Antimicrobial Activity of Calixarenes and Related Macrocycles
Abstract
:1. Introduction
2. Molecular Prodrugs and Drug Delivery Agents
2.1. Incorporation of Antibiotic Motifs
2.2. Incorporation of Oxazole, Thiadiazole, and Bithiazole Motifs
2.3. Functionalized Nanoparticles
2.4. Drug-Delivering Calixarenes
2.5. Metal-Binding Calixarenes
2.6. Sulfonamide-Containing Calixarenes
2.7. Antibiotic Pillar[5]arenes
3. Cell Destruction
3.1. Macrocyclon
3.2. Charged Calixarenes
3.3. Vancomycin Mimicking Calixarenes
3.4. Other Calixarenes
3.5. Pore-Forming Pillar[5]arenes
4. Biofilm Inhibition
4.1. Calixsugars
4.2. Biofilm Inhibiting Resorcinarenes
4.3. Biofilm Disruption through Drug Delivery
4.4. Biofilm-Inhibiting Pillar[n]arenes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Neri, P.; Sessler, J.L.; Wang, M.-X. (Eds.) Calixarenes and Beyond; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Perret, F.; Lazar, A.N.; Coleman, A.W. Biochemistry of the para-sulfonato-calix[n]arenes. Chem. Commun. 2006, 2425–2438. [Google Scholar] [CrossRef] [PubMed]
- Perret, F.; Coleman, A.W. Biochemistry of anionic calix[n]arenes. Chem. Commun. 2011, 47, 7303–7319. [Google Scholar] [CrossRef] [PubMed]
- Cragg, P.J. Pillar[n]arenes at the chemistry-biology interface. Isr. J. Chem. 2018, 58, 1158–1172. [Google Scholar] [CrossRef]
- Cornforth, J.W.; D’Arcy Hart, P.; Nicholls, G.A.; Rees, R.J.W.; Stock, J.A. Antituberculous effects of certain surface-active polyoxyethylene ethers. Br. J. Pharmacol. 1955, 10, 73–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamartine, R.; Tsukada, M.; Wilson, D.; Shirata, A. Antimicrobial activity of calixarenes. Comptes Rendus Chim. 2002, 5, 163–169. [Google Scholar] [CrossRef]
- Naseer, M.M.; Ahmed, M.; Hameed, S. Functionalized calix[4]arenes as potential therapeutic agents. Chem. Biol. Drug Des. 2017, 89, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Rodik, R.; Poberezhnyk, M.; Kalchenko, V. Calixarene derivatives for (nano)biotechnologies. Macroheterocycles 2017, 10, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Rodik, R.V. Aнтимiкpoбнa тa Пpoтивipycнa Aктивнicть Kaлiкcapeнiв. J. Org. Pharmaceut. Chem. 2015, 13, 67–78. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeny, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lalor, R.; Baillie-Johnson, H.; Redshaw, C.; Matthews, S.E.; Mueller, A. Cellular uptake of a fluorescent calix[4]arene derivative. J. Am. Chem. Soc. 2008, 130, 2892–2893. [Google Scholar] [CrossRef] [PubMed]
- Coleman, A.W.; Jebors, S.; Cecillon, C.; Perret, P.; Garin, D.; Marti-Battle, D.; Moulin, M. Toxicity and biodistribution of para-sulfonato-calix[4]arene in mice. New J. Chem. 2008, 32, 780–782. [Google Scholar] [CrossRef]
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef]
- Ben Salem, A.; Regnouf-de-Vains, J.-B. Synthesis and characterisation of a new podand based on a calixarene and a β-lactam. Tetrahedron Lett. 2001, 42, 7033–7036. [Google Scholar] [CrossRef]
- Korchowiec, B.; Ben Salem, A.; Corvis, Y.; Regnouf-de-Vains, J.-B.; Korchowiec, J.; Rogalska, E. Calixarenes in a membrane environment: A monolayer study on the miscibility of three p-tert-butylcalix[4]arene b-lactam derivatives with 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine. J. Phys. Chem. B 2007, 111, 13231–13242. [Google Scholar] [CrossRef]
- Ben Salem, A.; Sautrey, G.; Fontanay, S.; Duval, R.E.; Regnouf-de-Vains, J.-B. Molecular drug-organiser: Synthesis, characterization and biological evaluation of penicillin V and/or nalidixic acid calixarene-based podands. Bioorg. Med. Chem. 2011, 19, 7534–7540. [Google Scholar] [CrossRef]
- Ben Salem, A.; Regnouf-de-Vains, J.-B. Towards a new family of calixarene-based podands incorporating quinolone arms. An example using nalidixic acid. Tetrahedron Lett. 2003, 44, 6769–6771. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Nam, K.C. Calixarenes. 22. Synthesis, properties, and metal complexation of aminocalixarenes. J. Am. Chem. Soc. 1988, 110, 6153–6162. [Google Scholar] [CrossRef]
- Baker, T.J.; Tomioka, M.; Goodman, M.; Mergott, D.G.; Roush, W.R. Preparation and use of N,N′-di-BOC-N″-triflylguanidine. Org. Synth. 2000, 78, 91. [Google Scholar]
- Mourer, M.; Duval, R.E.; Finance, C.; Regnouf-de-Vains, J.-B. Functional organisation and gain of activity: The case of the antibacterial tetra-para-guanidinoethyl-calix[4]arene. Bioorg. Med. Chem. Lett. 2006, 16, 2960–2963. [Google Scholar] [CrossRef]
- Grare, M.; Mourer, M.; Fontanay, S.; Regnouf-de-Vains, J.-B.; Finance, C.; Duval, R.E. In vitro activity of para-guanidinoethylcalix[4]arene against susceptible and antibiotic-resistant Gram-negative and Gram-positive bacteria. J. Antimicrob. Chemother. 2007, 60, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Grare, M.; Massimba Dibama, H.; Lafosse, S.; Ribon, A.; Mourer, M.; Regnouf-de-Vains, J.-B.; Duval, R.E. Cationic compounds with activity against multidrug-resistant bacteria: Interest of a new compound compared with two older antiseptics, hexamidine and chlorhexidine. Clin. Microbiol. Infect. 2010, 16, 432–438. [Google Scholar] [CrossRef]
- Mourer, M.; Duval, R.E.; Constant, P.; Daffé, M.; Regnouf-de-Vains, J.-B. Impact of tetracationic calix[4]arene conformation-from conic structure to expanded bolaform-on their antibacterial and antimycobacterial activities. ChemBioChem 2019, 20, 911–921. [Google Scholar] [CrossRef]
- Massimba Dibama, H.; Clarot, I.; Fontanay, S.; Ben Salem, A.; Mourer, M.; Finance, C.; Duval, R.E.; Regnouf-de-Vains, J.-B. Towards calixarene-based prodrugs: Drug release and antibacterial behaviour of a water-soluble nalidixic acid/calix[4]arene ester adduct. Bioorg. Med. Chem. Lett. 2009, 19, 2679–2682. [Google Scholar] [CrossRef]
- Grare, M.; Mourer, M.; Regnouf-de-Vains, J.-B.; Finance, C.; Duval, R.E. Vers de nouvelles molécules antibactériennes. Intérêt du para-guanidinoéthylcalix[4]arène. Pathol. Biol. 2006, 54, 470–476. [Google Scholar] [CrossRef]
- Pur, F.N.; Dilmaghani, K.A. Calixpenams: Synthesis, characterization, and biological evaluation of penicillins V and X clustered by calixarene scaffold. Turk. J. Chem. 2014, 38, 288–296. [Google Scholar]
- Pur, F.N.; Dilmaghani, K.A. Calixcephems: Clustered cephalosporins analogous to calixpenams as novel potential anti-MRSA agents. Turk. J. Chem. 2014, 38, 850–858. [Google Scholar]
- Patel, M.B.; Modi, N.R.; Raval, J.P.; Menon, S.K. Calix[4]arene based 1,3,4-oxadiazole and thiadiazole derivatives: Design, synthesis, and biological evaluation. Org. Biomol. Chem. 2012, 10, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Gezelbash, Z.D.; Dilmaghani, K.A. Synthesis, antifungal and antibacterial activity of calix[4]arene-based 1,3,4-oxadiazole derivatives. J. Chin. Chem. Soc. 2020, 67, 1446–1452. [Google Scholar] [CrossRef]
- Mourer, M.; Psychogios, N.; Laumond, G.; Aubertin, A.-M.; Regnouf-de-Vains, J.-B. Synthesis and anti-HIV evaluation of water-soluble calixarene-based bithiazolyl podands. Bioorg. Med. Chem. 2010, 18, 36–45. [Google Scholar] [CrossRef]
- Perret, F.; Tauran, Y.; Suwinska, K.; Kim, B.; Chassain-Nely, C.; Boulet, M.; Coleman, A.W. Molecular recognition and transport of active pharmaceutical ingredients on anionic calix[4]arene-capped silver nanoparticles. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Boudebbouze, S.; Coleman, A.W.; Tauran, Y.; Mkaouar, H.; Perret, F.; Garnier, A.; Brioude, A.; Kim, B.; EMaguina, E.; Rhimi, M. Discriminatory antibacterial effects of calix[n]arene capped silver nanoparticles with regard to Gram positive and Gram negative bacteria. Chem. Commun. 2013, 49, 7150–7152. [Google Scholar] [CrossRef] [PubMed]
- Moussa, Y.E.; Ong, Y.Q.E.; Perry, J.D.; Cheng, Z.; Kayser, V.; Cruz, E.; Kim, R.R.; Sciortino, N.; Wheate, N.J. Demonstration of in vitro host-guest complex formation and safety of para-sulfonatocalix[8]arene as a delivery vehicle for two antibiotic drugs. J. Pharmaceut. Sci. 2018, 107, 3105–3111. [Google Scholar] [CrossRef] [PubMed]
- Consoli, G.M.L.; Granata, G.; Picciotto, R.; Blanco, A.R.; Geraci, C.; Marino, A.; Nostro, A. Design, synthesis and antibacterial evaluation of a polycationic calix[4]arene derivative alone and in combination with antibiotics. Med. Chem. Commun. 2018, 9, 160–164. [Google Scholar] [CrossRef]
- Memon, S.; Chandio, A.A.; Memon, A.A.; Nizamani, S.M.; Bhatti, A.A.; Brohi, N.A. Synthesis, characterization, and exploration of antimicrobial activity of copper complex of diamide derivative of p-tert-butylcalix[4]arene. Polycycl. Aromat. Compd. 2017, 37, 362–374. [Google Scholar] [CrossRef]
- Chandio, A.A.; Memon, A.A.; Memon, S.; Memon, F.N.; Panhwar, Q.K.; Durmaz, F.; Nizamani, S.M.; Brohi, N.A. Synthesis and antimicrobial assessment of Fe3+ inclusion complex of p-tert-butylcalix[4]arene diamide derivative. J. Chem. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Özkan, Ş.Ç.; Yilmaz, A.; Arslan, E.; Açık, L.; Sayın, Ü.; Mutlu, E.G. Novel copper (II) complexes of p-tert-butylcalix [4] arene diamide derivatives: Synthesis, antimicrobial and DNA cleavage activities. Supramol. Chem. 2015, 27, 255–267. [Google Scholar] [CrossRef]
- Noruzi, E.B.; Kheirkhahi, M.; Shaabani, B.; Geremia, S.; Hickey, N.; Asaro, F.; Nitti, P.; Kafil, H.S. Design of a thiosemicarbazide functionalized calix [4] arene ligand and related transition metal complexes: Synthesis, characterization and biological studies. Front. Chem. 2019, 7, 663. [Google Scholar] [CrossRef] [Green Version]
- Noruzi, E.B.; Shaabani, B.; Geremia, S.; Hickey, N.; Nitti, P.; Kafil, H.S. Synthesis, crystal structure, and biological activity of a multidentate calix[4]arene ligand doubly functionalized by 2-hydroxybenzeledene-thiosemicarbazone. Molecules 2020, 25, 370. [Google Scholar] [CrossRef] [Green Version]
- Roy, H.; Deolalkar, M.; Desai, A.S. Synthesis of calix-salen silver corates for evaluation of their antimicrobial and anticancer activities. ACS Omega 2019, 4, 21346–21352. [Google Scholar] [CrossRef] [Green Version]
- Ali, Y.; Bunnori, N.M.; Susanti, D.; Alhassan, A.M.; Hamid, S.A. Synthesis, in vitro and in silico studies of azo-based calix [4] arenes as antibacterial agent and neuraminidase inhibitor: A new look into an old scaffold. Front. Chem. 2018, 6, 210. [Google Scholar] [CrossRef] [Green Version]
- Barbera, L.; Franco, D.; De Plano, L.M.; Gattuso, G.; Guglielmino, S.P.P.; Lentini, G.; Manganaro, N.; Marino, N.; Pappalardo, S.; Parisi, M.F.; et al. A water-soluble pillar[5]arene as a new carrier for an old drug. Org. Biomol. Chem. 2017, 15, 3192–3195. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Abate, B.J.; Kang, S.L.; Ruffing, M.J.; Lerner, S.A.; Drusano, G.L. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob. Agents Chemother. 1999, 43, 1549–1555. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Xie, B.; Yang, X.; Dai, J.; Wei, G.; He, Y. Pillar[5]arene-based, dual pH and enzyme responsive supramolecular vesicles for targeted antibiotic delivery against intracellular MRSA. Chem. Commun. 2020, 56, 8115–8118. [Google Scholar] [CrossRef] [PubMed]
- Cornforth, J.W.; D’Arcy Hart, P.; Rees, R.J.W.; Stock, J.A. Antituberculous effect of certain surface-active polyoxyethylene ethers in mice. Nature 1951, 168, 150–153. [Google Scholar] [CrossRef]
- Zinke, A.; Zigeuner, G.; Hössinger, K.; Hoffmann, G. Zur Kenntnis des Härtungsprozesses von Phenol-Formaldehyd-Harzen. XVIII., vorläufige Mitteilung: Über cyclische Mehrkernphenole. Monatsh. Chem. 1948, 79, 438–439. [Google Scholar] [CrossRef]
- D’Arcy Hart, P.; Armstrong, J.A.; Brodaty, E. Calixarenes with host-mediated potency in experimental tuberculosis: Further evidence that macrophage lipids are involved in their mechanism of action. Infect. Immun. 1996, 64, 1491–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colston, M.J.; Hailes, H.C.; Stavropoulos, E.; Hervé, A.C.; Hervé, G.; Goodworth, K.J.; Hill, A.M.; Jenner, P.; D’Arcy Hart, P.; Tascon, R.E. Antimycobacterial calixarenes enhance innate defense mechanisms in murine macrophages and induce control of Mycobacterium tuberculosis infection in mice. Infect. Immun. 2004, 72, 6318–6323. [Google Scholar] [CrossRef] [Green Version]
- Goodworth, K.J.; Hervé, A.C.; Stavropoulos, E.; Hervé, G.; Casades, I.; Hill, A.M.; Weingarten, G.G.; Tascon, R.E.; Colston, M.J.; Hailes, H.C. Synthesis and in vivo biological activity of large-ringed calixarenes against Mycobacterium tuberculosis. Tetrahedron 2011, 67, 373–382. [Google Scholar] [CrossRef]
- Mourer, M.; Massimba Dibama, H.; Constant, P.; Daffé, M.; Regnouf-de-Vains, J.-B. Anti-mycobacterial activities of some cationic and anionic calix[4]arene derivatives. Bioorg. Med. Chem. 2012, 20, 2035–2041. [Google Scholar] [CrossRef]
- Mourer, M.; Massimba Dibama, H.; Fontanay, S.; Grare, M.; Duval, R.E.; Finance, C.; Regnouf-de-Vains, J.-B. p-Guanidinoethyl calixarene and parent phenol derivatives exhibiting antibacterial activities. Synthesis and biological evaluation. Bioorg. Med. Chem. 2009, 17, 5496–5509. [Google Scholar] [CrossRef]
- Mourer, M.; Fontanay, S.; Duval, R.E.; Regnouf-de-Vains, J.-B. Synthesis, characterization, and biological evaluation as antibacterial agents of water-soluble calix[4]arenes and phenol derivatives incorporating carboxylate groups. Helv. Chim. Acta 2012, 95, 1373–1386. [Google Scholar] [CrossRef]
- Yushchenko, T.I.; Germanyuk, T.A.; Chornoknyzhny, S.I.; Zaichko, N.V.; Korol, A.P.; Prokopchuk, Z.M.; Rodik, R.V.; Cheshun, E.A. Antibacterial and antiplatelet activity of calix[4,6]arene tetraalkylamines. Pharmacol. Drug Toxicol. 2012, 5, 79–88. [Google Scholar]
- Ukhatskaya, E.V.; Kurkov, S.V.; Hjálmarsdóttir, M.A.; Karginov, V.A.; Matthews, S.E.; Rodik, R.V.; Kalchenko, V.I.; Loftsson, T. Cationic quaternized aminocalix[4]arenes: Cytotoxicity, haemolytic and antibacterial activities. Int. J. Pharm. 2013, 458, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Melezhyk, I.O.; Rodik, R.V.; Iavorska, N.V.; Klymchenko, A.S.; Mely, Y.; Shepelevych, V.V.; Skivka, L.M.; Kalchenko, V.I. Antibacterial properties of tetraalkylammonium and imidazolium tetraalkoxycalix[4]arene derivatives. Anti-Infect. Agents 2015, 13, 87–94. [Google Scholar] [CrossRef]
- Di Bari, A.; Picciotto, R.; Granata, G.; Blanco, A.R.; Consoli, G.M.L.; Sortino, S. A bactericidal calix[4]arene-based nanoconstruct with amplified NO photorelease. Org. Biomol. Chem. 2016, 14, 8047–8052. [Google Scholar] [CrossRef] [PubMed]
- Consoli, G.M.L.; Di Bari, A.; Blanco, A.R.; Nostro, A.; D’Arrigo, M.; Pistarà, V.; Sortino, S. Design, synthesis, and antibacterial activity of a multivalent polycationic calix[4]arene-NO photodonor conjugate. ACS Med. Chem. Lett. 2017, 8, 881–885. [Google Scholar] [CrossRef]
- da Silva, C.M.; da Silva, D.L.; Magalhães, T.F.F.; Alves, R.B.; de Resende-Stoianoff, M.A.; Martins, F.T.; de Fátima, A. Iminecalix[4]arenes: Microwave-assisted synthesis, X-ray crystal structures, and anticandidal activity. Arab. J. Chem. 2019, 12, 4365–4376. [Google Scholar] [CrossRef] [Green Version]
- Casnati, A.; Fabbi, M.; Pelizzi, N.; Pochini, A.; Sansone, F.; Ungaro, R. Synthesis, antimicrobial activity and binding properties of calix[4]arene based vancomycin mimics. Bioorg. Med. Chem. Lett. 1996, 6, 2699–2704. [Google Scholar] [CrossRef]
- Mehta, V.; Athar, M.; Jha, P.C.; Panchal, M.; Modi, K.; Jain, V.K. Efficiently functionalized oxacalix[4]arenes: Synthesis, characterization and exploration of their biological profile as novel HDAC inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 1005–1010. [Google Scholar] [CrossRef]
- Soomro, A.M.; Oad, R.J.; Memon, S.; Qureshi, I. Bioactivity assessment of water soluble calix[4]arene derivative. Pak. J. Anal. Environ. Chem. 2012, 13, 36–39. [Google Scholar]
- Muneer, S.; Memon, S.; Panhwar, Q.K.; Bhatti, A.A.; Khokhar, T.S. Synthesis and investigation of antimicrobial properties of pyrrolidine appended calix[4]arene. J. Anal. Sci. Technol. 2017, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Muneer, S.; Memon, S.; Panhwar, Q.K.; Khushik, F.; Khokhar, T.S.; Noor, A.A. Synthesis and antimicrobial activity of p- tetranitrocalix[4]arene derivative. Polycycl. Aromat. Compd. 2016, 36, 554–563. [Google Scholar] [CrossRef]
- Galitskaya, P.; Fomin, V.; Stiokov, I.; Andreyko, E.; Selivanovskaya, S. Antimicrobial activity of nanoparticles from solid phase supramolecular assemblies based on stereoisomers of p-tert-butylthiacalix[4]arene with silver cations. Int. J. Pharm. Technol. 2016, 8, 15048–15053. [Google Scholar]
- Zhang, M.; Zhu, P.-P.; Xin, P.; Si, W.; Li, Z.-T.; Hou, J.-L. Synthetic channel specifically inserts into the lipid bilayer of gram- positive bacteria but not that of mammalian erythrocytes. Angew. Chem. Int. Ed. 2017, 56, 2999–3003. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Sun, Y.; Kong, H.; Wang, Y.; Tan, S.; Guo, J.; Jiang, T.; Dong, W.; Chen, C.-P. A unimolecular channel formed by dual helical peptide modified pillar[5]arene: Correlating transmembrane transport properties with antimicrobial activity and haemolytic toxicity. Chem. Commun. 2017, 53, 11492–11495. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Matthews, S.E. Calixsugars: Finally reaching their potential? In Calixarenes and Beyond; Neri, P., Sessler, J.L., Wang, M.-X., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 559–600. [Google Scholar]
- Baldini, L.; Casnati, A.; Sansone, F.; Ungaro, R. Peptido- and glycocalixarenes. In Calixarenes in the Nanoworld; Vicens, J., Harrowfield, J., Baklouti, L., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 233–257. [Google Scholar]
- Cecioni, S.; Lalor, R.; Blanchard, B.; Praly, J.-P.; Imberty, A.; Matthews, S.E.; Vidal, S. Achieving high affinity towards a bacterial lectin through multivalent topological isomers of calix[4]arene glycoconjugates. Chem. Eur. J. 2009, 15, 13232–13240. [Google Scholar] [CrossRef]
- Sicard, D.; Cecioni, S.; Iazykov, M.; Chevolot, Y.; Matthews, S.E.; Praly, J.-P.; Souteyrand, E.; Imberty, A.; Vidal, S.; Phaner-Goutorbe, M. AFM investigation of Pseudomonas aeruginosa lectin LecA (PA-IL) filaments induced by multivalent glycoclusters. Chem. Commun. 2011, 47, 9483–9485. [Google Scholar] [CrossRef]
- Cecioni, S.; Praly, J.-P.; Matthews, S.E.; Wimmerová, M.; Imberty, A.; Vidal, S. Rational design and synthesis of optimized glycoclusters for multivalent lectin–carbohydrate interactions: Influence of the linker arm. Chem. Eur. J. 2012, 18, 6250–6263. [Google Scholar] [CrossRef] [PubMed]
- Consoli, G.M.L.; Granata, G.; Cafiso, V.; Stefani, S.; Geraci, C. Multivalent calixarene-based C-fucosyl derivative: A new Pseudomonas aeruginosa biofilm inhibitor. Tetrahedron Lett. 2011, 52, 5831–5834. [Google Scholar] [CrossRef]
- Boukerb, A.M.; Rousset, A.; Galanos, N.; Meár, J.-B.; Thépaut, M.; Grandjean, T.; Gillon, E.; Cecioni, S.; Abderrahmen, C.; Faure, K.; et al. Antiadhesive properties of glycoclusters against Pseudomonas aeruginosa lung infection. J. Med. Chem. 2014, 57, 10275–10289. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Consoli, G.M.L.; Cafiso, V.; Stefani, S.; Geraci, C. Synthesis of a calix[4]arene derivative exposing multiple units of fucose and preliminary investigation as a potential broad-spectrum antibiofilm agent. Carb. Res. 2019, 476, 60–64. [Google Scholar] [CrossRef]
- Taouai, M.; Chakroun, K.; Sommer, R.; Michaud, G.; Giacalone, D.; Ben Maaouia, M.A.; Vallin-Butruille, A.; Mathiron, D.; Abidi, R.; Darbre, T.; et al. Glycocluster tetrahydroxamic acids exhibiting unprecedented inhibition of Pseudomonas aeruginosa biofilms. J. Med. Chem. 2019, 62, 7722–7738. [Google Scholar] [CrossRef] [PubMed]
- Guildford, A.; Morris, C.; Kitt, O.; Cooper, I. The effect of urinary Foley catheter substrate material on the antimicrobial potential of calixerene-based molecules. J. Appl. Microbiol. 2017, 124, 1047–1059. [Google Scholar] [CrossRef]
- Barlow, I.J.; Williams, N.H.; Stirling, C.J.M. Medical Devices and Coatings. Patent No. WO 2013/104916A2, 18 July 2013. [Google Scholar]
- Soomro, Z.H.; Cecioni, S.; Blanchard, H.; Praly, J.-P.; Imberty, A.; Vidal, S.; Matthews, S.E. CuAAC synthesis of resorcin[4]arene-based glycoclusters as multivalent ligands of lectins. Org. Biomol. Chem. 2011, 9, 6587–6597. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.R.; Razuvayeva, Y.S.; Ziganshina, A.Y.; Mukhitova, R.K.; Sapunova, A.S.; Voloshina, A.D.; Syakaev, V.V.; Latypov, S.K.; Nizameev, I.R.; Kadirov, M.K.; et al. N-Methyl-d-glucamine-calix[4]resorcinarene conjugates: Self-assembly and biological properties. Molecules 2019, 24, 1939. [Google Scholar] [CrossRef] [Green Version]
- Utomo, S.B.; Fujiyanti, M.; Lestari, W.P.; Mulyani, S. Antibacterial activity test of the C-4-methoxyphenylcalix[4]resorcinarene compound modified by hexadecyltrimethylammonium-bromide against Staphylococcus aureus and Escherichia coli bacteria. JKPK 2018, 3, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Dawn, A.; Chandra, H.; Ade-Browne, C.; Yadav, J.; Kumari, K. Multifaceted supramolecular interactions from C-methylresorcin[4]arene lead to an enhancement in in vitro antibacterial activity of gatifloxacin. Chem. Eur. J. 2017, 23, 18171–18179. [Google Scholar] [CrossRef]
- Abosadiya, H.M.; Hasbullah, S.A.; Mackeen, M.M.; Low, S.C.; Ibrahim, N.; Koketsu, M.; Yamin, B.M. Synthesis, characterization, X-ray structure and biological activities of C-5-bromo-2-hydroxyphenylcalix[4]-2-methyl resorcinarene. Molecules 2013, 18, 13369–13384. [Google Scholar] [CrossRef]
- Vagapova, L.; Nasirova, Z.; Burilova, E.; Zobov, V.; Burilov, A.; Amirov, R.; Pudovik, M. New salt structures based on aminomethylated calix[4]resorcinarenes and (1-hydroxyethane-1,1-diyl) bisphosphonic acid. Russ. J. Org. Chem. 2017, 53, 312–314. [Google Scholar] [CrossRef]
- Ali, I.; Imran, M.; Saifullah, S.; Tian, H.-W.; Guo, D.-S.; Shah, M.R. Amphiphilic p-sulfonatocalix[6]arene based self-assembled nanostructures for enhanced clarithromycin activity against resistant Streptococcus pneumoniae. Colloids Surf. B 2020, 186, 110676. [Google Scholar] [CrossRef] [PubMed]
- Galanos, N.; Gillon, E.; Imberty, A.; Matthews, S.E.; Vidal, S. Pentavalent pillar[5]arene-based glycoclusters and their multivalent binding to pathogenic bacterial lectins. Org. Biomol. Chem. 2016, 14, 3476–3481. [Google Scholar] [CrossRef]
- Buffet, K.; Nierengarten, I.; Galanos, N.; Gillon, E.; Holler, M.; Imberty, A.; Matthews, S.E.; Vidal, S.; Vincent, S.P.; Nierengarten, J.-F. Pillar[5]arene-based glycoclusters: Synthesis and multivalent binding to pathogenic bacterial lectins. Chem. Eur. J. 2016, 22, 2955–2963. [Google Scholar] [CrossRef]
- Vincent, S.P.; Buffet, K.; Nierengarten, I.; Imberty, A.; Nierengarten, J.-F. Biologically active heteroglycoclusters constructed on a pillar[5]arene-containing [2]rotaxane scaffold. Chem. Eur. J. 2016, 22, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Nierengarten, I.; Buffet, K.; Holler, M.; Vincent, S.; Nierengarten, J.-F. A mannosylated pillar[5]arene derivative: Chiral information transfer and antiadhesive properties against uropathogenic bacteria. Tetrahedron Lett. 2013, 54, 2398–2402. [Google Scholar] [CrossRef]
- Tikad, A.; Fu, H.; Sevrain, C.M.; Laurent, S.; Nierengarten, J.-F.; Vincent, S.P. Mechanistic insight into heptosyltransferase inhibition by using Kdo multivalent glycoclusters. Chem. Eur. J. 2016, 22, 13147–13155. [Google Scholar] [CrossRef]
- Yu, G.; Ma, Y.; Han, C.; Yao, Y.; Tang, G.; Mao, Z.; Gao, C.; Huang, F. A sugar-functionalized amphiphilic pillar[5]arene: Synthesis, self- assembly in water, and application in bacterial cell agglutination. J. Am. Chem. Soc. 2013, 135, 10310–10313. [Google Scholar] [CrossRef]
- Joseph, R.; Kaizerman, D.; Herzog, I.M.; Hadar, M.; Feldman, M.; Fridman, M.; Cohen, Y. Phosphonium pillar[5]arenes as a new class of efficient biofilm inhibitors: Importance of charge cooperativity and the pillar platform. Chem. Commun. 2016, 52, 10656–10659. [Google Scholar] [CrossRef]
- Joseph, R.; Naugolny, A.; Feldman, M.; Herzog, I.M.; Fridman, M.; Cohen, Y. Cationic pillararenes potently inhibit biofilm formation without affecting bacterial growth and viability. J. Am. Chem. Soc. 2016, 138, 754–757. [Google Scholar] [CrossRef]
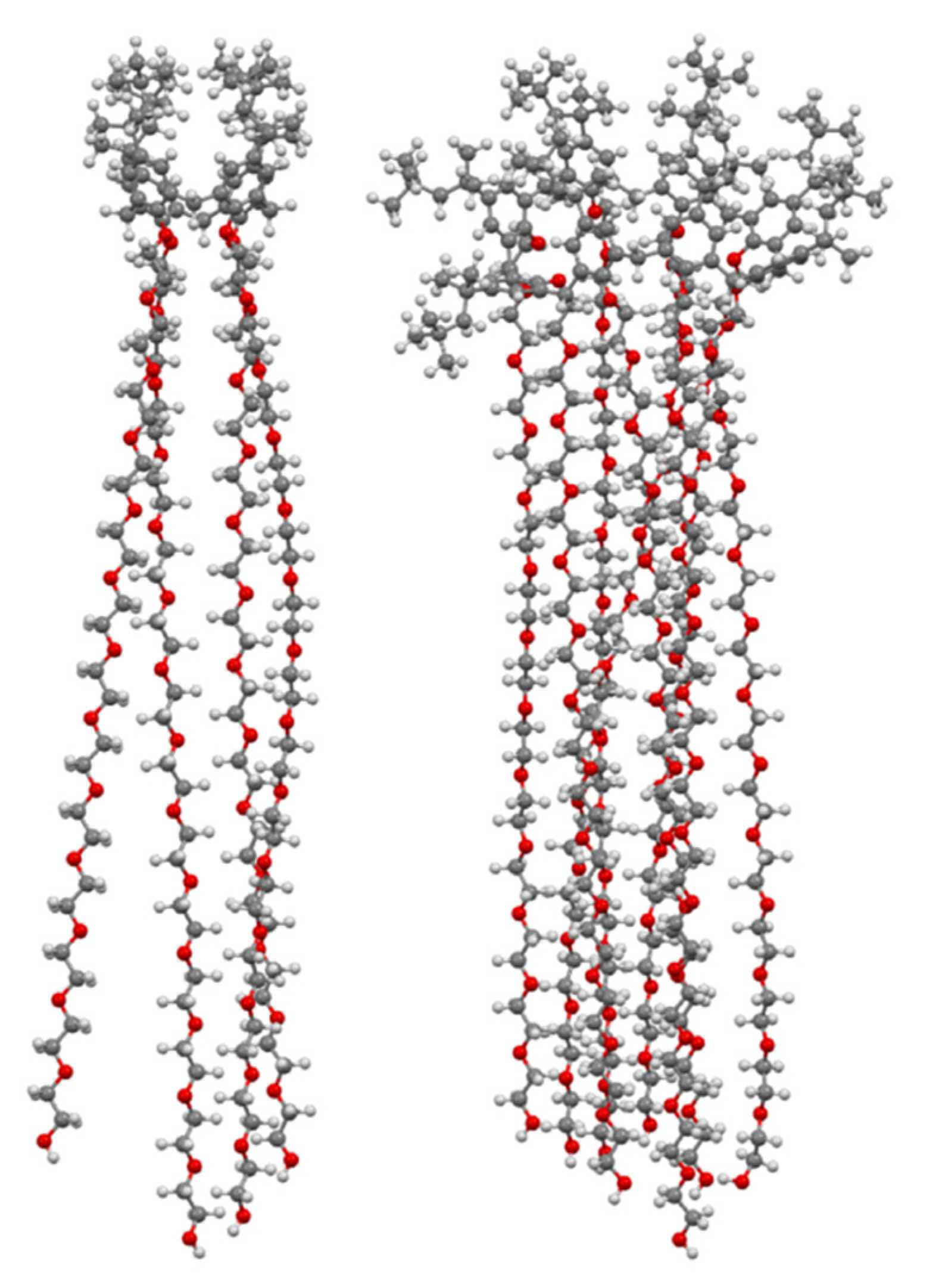




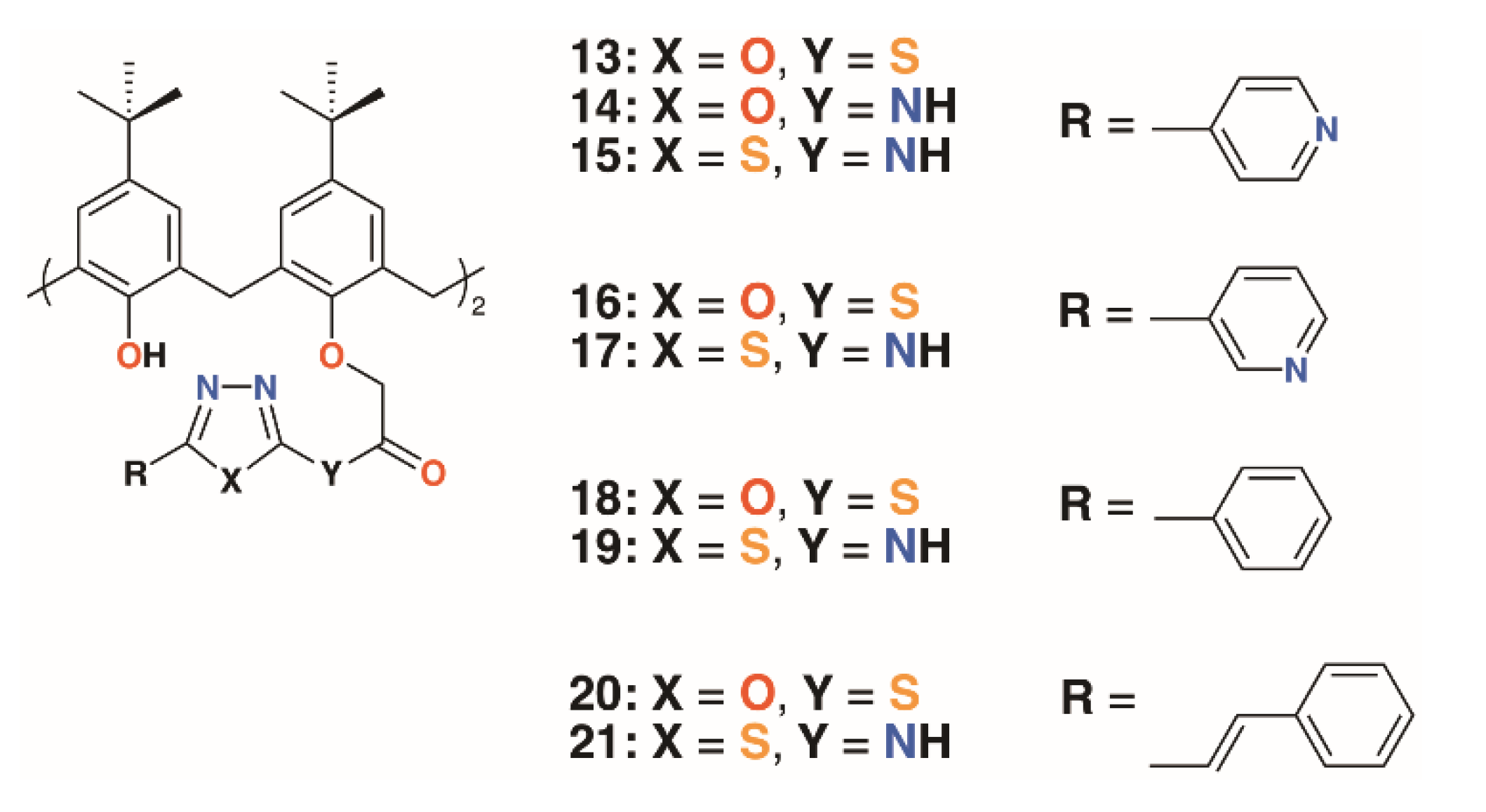















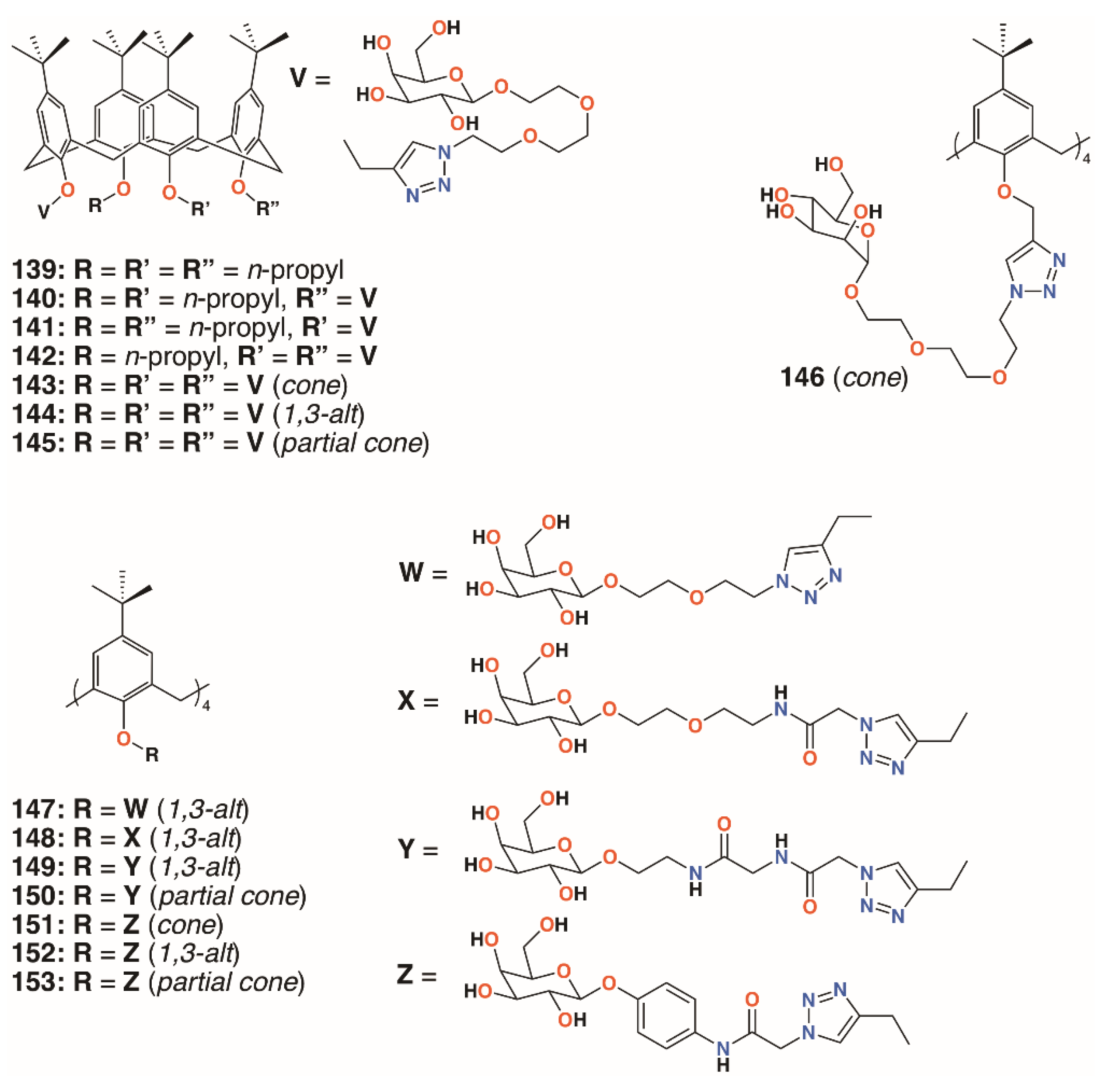











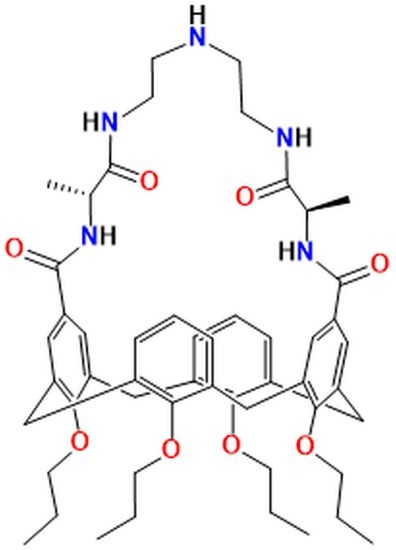
| Compound | Target | MIC/μg mL−1 | Ref |
|---|---|---|---|
| 5 | E. coli | 16 | [20] |
| S. aureus | 16 | [20] | |
| E. faecium | 16 | [20] | |
| 6 (cone) | E. faecalis | 8 | [23] |
| M. tuberculosis | 9.5 | [23] | |
| 6 (1,3-alt) | M. tuberculosis | 1.2 | [23] |
| 8 | E. coli | 4 | [25] |
| S. aureus | 8 | [25] | |
| Fe·47 | E. coli | 0.37 | [35] |
| S. albus | 0.37 | [35] | |
| R. stolonifera | 0.37 | [35] | |
| Cu2·47 | E. coli | 0.37 | [36] |
| S. albus | 0.37 | [36] | |
| R. stolonifera | 0.75 | [36] | |
| 59 | S. aureus | 7.8 | [41] |
| S. epidermidis | 7.8 | [41] | |
| MRSA | 15.6 | [41] | |
| B. subtilis | 15.6 | [41] | |
| P. aeruginosa | 15.6 | [41] | |
| 60 | S. aureus | 3.9 | [41] |
| S. epidermidis | 15.6 | [41] | |
| MRSA | 0.97 | [41] | |
| B. subtilis | 0.97 | [41] |
| Compound | Target | MIC/μg mL−1 | Ref |
|---|---|---|---|
| 5 | M. tuberculosis | 1.22 | [50] |
| 96 | M. tuberculosis | 1.89 | [50] |
| 105 | S. aureus | 1.95 | [53] |
| 109 | S. aureus | 1.95 | [55] |
| 122 | S. aureus | 4 | [59] |
| S. epidermidis | 16 | [59] | |
| 130 | S. aureus | 3.05 | [65] |
| S. epidermidis | 3.05 | [65] | |
| B. subtilis | 3.05 | [65] | |
| 131 | S. aureus | 4.32 | [65] |
| S. epidermidis | 4.32 | [65] | |
| B. subtilis | 4.32 | [65] | |
| 132 | S. aureus | 5.31 | [65] |
| S. epidermidis | 5.31 | [65] | |
| B. subtilis | 5.31 | [65] | |
| 133 | S. aureus | 6.64 | [65] |
| S. epidermidis | 6.64 | [65] | |
| B. subtilis | 6.64 | [65] |
| Compound | Target | MBIC/μg mL−1 | Ref |
|---|---|---|---|
| 154 | P. aeruginosa | 2 | [73] |
| 155 | P. aeruginosa | 1.1 | [74] |
| 158 | P. aeruginosa | 5.4 | [76] |
| 165 | S. aureus | 17.2 | [80] |
| 168 | L. pneumophila | 0.78 | [82] |
| 168·gatifloxacin | S. aureus | 0.16 | [82] |
| 169 | S. aureus | 6.25 | [83] |
| E. faecalis | 6.25 | [83] | |
| MRSA | 1.56 | [83] | |
| 172·clarithromycin | S. pneumoniae | 13.69 | [85] |
| 191 | S. aureus | 0.17 | [92] |
| E. faecalis | 0.47 | [92] | |
| 192 | S. aureus | 0.17 | [92] |
| E. faecalis | 0.47 | [92] | |
| 193 | S. aureus | 0.17 | [92] |
| E. faecalis | 0.47 | [92] | |
| 194 | S. aureus | 0.17 | [92] |
| E. faecalis | 0.47 | [92] | |
| 195 | S. aureus | 0.20 | [92] |
| E. faecalis | 0.20 | [92] | |
| S. mutans | 0.50 | [92] | |
| 198 | S. aureus | 0.11 | [92] |
| E. faecalis | 0.11 | [92] | |
| S. epidermidis | 0.23 | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurpik, D.N.; Padnya, P.L.; Stoikov, I.I.; Cragg, P.J. Antimicrobial Activity of Calixarenes and Related Macrocycles. Molecules 2020, 25, 5145. https://doi.org/10.3390/molecules25215145
Shurpik DN, Padnya PL, Stoikov II, Cragg PJ. Antimicrobial Activity of Calixarenes and Related Macrocycles. Molecules. 2020; 25(21):5145. https://doi.org/10.3390/molecules25215145
Chicago/Turabian StyleShurpik, Dmitriy N., Pavel L. Padnya, Ivan I. Stoikov, and Peter J. Cragg. 2020. "Antimicrobial Activity of Calixarenes and Related Macrocycles" Molecules 25, no. 21: 5145. https://doi.org/10.3390/molecules25215145
APA StyleShurpik, D. N., Padnya, P. L., Stoikov, I. I., & Cragg, P. J. (2020). Antimicrobial Activity of Calixarenes and Related Macrocycles. Molecules, 25(21), 5145. https://doi.org/10.3390/molecules25215145