos-Martínez N, Ordaz-Rosado D, García-Becerra R, Vazquez MA. Design of Fluorescent Coumarin-Hydroxamic Acid Derivatives as Inhibitors of HDACs: Synthesis, Anti-Proliferative Evaluation and Docking Studies. Molecules. 2020; 25(21):5134. https://doi.org/10.3390/molecules25215134
Chicago/Turabian StyleGarcía, Santiago, Itzel Mercado-Sánchez, Luis Bahena, Yolanda Alcaraz, Marco A. García-Revilla, Juvencio Robles, Nancy Santos-Martínez, David Ordaz-Rosado, Rocío García-Becerra, and Miguel A. Vazquez. 2020. "Design of Fluorescent Coumarin-Hydroxamic Acid Derivatives as Inhibitors of HDACs: Synthesis, Anti-Proliferative Evaluation and Docking Studies" Molecules 25, no. 21: 5134. https://doi.org/10.3390/molecules25215134
APA StyleGarcía, S., Mercado-Sánchez, I., Bahena, L., Alcaraz, Y., García-Revilla, M. A., Robles, J., Santos-Martínez, N., Ordaz-Rosado, D., García-Becerra, R., & Vazquez, M. A. (2020). Design of Fluorescent Coumarin-Hydroxamic Acid Derivatives as Inhibitors of HDACs: Synthesis, Anti-Proliferative Evaluation and Docking Studies. Molecules, 25(21), 5134. https://doi.org/10.3390/molecules25215134








